(Created page with "<!-- metadata commented in wiki content <div class="center" style="width: auto; margin-left: auto; margin-right: auto;"> '''Neuroprotective Proficiency: Evaluating Sinapic A...") |
m (Catherinechen moved page Draft Chen 477408004 to Review 545687567396) |
(No difference)
| |
Revision as of 14:20, 30 January 2025
Abstract
The prevalence of Parkinson's Disease (PD) has doubled in the last 25 years, making it the second most common neurodegenerative disease in the US. Central to PD pathology is the degeneration of dopaminergic neurons within the substantia nigra pars compacta. Levodopa, a primary therapeutic agent, aids in dopamine production to alleviate symptoms of PD. However, it has side effects due to premature conversion of L-dopa into dopamine before passing the blood-brain barrier. Compounds such as sinapic acid, characterized by their methoxy and hydroxyl groups, possess antioxidant properties that can mitigate oxidative stress damaging mitochondrial DNA. This study aimed to mitigate parkinsonism effects from ɑ-synuclein mutation using sinapic acid (10, 20, 30 µM), levodopa 30µM, and sinapic acid 30µM + levodopa 30µM, assessing its impact on lifespan, dopamine concentration, movement speed, and memory, comparing it with Levodopa. 30125, 8848, and 8146 strained drosophila were crossed to obtain Mutant ɑ-synuclein Drosophila expressing GFP in dopaminergic neurons. Drosophila (wild and PD) were exposed to treatments, with assessments at 5 and 35 days old. Two-way ANOVA and post hoc Tukey/Scheffe analyses revealed that all 30µM treatment groups significantly reduced PD symptoms of PD strain drosophila (p<0.05). Sinapic acid 30µM + Levodopa 30µM exhibited the best results, attributed to sinapic acid's antioxidant abilities. It had a 43.7% increase in movement speed, a 56% increase in lifespan, and a 62.5% increase in memory. Long-term Levodopa showed increased detrimental effects due to its adverse impacts during consumption. Limitations included the absence of various chemicals (carbidopa and paraquat), suggesting the need for future studies with these chemicals and other model organisms (rats and C.elegans).
1. Introduction
Globally, the amount of Parkinson's Disease cases has doubled in the past 25 years, with the number of new cases annually increasing from 60,000 to nearly 90,000 [1]. Furthermore, in 2019, there were approximately 8.5 million individuals with Parkinson's Disease in the world. It is estimated that Parkinson's Disease causes 5.8 million disability-adjusted life years (DALYs), which is an increase of 81% compared to 2000. This led to 329,000 deaths related to Parkinson's Disease, which was an increase of over 100% since 2000 [2].
Parkinson’s Disease (PD) is the second most common neurodegenerative disease, where dopaminergic neurons are lost in the substantia nigra pars compacta[3]. Parkinson's disease leads to impaired motor function (tremors, slowness of movement, etc), and it is suggested that elevated oxidative stress and neuroinflammation may be responsible for dopaminergic neuronal atrophy and, ultimately, the clinical manifestation of PD [4]. To add on, although Parkinson's is a common neurodegenerative disease, there is no complete cure. However, modern medicine, surgical treatment, and other therapies can treat the symptoms. A standard medicine used for PD is Levodopa; Levodopa helps the nerve cells make dopamine, which is lacking in the brain of patients with PD. However, it has severe side effects on the patients, and if the treatment is stopped, it can also cause serious side effects, such as being unable to move or difficulty breathing [5]. Some side effects of patients taking Levodopa are hallucinations and delusions since Levodopa increases homocysteine levels. These effects are due to Levodopa prematurely turning into dopamine before reaching the blood-brain barrier. In addition, a high concentration of Levodopa is shown to be toxic, which can damage neuronal cells and can lead to apoptosis [6].
