m (Catherinechen moved page Draft Chen 477408004 to Review 545687567396) |
m (Tomamil moved page Review 545687567396 to Chen et al 2025c) |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 22: | Line 22: | ||
Parkinson’s Disease (PD) is the second most common neurodegenerative disease, where dopaminergic neurons are lost in the substantia nigra pars compacta[3]. Parkinson's disease leads to impaired motor function (tremors, slowness of movement, etc), and it is suggested that elevated oxidative stress and neuroinflammation may be responsible for dopaminergic neuronal atrophy and, ultimately, the clinical manifestation of PD [4]. To add on, although Parkinson's is a common neurodegenerative disease, there is no complete cure. However, modern medicine, surgical treatment, and other therapies can treat the symptoms. A standard medicine used for PD is Levodopa; Levodopa helps the nerve cells make dopamine, which is lacking in the brain of patients with PD. However, it has severe side effects on the patients, and if the treatment is stopped, it can also cause serious side effects, such as being unable to move or difficulty breathing [5]. Some side effects of patients taking Levodopa are hallucinations and delusions since Levodopa increases homocysteine levels. These effects are due to Levodopa prematurely turning into dopamine before reaching the blood-brain barrier. In addition, a high concentration of Levodopa is shown to be toxic, which can damage neuronal cells and can lead to apoptosis [6]. | Parkinson’s Disease (PD) is the second most common neurodegenerative disease, where dopaminergic neurons are lost in the substantia nigra pars compacta[3]. Parkinson's disease leads to impaired motor function (tremors, slowness of movement, etc), and it is suggested that elevated oxidative stress and neuroinflammation may be responsible for dopaminergic neuronal atrophy and, ultimately, the clinical manifestation of PD [4]. To add on, although Parkinson's is a common neurodegenerative disease, there is no complete cure. However, modern medicine, surgical treatment, and other therapies can treat the symptoms. A standard medicine used for PD is Levodopa; Levodopa helps the nerve cells make dopamine, which is lacking in the brain of patients with PD. However, it has severe side effects on the patients, and if the treatment is stopped, it can also cause serious side effects, such as being unable to move or difficulty breathing [5]. Some side effects of patients taking Levodopa are hallucinations and delusions since Levodopa increases homocysteine levels. These effects are due to Levodopa prematurely turning into dopamine before reaching the blood-brain barrier. In addition, a high concentration of Levodopa is shown to be toxic, which can damage neuronal cells and can lead to apoptosis [6]. | ||
| − | Hydroxycinnamic acids are phenolic compounds found in fruits and vegetables. Sinapic acid, one type of hydroxycinnamic acid, is most naturally found in Brassica family plants and has antioxidant, anti-inflammatory, anticancer, antimutagenic, antiglycemic, neuroprotective, and antibacterial properties [7]. Thus, with the lack of treatments for PD and the harmful side effects of current medicine, Levodopa, it is necessary to study effective and harmless treatments. The purpose of this experiment is to alleviate the Parkinsonian effects from ɑ-synuclein mutation using Sinapic acid (10, 20, 30 µM), Levodopa (30 µM), and sinapic acid + Levodopa (30µM) to show its phase-specific effect on lifespan, dopamine concentration, movement speed, and memory, and its comparison to Levodopa. For the alternative hypothesis, Sinapic acid will have a neuroprotective impact and reduce the severity of PD-associated defects in drosophila (climbing, memory, and dopamine). Additionally, long-term sinapic acid will have a more significant effect than Levodopa, while levodopa and sinapic acid will be the most effective. As for our null hypothesis, Sinapic acid does not affect the flies and shouldn't be used as an alternative to Levodopa. | + | Hydroxycinnamic acids are phenolic compounds found in fruits and vegetables. Sinapic acid, one type of hydroxycinnamic acid, is most naturally found in Brassica family plants and has antioxidant, anti-inflammatory, anticancer, antimutagenic, antiglycemic, neuroprotective, and antibacterial properties [7]. Thus, with the lack of treatments for PD and the harmful side effects of current medicine, Levodopa, it is necessary to study effective and harmless treatments. |
| + | - The purpose of this experiment is to alleviate the Parkinsonian effects from ɑ-synuclein mutation using Sinapic acid (10, 20, 30 µM), Levodopa (30 µM), and sinapic acid + Levodopa (30µM) to show its phase-specific effect on lifespan, dopamine concentration, movement speed, and memory, and its comparison to Levodopa. | ||
| + | - For the alternative hypothesis, Sinapic acid will have a neuroprotective impact and reduce the severity of PD-associated defects in drosophila (climbing, memory, and dopamine). Additionally, long-term sinapic acid will have a more significant effect than Levodopa, while levodopa and sinapic acid will be the most effective. - As for our null hypothesis, Sinapic acid does not affect the flies and shouldn't be used as an alternative to Levodopa. | ||
| − | = | + | ==Drosophila melanogaster Strains == |