Hydroxycinnamic acids are phenolic compounds found in fruits and vegetables. Sinapic acid, one type of hydroxycinnamic acid, is most naturally found in Brassica family plants and has antioxidant, anti-inflammatory, anticancer, antimutagenic, antiglycemic, neuroprotective, and antibacterial properties [7]. Thus, with the lack of treatments for PD and the harmful side effects of current medicine, Levodopa, it is necessary to study effective and harmless treatments. The purpose of this experiment is to alleviate the Parkinsonian effects from ɑ-synuclein mutation using Sinapic acid (10, 20, 30 µM), Levodopa (30 µM), and sinapic acid + Levodopa (30µM) to show its phase-specific effect on lifespan, dopamine concentration, movement speed, and memory, and its comparison to Levodopa. For the alternative hypothesis, Sinapic acid will have a neuroprotective impact and reduce the severity of PD-associated defects in drosophila (climbing, memory, and dopamine). Additionally, long-term sinapic acid will have a more significant effect than Levodopa, while levodopa and sinapic acid will be the most effective. As for our null hypothesis, Sinapic acid does not affect the flies and shouldn't be used as an alternative to Levodopa.
2. Methodology
Drosophila melanogaster Strains [8]
Drosophila expressing UAS:mCD8-GFP under P{UAS(FRT.stop)mCD8-GFP.H}11 (stock #30125) with TH-GAL4 under P{ple-GAL4.F}3 (stock #8848), and UAS-Hsap under P{UAS-Hsap\SNCA.F}5B (stock #8146) Drosophila were obtained from Bloomington Stock Center.
Crossing the flies [9]
UAS-GFP was crossed with GAL4 for 24 hours, and the adult flies were removed. Drosophila expressing GFP in dopaminergic neurons was obtained. These were used as the control group drosophila. TH-GAL4 was crossed with UAS-Hsap to obtain drosophila expressing mutant ɑ-synuclein in dopaminergic neurons. The two resultant crosses were crossed to obtain Mutant ɑ-synuclein Drosophila expressing GFP in dopaminergic neurons. Adult flies were transferred to new vials every seven days and sexed. The experimental groups were sinapic acid of 10, 20, 30 µM, levodopa of 30 µM, sinapic acid and levodopa of 30 µM. The control group had only Carolina 4-24 drosophila media. These chemicals were placed on two groups of drosophila: Drosophila expressing GFP in dopaminergic neurons (control) and crossed PD drosophila. Each assay was done for each group 2 times (when the PD flies were 5 and 35 days old). There were three trials for each group/concentration. The independent variables are the concentrations of the chemicals stated. The dependent variables are the characteristics tested.
Rapid Iterative Negative Geotaxis (RING) assay [10]
Vials containing flies were assembled into the RING apparatus. The flies were left undisturbed for 15-20 minutes to familiarize themselves with the environment. A digital camera was placed 1 m in front of the apparatus to align the camera at the mid-height of the vials. A timer was set for 3 seconds. The apparatus was sharply tapped onto a table three times to ensure all the flies were knocked onto the bottom of the vials. The three-second timer was started simultaneously with the third tap. Reset the timer for 1 minute and start. During this time, the camera was reset and focused on the apparatus, and another channel of the timer was set for three seconds. After 1 minute, the steps were repeated. After 5-6 trials, images were uploaded onto a computer, and an image viewer was used to open and count the amount of flies past the 8 cm line.
Aversive phototaxic suppression assay (APS assay/memory) [11]
Flies of each group were placed in an empty polystyrene vial with water-moistened filter paper for six hours before the assay was conducted to make the flies more receptive to aversive tastes. The T-maze had two chambers (light and dark). The preparation of the chambers involved taking three drosophila vials attached to a t-connector with three openings. The one perpendicular to the other two was used as the entrance vial (where the drosophila was initially placed). The tube on the left was placed near a light source (a flashlight), while the other tube was covered with red film to serve as the "dark" chamber since drosophila is blind to red light. Filter paper was loaded with 180uL of either distilled water or a 0.1M quinine solution and placed in the light chamber. Flies were transferred into the perpendicular vial. The light source was turned on. The drosophila was banged down to establish the negative geotaxis response. If the fly moved to the lighted chamber within 10 seconds, it was positive phototactic and was prepared for the assay. If not, the fly was removed due to indications of visual system issues. For Aversive Phototaxic Suppression (APS) training, the fly that exhibited positive phototaxis was gently tapped back into the dark chamber. Filter paper containing a 0.1M quinine solution was placed in the lighted chamber. The light turned on, allowing the fly to walk into the lighted chamber coated with quinine. After one minute, the fly was tapped back into the dark chamber, and this process was repeated nine more times. For a short term, after training, the flies were placed back into their original vial for 30 min. After 30 min, the flies repeated three trials, as stated before, and recorded the number of times the flies avoided or went into the lighted vial. For the long term, the drosophila were tested after 24 hours.