| + | Drosophila expressing UAS:mCD8-GFP under P{UAS(FRT.stop)mCD8-GFP.H}11 (stock #30125), TH-GAL4 under P{ple-GAL4.F}3 (stock #8848), and UAS-Hsap under P{UAS-Hsap\SNCA.F}5B (stock #8146) were obtained from the Bloomington Stock Center. All flies were maintained at 25 ± 1°C and 60% relative humidity, under a 12:12 light-dark cycle to ensure consistent environmental conditions across all assays. | ||
| − | == | + | ==Crossing and Generation of PD Model Flies == |
| + | To generate the experimental lines, UAS-GFP was crossed with GAL4 for 24 hours, after which the adult flies were removed. This produced Drosophila expressing GFP in dopaminergic neurons, which were used as the control group. Separately, TH-GAL4 was crossed with UAS-Hsap to obtain Drosophila expressing mutant α-synuclein in dopaminergic neurons. These two resultant crosses were then mated to obtain the PD model: mutant α-synuclein Drosophila expressing GFP in dopaminergic neurons. Adult flies were sexed and transferred to new vials every seven days to maintain consistent density and nutrition. | ||
| − | + | ==Chemical Treatment and Grouping== | |
| + | The experimental treatment groups consisted of sinapic acid at 10, 20, and 30 µM, levodopa at 30 µM, and a combined treatment of sinapic acid and levodopa, each at 30 µM. Chemicals were administered through the media, and treatments were applied to both the GFP-expressing control flies and the PD model flies. The control group received only Carolina 4-24 Drosophila media. Each behavioral and physiological assay was performed at two time points: when the PD flies were 5 days old (young) and 35 days old (aged), with three biological replicates for each concentration and group. The independent variables were the concentrations and combinations of sinapic acid and levodopa, while the dependent variables were climbing ability, memory performance, and lifespan. | ||
| − | == | + | ==Rapid Iterative Negative Geotaxis (RING) Assay == |
| + | The RING assay was used to measure locomotor ability. Vials containing flies were assembled into the RING apparatus and left undisturbed for 15–20 minutes to allow acclimation. A digital camera was placed 1 meter in front of the apparatus, aligned at mid-vial height. A timer was set for 3 seconds. The apparatus was tapped sharply on a table three times to knock all flies to the bottom of the vials, and the timer was started simultaneously with the third tap. After a 1-minute reset period, the procedure was repeated. This sequence was carried out for 5–6 trials per group. Images were uploaded to a computer and analyzed using an image viewer to count the number of flies that climbed past the 8 cm line. | ||
| − | + | ==Aversive Phototaxic Suppression (APS) Assay == | |
| − | == | + | ===Preparation and Conditioning=== |
| + | To assess memory performance, flies from each experimental group were first conditioned to enhance responsiveness to aversive stimuli. Each group of flies was placed in an empty polystyrene vial lined with water-moistened filter paper for six hours. This period of mild deprivation increased their receptiveness to the bitter compound used in the assay. | ||
| − | + | ===T-Maze Setup=== | |
| + | The APS assay was performed using a T-maze apparatus composed of three interconnected drosophila vials arranged in a T-formation. The perpendicular vial served as the entrance chamber where flies were initially placed. One arm of the T-maze was left transparent and placed near a flashlight to create the "light" chamber, while the opposite arm was covered with red transparency film to create a "dark" chamber, since drosophila are blind to red light. | ||
| − | + | Each light chamber vial contained a filter paper saturated with either distilled water or a 0.1 M quinine solution (a bitter tastant). The quinine solution was used during the training trials to establish a negative association with the light chamber. | |
| − | ' | + | |
| + | ===Phototaxis Screening and Fly Selection=== | ||
| + | Flies were tapped down into the entry vial and allowed to respond to the light stimulus. If a fly entered the light chamber within 10 seconds, it was considered positively phototactic and selected for the training trials. Flies that failed to respond were excluded due to potential defects in the visual system, which could affect the assay's reliability. | ||
| − | == | + | ===Training Trials=== |
| + | Positively phototactic flies were gently tapped into the dark chamber to begin the training protocol. The light chamber was prepared with 180 µL of 0.1 M quinine-soaked filter paper, and the light source was turned on. Each fly was allowed to voluntarily enter the quinine-treated light chamber, remain there for one minute, and was then tapped back into the dark chamber. This process was repeated for a total of ten training trials to establish the aversive association between light and quinine exposure. | ||
| − | + | ===Short-Term Memory Test (30 Minutes Post-Training)=== | |
| + | After training, flies were returned to their original vials for 30 minutes. Following this rest period, each fly underwent three test trials using the same T-maze setup, without additional quinine exposure. The number of flies that avoided or entered the light chamber during these trials was recorded and used as a measure of short-term memory retention. | ||
| − | == | + | ===Long-Term Memory Test (24 Hours Post-Training)=== |
| + | To evaluate long-term memory, the same flies were tested again 24 hours after training using the same protocol. Flies were reintroduced to the T-maze, and their responses to the light chamber were recorded across three trials. Avoidance of the light chamber indicated successful memory retention of the aversive experience. | ||
| − | |||
| − | == | + | ==Lifespan Assay == |
| + | Three biologically identical groups were maintained for each condition to ensure statistical reliability. Each group consisted of 50–100 synchronized flies. Flies were transferred to new vials one day after birth and weekly thereafter. The number of surviving flies was recorded every 5 days until Day 35. Environmental conditions were kept constant throughout the assay. | ||
| − | + | ==Statistical Analysis== | |
| + | Data from all assays were analyzed using two-way ANOVA in SPSS, with significance set at p < 0.05. Post hoc analyses were conducted using the Scheffe test for the RING and APS assays, and the Tukey test for the lifespan assay. Each experimental condition was tested with at least three replicates, and standard error values were reported where appropriate. | ||
=3. Results= | =3. Results= | ||
| Line 58: | Line 72: | ||
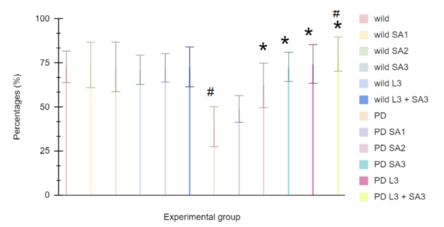
| − | [[ | + | [[File:Figure number 1.png|centre|444x444px|Figure #1: Climbing ability of young wild type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. SA1 = 10 μM; SA2 = 20 μM; SA3 = 30 μM; L3 = 30μM. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3.]] |
| − | + | ||
Figure #1: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. SA1 = 10 μM; SA2 = 20 μM; SA3 = 30 μM; L3 = 30μM. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3. | Figure #1: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. SA1 = 10 μM; SA2 = 20 μM; SA3 = 30 μM; L3 = 30μM. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3. | ||
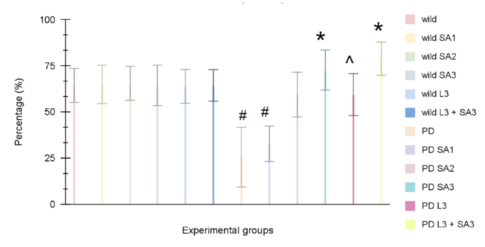
| − | [[ | + | [[File:Figure number 2 .png|centre|485x485px|Figure #2: Climbing ability of old wild type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3]] |
| − | + | ||
Figure #2: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 | Figure #2: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 | ||
| Line 72: | Line 84: | ||
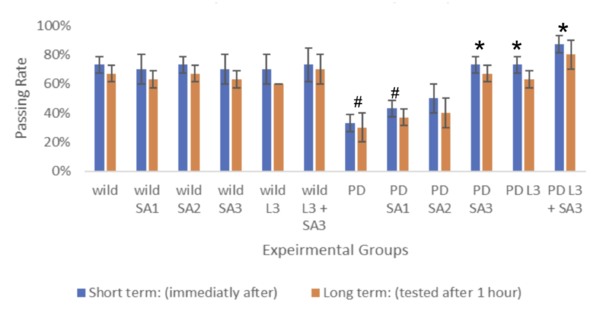
| − | [[ | + | [[File:FIgure number 3.png|centre|600x600px|Figure #3: Memory formation index for young drosophila (n=30) #Significant to control(p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 . ]] |
| − | + | ||
Figure #3: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to | Figure #3: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to | ||
| Line 79: | Line 90: | ||
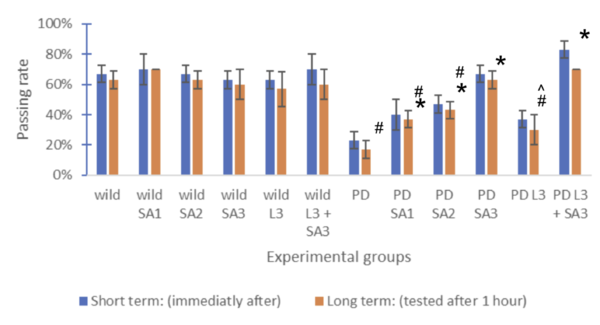
| − | [[ | + | [[File:Figure number 4.png|centre|600x600px|Figure #4: Memory formation index for old drosophila (n=30) #Significant to control(p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 .]] |
| − | + | ||
Figure #4: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 . | Figure #4: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 . | ||
| Line 87: | Line 97: | ||
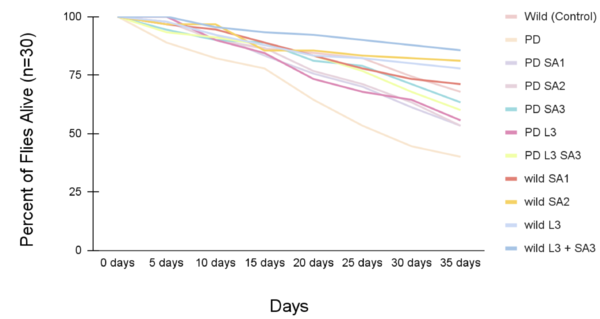
Figures 5 and 6 show the lifespan of the Drosophila flies (n=30) as measured via the lifespan assay over time. | Figures 5 and 6 show the lifespan of the Drosophila flies (n=30) as measured via the lifespan assay over time. | ||
| − | [[ | + | [[File:Figure number 5.png|600x600px]] |
''' ''' | ''' ''' | ||
| Line 93: | Line 103: | ||
| − | [[ | + | [[File:Figure number 6.png|centre|599x599px|Figure #6: Percent of drosophila alive at day 35 (lifespan)(n=30). #Significant to control (p<0.05)*Significant to PD (p<0.05).]] |
| − | + | ||
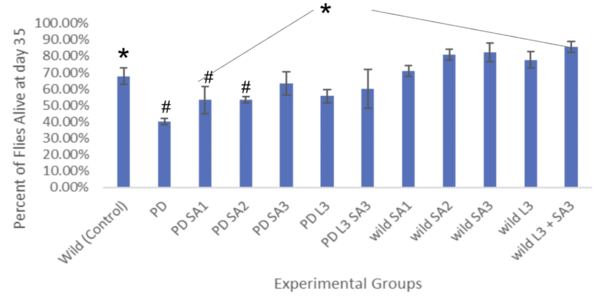
Figure #6: Percent of drosophila alive at day 35 (lifespan)(n=30). #Significant to control (p<0.05)*Significant to PD (p<0.05). | Figure #6: Percent of drosophila alive at day 35 (lifespan)(n=30). #Significant to control (p<0.05)*Significant to PD (p<0.05). | ||
| Line 101: | Line 110: | ||
=4. Discussion= | =4. Discussion= | ||
| − | SA | + | Sinapic acid (SA) at 30 µM, Levodopa (LD) at 30 µM, and the combined treatment of SA 30 µM + LD 30 µM significantly alleviated the detrimental effects associated with Parkinson’s Disease (PD) in mutant α-synuclein Drosophila (p < 0.05), while showing no significant effect on wild-type flies. These findings support the neuroprotective role of sinapic acid and highlight the additive or possibly synergistic effect when combined with levodopa. |
| − | + | Across all behavioral assays—movement (RING), memory (APS), and lifespan-flies treated with SA 30 µM + LD 30 µM demonstrated the greatest improvement, with performance metrics approaching or exceeding wild-type controls. Notably, movement speed increased by 43.7%, memory retention by 62.5%, and lifespan by 56% compared to untreated PD flies. These effect sizes underscore the robustness of the treatment, especially in contrast to LD-only groups, which saw greater declines over time, particularly in older flies where movement speed dropped to 59.44%. | |
| + | |||
| + | Mechanistically, the results suggest that SA’s effectiveness is rooted in its antioxidant properties. Dopaminergic neurons are highly energy-dependent and thus particularly susceptible to oxidative damage from reactive oxygen species (ROS), which can disrupt mitochondrial ATP synthesis. Sinapic acid contains both methoxy (-OCH₃) and hydroxyl (-OH) groups, which contribute to its free radical-scavenging ability. The hydroxyl group can donate hydrogen atoms to neutralize radicals, while methoxylation enhances molecular stability and electron distribution, making phenolic rings less prone to oxidation. This aligns with prior research on phenolic antioxidants reducing ROS, stabilizing mitochondrial function, and remodeling toxic α-synuclein aggregates into less harmful forms [13]. | ||