Measuring lifespan [12]
Three identical groups were maintained for statistical accuracy. Lifespan was represented using 50-100 flies. The flies were synchronized and counted every 5 days. Flies were transferred to new vials one day after birth and once a week.
Statistical analysis
For all assays, a two-way ANOVA was run on SPSS. Then, the RING and APS assays were analyzed using the post-hoc Scheffe test. Similarly, lifespan was analyzed using the posthoc Tukey test. All tests were tested to a significant p-value of 0.05.
3. Results
The RING assay was conducted which evaluated the climbing ability of young and old Drosophila melanogaster (5-10 days old) in control, Parkinson-like, and treated groups.
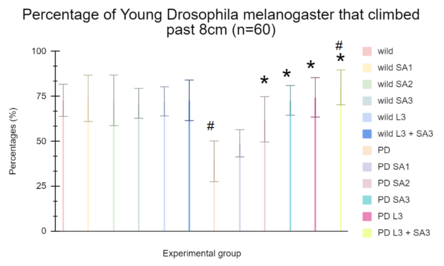
Figure #1: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. SA1 = 10 μM; SA2 = 20 μM; SA3 = 30 μM; L3 = 30μM. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3.
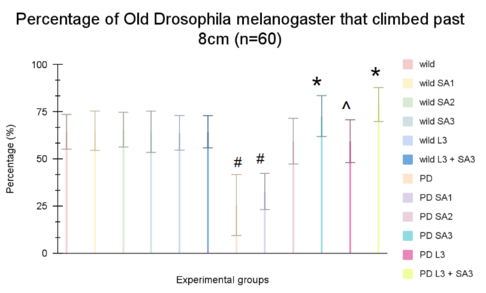
Figure #2: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3
It has been observed in Figure 1 that the control group averaged 72.77%, while wild flies treated with Sinapic acid (10, 20, 30μM), Levodopa (30μM), and combined Levodopa + Sinapic acid (30μM) showed averages of 73.88%, 72.77%, 71.11%, 72.22%, and 72.77%, respectively. Parkinson-like flies averaged 38.88%, and with the same treatments, they climbed 48.88%, 62.22%, 72.77%, 74.44%, and 80%. Two-way ANOVA and post hoc Scheffe test found that the Parkinson-only group and the combined Levodopa + Sinapic acid treatment differed significantly from controls (p < 0.05). All treated Parkinson groups showed significant improvement compared to untreated Parkinson flies (p < 0.05).
The memory retaining ability of the Drosophila flies was assessed using the APS assay at a young and old age.
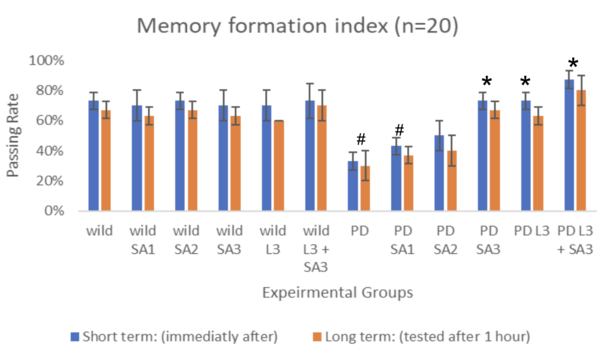
Figure #3: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to
PD(p<0.05). ^Significant to PD L3 + SA3 .