| + | |||
| + | While both SA and LD improved short-term outcomes in PD flies, long-term effects revealed a divergence. Chronic LD exposure led to reduced efficacy and signs of neurotoxicity, consistent with known adverse effects such as hallucinations, neuronal apoptosis, and premature peripheral dopamine conversion [14][15]. In contrast, long-term SA treatment sustained or improved behavioral and survival outcomes, indicating superior tolerability and efficacy. | ||
| + | |||
| + | Potential variability, such as inter-trial differences in fly behavior or micro-environmental changes during aging, was minimized through replication (n=30 per group) and statistical validation (Two-way ANOVA and post hoc tests). However, one limitation is the absence of co-administered carbidopa, which could have mitigated LD’s premature conversion and allowed a more accurate comparison. Furthermore, while Drosophila offers a genetically tractable model, extrapolation to humans requires validation in higher organisms, such as C. elegans or rodent models, especially for understanding pharmacokinetics and blood-brain barrier dynamics. Although treatment effects were measured across multiple physiological and behavioral dimensions, the manuscript could benefit from acknowledging additional potential confounding factors. Environmental variables such as temperature, humidity, and light cycles, which can significantly influence Drosophila behavior and survival, were not explicitly controlled or reported. | ||
=5. Conclusion= | =5. Conclusion= | ||
| Line 114: | Line 129: | ||
=6. Bibliography= | =6. Bibliography= | ||
| + | [1] Ja, W. W., Carvalho, G. B., Mak, E. M., De La Rosa, N. N., Fang, A. Y., Liong, J. C., ... & Benzer, S. (2007). Prandiology of Drosophila and the CAFE assay. Proceedings of the National Academy of Sciences, 104(20), 8253–8256. https://doi.org/10.1073/pnas.0702726104 | ||
| − | + | [2] World Health Organization. (2023). Parkinson disease. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease | |
| − | + | ||
| − | [2] | + | |
| − | [3] | + | [3] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson's disease. Food & Function, 12(17), 7816–7824. https://doi.org/10.1039/D1FO01244G |
| − | [4] Johnson, S. L., Park, H. Y., DaSilva, N. A., Vattem, D. A., Ma, H., & Seeram, N. P. (2018). Levodopa- | + | [4] Johnson, S. L., Park, H. Y., DaSilva, N. A., Vattem, D. A., Ma, H., & Seeram, N. P. (2018). Levodopa-reduced seed extract shows neuroprotective effects against Parkinson's disease in murine microglia and human neuroblastoma cells. Nutrients, 10(9), 1139. https://doi.org/10.3390/nu10091139 |
| − | [5] U.S. Department of Health and Human Services. (2022, April 14). Parkinson’s disease: Causes, symptoms, and treatments. National Institute on Aging. | + | [5] U.S. Department of Health and Human Services. (2022, April 14). Parkinson’s disease: Causes, symptoms, and treatments. National Institute on Aging. https://www.nia.nih.gov/health/parkinsons-disease |
| − | [6] Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa | + | [6] Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa. In StatPearls. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK482140/ |
| − | [7] | + | [7] Nguyen, V. P. T., Stewart, J. D., Ioannou, I., & Allais, F. (2021). Sinapic acid and sinapate esters in Brassica: Innate accumulation, biosynthesis, accessibility via chemical synthesis or recovery from biomass, and biological activities. Frontiers in Chemistry, 9, Article 664602. https://doi.org/10.3389/fchem.2021.664602 |
| − | [8] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the | + | [8] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson's disease. Food & Function, 12(17), 7816–7824. https://doi.org/10.1039/D1FO01244G |
| − | [9] Pulver, S. R., & Berni, J. (2012 | + | [9] Pulver, S. R., & Berni, J. (2012). The fundamentals of flying: Simple and inexpensive strategies for employing Drosophila genetics in neuroscience teaching laboratories. Journal of Undergraduate Neuroscience Education, 11(1), A139–A148. https://pubmed.ncbi.nlm.nih.gov/23493248/ |
| − | [10] Nichols, | + | [10] Nichols, C. D., Becnel, J., & Pandey, U. B. (2012). Methods to assay Drosophila behavior. Journal of Visualized Experiments, 61, e3679. https://doi.org/10.3791/3679 |
| − | [11] Ali, | + | [11] Ali, Y. O., Escala, W., Ruan, K., & Zhai, R. G. (2011). Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. Journal of Visualized Experiments, 49, e2504. https://doi.org/10.3791/2504 |
| − | [12] Linford | + | [12] Linford, N. J., Bilgir, C., Ro, J., & Pletcher, S. D. (2013). Measurement of lifespan in Drosophila melanogaster. Journal of Visualized Experiments, 71, e50068. https://doi.org/10.3791/50068 |
| − | [13] Siddique, Y. H., Naz, F., & Jyoti, S. (2014). Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic | + | [13] Siddique, Y. H., Naz, F., & Jyoti, S. (2014). Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. BioMed Research International, 2014, Article 606928. https://doi.org/10.1155/2014/606928 |
| − | [14]Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa | + | [14] Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa. In StatPearls. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK482140/ |
| − | [15] Bodkhe, S. N. (2020, September). A review on levodopa/carbidopa drug for the treatment of | + | [15] Bodkhe, S. N. (2020, September). A review on levodopa/carbidopa drug for the treatment of Parkinson’s disease. International Journal of Scientific Development and Research, 5(9), 233–237. https://www.ijsdr.org/papers/IJSDR2009079.pdf |
Latest revision as of 15:46, 23 June 2025
Abstract
The prevalence of Parkinson's Disease (PD) has doubled in the last 25 years, making it the second most common neurodegenerative disease in the US. Central to PD pathology is the degeneration of dopaminergic neurons within the substantia nigra pars compacta. Levodopa, a primary therapeutic agent, aids in dopamine production to alleviate symptoms of PD. However, it has side effects due to premature conversion of L-dopa into dopamine before passing the blood-brain barrier. Compounds such as sinapic acid, characterized by their methoxy and hydroxyl groups, possess antioxidant properties that can mitigate oxidative stress damaging mitochondrial DNA. This study aimed to mitigate parkinsonism effects from ɑ-synuclein mutation using sinapic acid (10, 20, 30 µM), levodopa 30µM, and sinapic acid 30µM + levodopa 30µM, assessing its impact on lifespan, dopamine concentration, movement speed, and memory, comparing it with Levodopa. 30125, 8848, and 8146 strained drosophila were crossed to obtain Mutant ɑ-synuclein Drosophila expressing GFP in dopaminergic neurons. Drosophila (wild and PD) were exposed to treatments, with assessments at 5 and 35 days old. Two-way ANOVA and post hoc Tukey/Scheffe analyses revealed that all 30µM treatment groups significantly reduced PD symptoms of PD strain drosophila (p<0.05). Sinapic acid 30µM + Levodopa 30µM exhibited the best results, attributed to sinapic acid's antioxidant abilities. It had a 43.7% increase in movement speed, a 56% increase in lifespan, and a 62.5% increase in memory. Long-term Levodopa showed increased detrimental effects due to its adverse impacts during consumption. Limitations included the absence of various chemicals (carbidopa and paraquat), suggesting the need for future studies with these chemicals and other model organisms (rats and C.elegans).
1. Introduction
Globally, the amount of Parkinson's Disease cases has doubled in the past 25 years, with the number of new cases annually increasing from 60,000 to nearly 90,000 [1]. Furthermore, in 2019, there were approximately 8.5 million individuals with Parkinson's Disease in the world. It is estimated that Parkinson's Disease causes 5.8 million disability-adjusted life years (DALYs), which is an increase of 81% compared to 2000. This led to 329,000 deaths related to Parkinson's Disease, which was an increase of over 100% since 2000 [2].
Parkinson’s Disease (PD) is the second most common neurodegenerative disease, where dopaminergic neurons are lost in the substantia nigra pars compacta[3]. Parkinson's disease leads to impaired motor function (tremors, slowness of movement, etc), and it is suggested that elevated oxidative stress and neuroinflammation may be responsible for dopaminergic neuronal atrophy and, ultimately, the clinical manifestation of PD [4]. To add on, although Parkinson's is a common neurodegenerative disease, there is no complete cure. However, modern medicine, surgical treatment, and other therapies can treat the symptoms. A standard medicine used for PD is Levodopa; Levodopa helps the nerve cells make dopamine, which is lacking in the brain of patients with PD. However, it has severe side effects on the patients, and if the treatment is stopped, it can also cause serious side effects, such as being unable to move or difficulty breathing [5]. Some side effects of patients taking Levodopa are hallucinations and delusions since Levodopa increases homocysteine levels. These effects are due to Levodopa prematurely turning into dopamine before reaching the blood-brain barrier. In addition, a high concentration of Levodopa is shown to be toxic, which can damage neuronal cells and can lead to apoptosis [6].
Hydroxycinnamic acids are phenolic compounds found in fruits and vegetables. Sinapic acid, one type of hydroxycinnamic acid, is most naturally found in Brassica family plants and has antioxidant, anti-inflammatory, anticancer, antimutagenic, antiglycemic, neuroprotective, and antibacterial properties [7]. Thus, with the lack of treatments for PD and the harmful side effects of current medicine, Levodopa, it is necessary to study effective and harmless treatments. - The purpose of this experiment is to alleviate the Parkinsonian effects from ɑ-synuclein mutation using Sinapic acid (10, 20, 30 µM), Levodopa (30 µM), and sinapic acid + Levodopa (30µM) to show its phase-specific effect on lifespan, dopamine concentration, movement speed, and memory, and its comparison to Levodopa. - For the alternative hypothesis, Sinapic acid will have a neuroprotective impact and reduce the severity of PD-associated defects in drosophila (climbing, memory, and dopamine). Additionally, long-term sinapic acid will have a more significant effect than Levodopa, while levodopa and sinapic acid will be the most effective. - As for our null hypothesis, Sinapic acid does not affect the flies and shouldn't be used as an alternative to Levodopa.