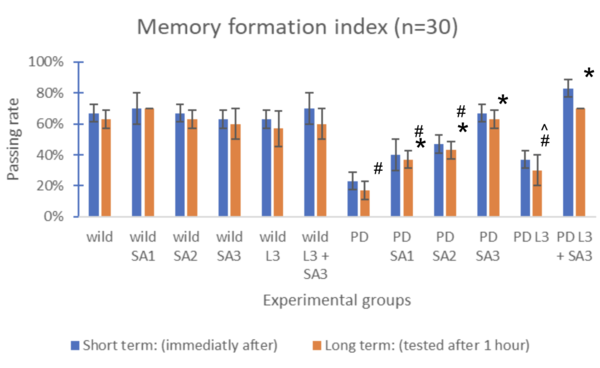
Figure #4: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 .
Drosophila melanogaster was assessed both short-term (immediately after) and long-term (tested after 1 hour), represented through blue and red bars, respectively. The control group (wild without treatment) averaged 73% for the short term and 67% for the long term. The Parkinson's group (PD) with no exposure to treatments averaged 33% for the short term and 30% for the long term. The same treatments were applied to flies expressing Parkison’s. The percentage of flies that successfully went to the dark vial for the short-term group was 43%, 50%, 73%, 73%, and 87%, respectively, and for the long-term group, 30%, 37%, 40%, 67%, 63%, and 80% respectively. The groups were compared and tested for significance using Two-way ANOVA and the post hoc Scheffe test. Through the analysis, compared to the control, PD flies only and PD with Sinapic acid 10μM were significant (p<0.05). Compared to the PD flies, only PD Sinaic acid 30μM, Levodopa 30μM, and SInapic acid and levodopa 30μM were significantly higher (p<0.05). In addition, there was significance (p<0.05) between the treatment groups of Levodopa 30μM and combined Levodopa + Sinapic acid. All significance was the same for both long and short-term tests. Treatment of sinapic acid was similar to treatment of Levodopa, while the treatment of sinapic acid + levodopa was the most effective (19.17% increase compared to just Levodopa for the short term). The older drosophila showed similar results; however, the passing rate of only the LD-treated drosophila decreased significantly to 37% for the short term and 30% for the long term.
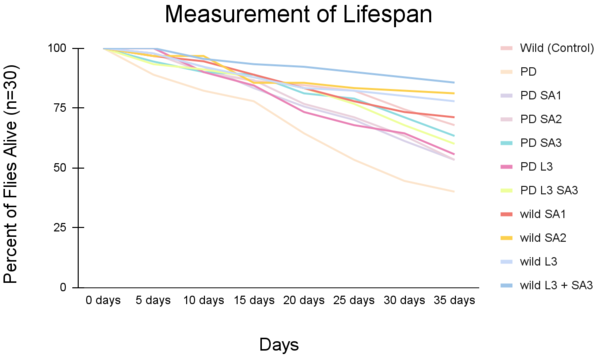
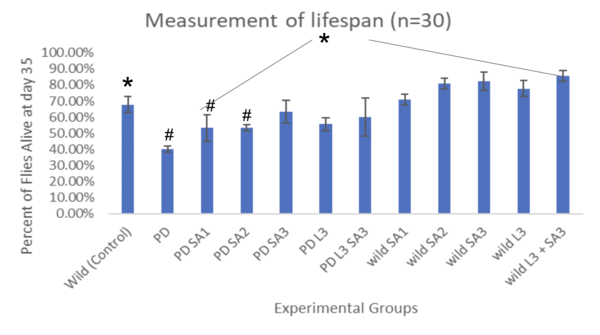
Figures 5 and 6 show the lifespan of the Drosophila flies (n=30) as measured via the lifespan assay over time.
Figure #5: Percent of drosophila alive through time (lifespan)(n=30). Statistical significance was determined using Anova: Two-Factor With Replication with a post-hoc Tukey Test (p<0.05). #Significant to control (p<0.05) *Significant to PD (p<0.05).
Figure #6: Percent of drosophila alive at day 35 (lifespan)(n=30). #Significant to control (p<0.05)*Significant to PD (p<0.05).