Drosophila melanogaster Strains
Drosophila expressing UAS:mCD8-GFP under P{UAS(FRT.stop)mCD8-GFP.H}11 (stock #30125), TH-GAL4 under P{ple-GAL4.F}3 (stock #8848), and UAS-Hsap under P{UAS-Hsap\SNCA.F}5B (stock #8146) were obtained from the Bloomington Stock Center. All flies were maintained at 25 ± 1°C and 60% relative humidity, under a 12:12 light-dark cycle to ensure consistent environmental conditions across all assays.
Crossing and Generation of PD Model Flies
To generate the experimental lines, UAS-GFP was crossed with GAL4 for 24 hours, after which the adult flies were removed. This produced Drosophila expressing GFP in dopaminergic neurons, which were used as the control group. Separately, TH-GAL4 was crossed with UAS-Hsap to obtain Drosophila expressing mutant α-synuclein in dopaminergic neurons. These two resultant crosses were then mated to obtain the PD model: mutant α-synuclein Drosophila expressing GFP in dopaminergic neurons. Adult flies were sexed and transferred to new vials every seven days to maintain consistent density and nutrition.
Chemical Treatment and Grouping
The experimental treatment groups consisted of sinapic acid at 10, 20, and 30 µM, levodopa at 30 µM, and a combined treatment of sinapic acid and levodopa, each at 30 µM. Chemicals were administered through the media, and treatments were applied to both the GFP-expressing control flies and the PD model flies. The control group received only Carolina 4-24 Drosophila media. Each behavioral and physiological assay was performed at two time points: when the PD flies were 5 days old (young) and 35 days old (aged), with three biological replicates for each concentration and group. The independent variables were the concentrations and combinations of sinapic acid and levodopa, while the dependent variables were climbing ability, memory performance, and lifespan.
Rapid Iterative Negative Geotaxis (RING) Assay
The RING assay was used to measure locomotor ability. Vials containing flies were assembled into the RING apparatus and left undisturbed for 15–20 minutes to allow acclimation. A digital camera was placed 1 meter in front of the apparatus, aligned at mid-vial height. A timer was set for 3 seconds. The apparatus was tapped sharply on a table three times to knock all flies to the bottom of the vials, and the timer was started simultaneously with the third tap. After a 1-minute reset period, the procedure was repeated. This sequence was carried out for 5–6 trials per group. Images were uploaded to a computer and analyzed using an image viewer to count the number of flies that climbed past the 8 cm line.
Aversive Phototaxic Suppression (APS) Assay
Preparation and Conditioning
To assess memory performance, flies from each experimental group were first conditioned to enhance responsiveness to aversive stimuli. Each group of flies was placed in an empty polystyrene vial lined with water-moistened filter paper for six hours. This period of mild deprivation increased their receptiveness to the bitter compound used in the assay.
T-Maze Setup
The APS assay was performed using a T-maze apparatus composed of three interconnected drosophila vials arranged in a T-formation. The perpendicular vial served as the entrance chamber where flies were initially placed. One arm of the T-maze was left transparent and placed near a flashlight to create the "light" chamber, while the opposite arm was covered with red transparency film to create a "dark" chamber, since drosophila are blind to red light.
Each light chamber vial contained a filter paper saturated with either distilled water or a 0.1 M quinine solution (a bitter tastant). The quinine solution was used during the training trials to establish a negative association with the light chamber.
Phototaxis Screening and Fly Selection
Flies were tapped down into the entry vial and allowed to respond to the light stimulus. If a fly entered the light chamber within 10 seconds, it was considered positively phototactic and selected for the training trials. Flies that failed to respond were excluded due to potential defects in the visual system, which could affect the assay's reliability.
Training Trials
Positively phototactic flies were gently tapped into the dark chamber to begin the training protocol. The light chamber was prepared with 180 µL of 0.1 M quinine-soaked filter paper, and the light source was turned on. Each fly was allowed to voluntarily enter the quinine-treated light chamber, remain there for one minute, and was then tapped back into the dark chamber. This process was repeated for a total of ten training trials to establish the aversive association between light and quinine exposure.
Short-Term Memory Test (30 Minutes Post-Training)
After training, flies were returned to their original vials for 30 minutes. Following this rest period, each fly underwent three test trials using the same T-maze setup, without additional quinine exposure. The number of flies that avoided or entered the light chamber during these trials was recorded and used as a measure of short-term memory retention.
Long-Term Memory Test (24 Hours Post-Training)
To evaluate long-term memory, the same flies were tested again 24 hours after training using the same protocol. Flies were reintroduced to the T-maze, and their responses to the light chamber were recorded across three trials. Avoidance of the light chamber indicated successful memory retention of the aversive experience.
Lifespan Assay
Three biologically identical groups were maintained for each condition to ensure statistical reliability. Each group consisted of 50–100 synchronized flies. Flies were transferred to new vials one day after birth and weekly thereafter. The number of surviving flies was recorded every 5 days until Day 35. Environmental conditions were kept constant throughout the assay.
Statistical Analysis
Data from all assays were analyzed using two-way ANOVA in SPSS, with significance set at p < 0.05. Post hoc analyses were conducted using the Scheffe test for the RING and APS assays, and the Tukey test for the lifespan assay. Each experimental condition was tested with at least three replicates, and standard error values were reported where appropriate.
3. Results
The RING assay was conducted which evaluated the climbing ability of young and old Drosophila melanogaster (5-10 days old) in control, Parkinson-like, and treated groups.
Figure #1: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. SA1 = 10 μM; SA2 = 20 μM; SA3 = 30 μM; L3 = 30μM. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3.
Figure #2: Climbing ability of young wild-type Drosophila melanogaster. Data is the percent (± 1 SE) of the number of flies. #Significant to control (p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3
It has been observed in Figure 1 that the control group averaged 72.77%, while wild flies treated with Sinapic acid (10, 20, 30μM), Levodopa (30μM), and combined Levodopa + Sinapic acid (30μM) showed averages of 73.88%, 72.77%, 71.11%, 72.22%, and 72.77%, respectively. Parkinson-like flies averaged 38.88%, and with the same treatments, they climbed 48.88%, 62.22%, 72.77%, 74.44%, and 80%. Two-way ANOVA and post hoc Scheffe test found that the Parkinson-only group and the combined Levodopa + Sinapic acid treatment differed significantly from controls (p < 0.05). All treated Parkinson groups showed significant improvement compared to untreated Parkinson flies (p < 0.05).
The memory retaining ability of the Drosophila flies was assessed using the APS assay at a young and old age.
Figure #3: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to
PD(p<0.05). ^Significant to PD L3 + SA3 .
Figure #4: Memory formation index (n=30) #Significant to control(p<0.05) *Significant to PD(p<0.05). ^Significant to PD L3 + SA3 .
Drosophila melanogaster was assessed both short-term (immediately after) and long-term (tested after 1 hour), represented through blue and red bars, respectively. The control group (wild without treatment) averaged 73% for the short term and 67% for the long term. The Parkinson's group (PD) with no exposure to treatments averaged 33% for the short term and 30% for the long term. The same treatments were applied to flies expressing Parkison’s. The percentage of flies that successfully went to the dark vial for the short-term group was 43%, 50%, 73%, 73%, and 87%, respectively, and for the long-term group, 30%, 37%, 40%, 67%, 63%, and 80% respectively. The groups were compared and tested for significance using Two-way ANOVA and the post hoc Scheffe test. Through the analysis, compared to the control, PD flies only and PD with Sinapic acid 10μM were significant (p<0.05). Compared to the PD flies, only PD Sinaic acid 30μM, Levodopa 30μM, and SInapic acid and levodopa 30μM were significantly higher (p<0.05). In addition, there was significance (p<0.05) between the treatment groups of Levodopa 30μM and combined Levodopa + Sinapic acid. All significance was the same for both long and short-term tests. Treatment of sinapic acid was similar to treatment of Levodopa, while the treatment of sinapic acid + levodopa was the most effective (19.17% increase compared to just Levodopa for the short term). The older drosophila showed similar results; however, the passing rate of only the LD-treated drosophila decreased significantly to 37% for the short term and 30% for the long term.
Figures 5 and 6 show the lifespan of the Drosophila flies (n=30) as measured via the lifespan assay over time.
Figure #5: Percent of drosophila alive through time (lifespan)(n=30). Statistical significance was determined using Anova: Two-Factor With Replication with a post-hoc Tukey Test (p<0.05). #Significant to control (p<0.05) *Significant to PD (p<0.05).
Figure #6: Percent of drosophila alive at day 35 (lifespan)(n=30). #Significant to control (p<0.05)*Significant to PD (p<0.05).
The lifespan of drosophila flies (n=30) was also evaluated as percentages (± 1 SE) every five days for 35 days (figure #5). On day 20, the control group had 84.5% flies alive, and the PD (with no treatment) had 64.4% alive. The percent of drosophila alive at day 20 were 75.6%, 76.7%, 81.1%, 73.3%, and 83.3% respectively. At day 30, the control group had 74.4%, and the PD (with no treatment) had 44.5% survival. The wild groups that received the treatment had averaged survival of 73.3%, 82.2%, 84.3%, 80%, and 87.8 respectively. The PD flies exposed to the treatment at day 30 had 61.2%, 63.3%, 71.1%, 64.4%, and 67.8%, respectively. The life span all showed a decreasing trend as the days increased. The most significant percentage of flies alive after 35 days was the wild flies treated with combined Levodopa + Sinapic acid. The PD flies given Sinapic acid 30μM in their diet were the most effective treatment in increasing the survival rate against Parkinson's. Similarly, groups given the treatments of Sinapic acid 10, 20μM, and Levodopa 30μM showed similar decreasing trends of lifespan. Additional significance was tested using Two-way ANOVA and the post hoc Tukey test (p<0.05). Significant differences were at day 35 and are indicated in Figure #6.
4. Discussion
Sinapic acid (SA) at 30 µM, Levodopa (LD) at 30 µM, and the combined treatment of SA 30 µM + LD 30 µM significantly alleviated the detrimental effects associated with Parkinson’s Disease (PD) in mutant α-synuclein Drosophila (p < 0.05), while showing no significant effect on wild-type flies. These findings support the neuroprotective role of sinapic acid and highlight the additive or possibly synergistic effect when combined with levodopa.
Across all behavioral assays—movement (RING), memory (APS), and lifespan-flies treated with SA 30 µM + LD 30 µM demonstrated the greatest improvement, with performance metrics approaching or exceeding wild-type controls. Notably, movement speed increased by 43.7%, memory retention by 62.5%, and lifespan by 56% compared to untreated PD flies. These effect sizes underscore the robustness of the treatment, especially in contrast to LD-only groups, which saw greater declines over time, particularly in older flies where movement speed dropped to 59.44%.
Mechanistically, the results suggest that SA’s effectiveness is rooted in its antioxidant properties. Dopaminergic neurons are highly energy-dependent and thus particularly susceptible to oxidative damage from reactive oxygen species (ROS), which can disrupt mitochondrial ATP synthesis. Sinapic acid contains both methoxy (-OCH₃) and hydroxyl (-OH) groups, which contribute to its free radical-scavenging ability. The hydroxyl group can donate hydrogen atoms to neutralize radicals, while methoxylation enhances molecular stability and electron distribution, making phenolic rings less prone to oxidation. This aligns with prior research on phenolic antioxidants reducing ROS, stabilizing mitochondrial function, and remodeling toxic α-synuclein aggregates into less harmful forms [13].