The lifespan of drosophila flies (n=30) was also evaluated as percentages (± 1 SE) every five days for 35 days (figure #5). On day 20, the control group had 84.5% flies alive, and the PD (with no treatment) had 64.4% alive. The percent of drosophila alive at day 20 were 75.6%, 76.7%, 81.1%, 73.3%, and 83.3% respectively. At day 30, the control group had 74.4%, and the PD (with no treatment) had 44.5% survival. The wild groups that received the treatment had averaged survival of 73.3%, 82.2%, 84.3%, 80%, and 87.8 respectively. The PD flies exposed to the treatment at day 30 had 61.2%, 63.3%, 71.1%, 64.4%, and 67.8%, respectively. The life span all showed a decreasing trend as the days increased. The most significant percentage of flies alive after 35 days was the wild flies treated with combined Levodopa + Sinapic acid. The PD flies given Sinapic acid 30μM in their diet were the most effective treatment in increasing the survival rate against Parkinson's. Similarly, groups given the treatments of Sinapic acid 10, 20μM, and Levodopa 30μM showed similar decreasing trends of lifespan. Additional significance was tested using Two-way ANOVA and the post hoc Tukey test (p<0.05). Significant differences were at day 35 and are indicated in Figure #6.
4. Discussion
SA 30µm, LD 30µm, and SA 30µm + LD 30µm significantly decreased the detrimental effects of PD on PD strain drosophila(p<0.05) compared to the unexposed PD drosophila. All treatments had no significant effect on wild types.
In the short term, SA 30µm had a similar effect compared to LD 30µm for all cases; SA 30µm + LD 30µm had the best results, all similar to the wild type. Dopaminergic neurons rely heavily on the ATP produced in mitochondria, which ROS can disturb. Antioxidants (SA) lower levels of oxidative stress, though when extreme levels are reached, can damage mitochondrial DNA, which in turn contributes to mitochondrial dysfunction. The antioxidants can also be protected against oxidative stress by remodeling a-synuclein aggregates into nontoxic fibrils and reducing ROS (reduced free radicals and cell damage) [13]. For sinapic acid, the presence of methoxy- and hydroxyl-- groups in the structure helps their antioxidant abilities. Sinapic acid, which contains a hydroxyl group, the OH, can donate a hydrogen atom to the free radical, which neutralizes. Sinapic acid reacts with free radicals, donating a hydrogen atom, forming water and a free molecule. Stabilizing the free radical prevents oxidative stress. A methoxy group with an OCH3 allows the methoxylation of phenolic compounds such as sinapic acid, which can increase their stability and enhance their antioxidant abilities by altering the electronic properties of the aromatic ring, making it less susceptible to oxidation. They are a part of the phenolic family, which has incredible antioxidant activity. ROS leads to oxidative damage of mitochondrial DNA, protein, and membrane, hindering its ability to synthesize DNA. Since PD is typically prevalent in the older population, the long-term effects of these treatments were also tested. Long-term consumption of SA caused a more considerable decrease in detrimental effects of PD compared to long-term consumption of LD. LD contains many drawbacks, such as hallucinations, delusions, damage to neural cells, and apoptosis of cells[14]. There is a premature change of Levodopa to dopamine before passing the blood-brain barrier [15]
5. Conclusion
(SA) shows potential neuroprotective activity in mitigating the effects of parkinsonism like-effects in a drosophila model, thus supporting the alternative hypothesis. Contrary to expectations, due to its side effects, sinapic acid performed better than Levodopa in the long term. This differs from other researchers because they tested Levodopa to be more beneficial than their similar antioxidants. This creates essential implications for sinapic acid in the long term to show its therapeutic benefits for PD treatments. The study also demonstrates that long-term Sinapic Acid consumption yields more significant benefits than Levodopa. This helps advance past research about using only Levodopa to treat Parkinson's disease by adding a more effective therapeutic approach (sinapic acid) in the long term. These findings highlight the potential of Sinapic Acid as a therapeutic approach for PD treatment, offering a safer and more effective alternative to conventional medications like Levodopa. Old Drosophila showed similar effects to the young ones, except the movement speed of the only Levodopa-treated drosophila decreased to 59.44%.
ACKNOWLEDGEMENT
We would like to acknowledge Alison Huenger, Dr. Allal Boutajangout, Melanie Greenwald, Marissa Alamo, Stephanie DiPreta, and Sheryl Idzik for their guidance and support in conducting this research. We also extend our thanks to the Manhasset Science Research Program for providing us with the opportunity to conduct this research.