While both SA and LD improved short-term outcomes in PD flies, long-term effects revealed a divergence. Chronic LD exposure led to reduced efficacy and signs of neurotoxicity, consistent with known adverse effects such as hallucinations, neuronal apoptosis, and premature peripheral dopamine conversion [14][15]. In contrast, long-term SA treatment sustained or improved behavioral and survival outcomes, indicating superior tolerability and efficacy.
Potential variability, such as inter-trial differences in fly behavior or micro-environmental changes during aging, was minimized through replication (n=30 per group) and statistical validation (Two-way ANOVA and post hoc tests). However, one limitation is the absence of co-administered carbidopa, which could have mitigated LD’s premature conversion and allowed a more accurate comparison. Furthermore, while Drosophila offers a genetically tractable model, extrapolation to humans requires validation in higher organisms, such as C. elegans or rodent models, especially for understanding pharmacokinetics and blood-brain barrier dynamics. Although treatment effects were measured across multiple physiological and behavioral dimensions, the manuscript could benefit from acknowledging additional potential confounding factors. Environmental variables such as temperature, humidity, and light cycles, which can significantly influence Drosophila behavior and survival, were not explicitly controlled or reported.
5. Conclusion
(SA) shows potential neuroprotective activity in mitigating the effects of parkinsonism like-effects in a drosophila model, thus supporting the alternative hypothesis. Contrary to expectations, due to its side effects, sinapic acid performed better than Levodopa in the long term. This differs from other researchers because they tested Levodopa to be more beneficial than their similar antioxidants. This creates essential implications for sinapic acid in the long term to show its therapeutic benefits for PD treatments. The study also demonstrates that long-term Sinapic Acid consumption yields more significant benefits than Levodopa. This helps advance past research about using only Levodopa to treat Parkinson's disease by adding a more effective therapeutic approach (sinapic acid) in the long term. These findings highlight the potential of Sinapic Acid as a therapeutic approach for PD treatment, offering a safer and more effective alternative to conventional medications like Levodopa. Old Drosophila showed similar effects to the young ones, except the movement speed of the only Levodopa-treated drosophila decreased to 59.44%.
ACKNOWLEDGEMENT
We would like to acknowledge Alison Huenger, Dr. Allal Boutajangout, Melanie Greenwald, Marissa Alamo, Stephanie DiPreta, and Sheryl Idzik for their guidance and support in conducting this research. We also extend our thanks to the Manhasset Science Research Program for providing us with the opportunity to conduct this research.
6. Bibliography
[1] Ja, W. W., Carvalho, G. B., Mak, E. M., De La Rosa, N. N., Fang, A. Y., Liong, J. C., ... & Benzer, S. (2007). Prandiology of Drosophila and the CAFE assay. Proceedings of the National Academy of Sciences, 104(20), 8253–8256. https://doi.org/10.1073/pnas.0702726104
[2] World Health Organization. (2023). Parkinson disease. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease
[3] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson's disease. Food & Function, 12(17), 7816–7824. https://doi.org/10.1039/D1FO01244G
[4] Johnson, S. L., Park, H. Y., DaSilva, N. A., Vattem, D. A., Ma, H., & Seeram, N. P. (2018). Levodopa-reduced seed extract shows neuroprotective effects against Parkinson's disease in murine microglia and human neuroblastoma cells. Nutrients, 10(9), 1139. https://doi.org/10.3390/nu10091139
[5] U.S. Department of Health and Human Services. (2022, April 14). Parkinson’s disease: Causes, symptoms, and treatments. National Institute on Aging. https://www.nia.nih.gov/health/parkinsons-disease
[6] Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa. In StatPearls. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK482140/
[7] Nguyen, V. P. T., Stewart, J. D., Ioannou, I., & Allais, F. (2021). Sinapic acid and sinapate esters in Brassica: Innate accumulation, biosynthesis, accessibility via chemical synthesis or recovery from biomass, and biological activities. Frontiers in Chemistry, 9, Article 664602. https://doi.org/10.3389/fchem.2021.664602
[8] He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., Li, X., & Liu, Y. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson's disease. Food & Function, 12(17), 7816–7824. https://doi.org/10.1039/D1FO01244G
[9] Pulver, S. R., & Berni, J. (2012). The fundamentals of flying: Simple and inexpensive strategies for employing Drosophila genetics in neuroscience teaching laboratories. Journal of Undergraduate Neuroscience Education, 11(1), A139–A148. https://pubmed.ncbi.nlm.nih.gov/23493248/
[10] Nichols, C. D., Becnel, J., & Pandey, U. B. (2012). Methods to assay Drosophila behavior. Journal of Visualized Experiments, 61, e3679. https://doi.org/10.3791/3679
[11] Ali, Y. O., Escala, W., Ruan, K., & Zhai, R. G. (2011). Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. Journal of Visualized Experiments, 49, e2504. https://doi.org/10.3791/2504
[12] Linford, N. J., Bilgir, C., Ro, J., & Pletcher, S. D. (2013). Measurement of lifespan in Drosophila melanogaster. Journal of Visualized Experiments, 71, e50068. https://doi.org/10.3791/50068
[13] Siddique, Y. H., Naz, F., & Jyoti, S. (2014). Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. BioMed Research International, 2014, Article 606928. https://doi.org/10.1155/2014/606928
[14] Gandhi, K. R., & Saadabadi, A. (2023, April 17). Levodopa. In StatPearls. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK482140/
[15] Bodkhe, S. N. (2020, September). A review on levodopa/carbidopa drug for the treatment of Parkinson’s disease. International Journal of Scientific Development and Research, 5(9), 233–237. https://www.ijsdr.org/papers/IJSDR2009079.pdf
Document information
Published on 23/06/25
Submitted on 30/01/25
Volume 7, 2025
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?