6. Bibliography
[1] Ja, William W., et al. “Prandiology of Drosophila and the CAFE Assay.” PNAS, National Academy of Sciences, 15 May 2007, <www.pnas.org/content/104/20/8253>. <www.pnas.org/content/104/20/8253>.
[2] World Health Organization. (2023). Parkinson disease. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease#:~:text=Assessment%20and%20disease%20burden&text=Global%20estimates%20in%202019%20showed,of%20over%20100%25%20since%202000.
[3] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the drosophila PINK1 model of Parkinson's disease. Food & Function, 12(17), 7816–7824.
[4] Johnson, S. L., Park, H. Y., DaSilva, N. A., Vattem, D. A., Ma, H., & Seeram, N. P. (2018). Levodopa-Reduced Seed Extract Shows Neuroprotective Effects against Parkinson's Disease in Murine Microglia and Human Neuroblastoma Cells, and., (9), 1139.
[5] U.S. Department of Health and Human Services. (2022, April 14). Parkinson’s disease: Causes, symptoms, and treatments. National Institute on Aging. https://www. nia.nih.gov/health/parkinsons-disease#:~:text=Parkinson’s%20disease%20is%20a%20brain,have%20difficulty%20walking%20and%20talking.
[6] Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa (Levodopa) - statpearls - NCBI bookshelf. National Library of Medicine. https://www.ncbi.nlm. nih.gov/books /NBK482140/
[7] Nguyen, V. P. T., Stewart, J. D., Ioannou, I., & Allais, F. (2021). Sinapic acid and sinapate esters in Brassica: innate accumulation, biosynthesis, accessibility via chemical synthesis or recovery from biomass, and biological activities. Frontiers in Chemistry, 9. https://doi.org/10.3389/fchem.2021.664602
[8] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the drosophila PINK1 model of Parkinson's disease. Food & Function, 12(17), 7816–7824.
[9] Pulver, S. R., & Berni, J. (2012, October). The fundamentals of flying: Simple and inexpensive strategies for employing drosophila genetics in neuroscience teaching laboratories. Journal of undergraduate neuroscience education : JUNE : a publication of FUN, Faculty for Undergraduate Neuroscience. https://pubmed.ncbi.nlm.nih.gov/23493248/
[10] Nichols, Charles D., et al. “Methods to Assay Drosophila Behavior.” Advances in Pediatrics., U.S. National Library of Medicine, 7 Mar. 2012, <www.ncbi.nlm. <www.ncbi.nlm. nih.gov/pmc/articles/PMC3671839/>.
[11] Ali, Yousuf O., et al. “Assaying Locomotor, Learning, and Memory Deficits in Drosophila Models of Neurodegeneration | Protocol.” JoVE, 11 Mar. 2011, <www.jove.com/video/2504/assaying-locomotor-learning-memory-deficits-drosophila-models>. <www.jove.com/video/2504/assaying-locomotor-learning-memory-deficits-drosophila-models>.
[12] Linford NJ, Bilgir C, Ro J, Pletcher SD. Measurement of lifespan in Drosophila melanogaster. J Vis Exp. 2013 Jan 7;(71):50068. doi: 10.3791/50068. PMID: 23328955; PMCID: PMC3582515.
[13] Siddique, Y. H., Naz, F., & Jyoti, S. (2014). Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic drosophila model of Parkinson's disease. BioMed Research International, 2014, 1–6. https://doi.org/https://pubmed.ncbi.nlm.nih.gov/24860828/
[14]Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa (Levodopa) - statpearls - NCBI bookshelf. National Library of Medicine. https://www.ncbi.nlm. nih.gov/books /NBK482140/
[15] Bodkhe, S. N. (2020, September). A review on levodopa/carbidopa drug for the treatment of Parkinson... - IJSDR. International Journal of Scientific Development and Research. https://www.ijsdr.org/papers/IJSDR2009079.pdf
Document information
Published on 23/06/25
Submitted on 30/01/25
Volume 7, 2025
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?