Summary
Background/Objective
Pancreatic fistula (PF) is the most common and challenging complication after pancreaticoduodenectomy (PD). This meta-analysis aimed to evaluate the impact of pancreaticogastrostomy (PG) versus pancreaticojejunostomy (PJ) on occurrences of postoperative PF.
Methods
A systematic literature search in the Medline, EMBASE, OVID, and Cochrane databases was performed to identify all eligible randomized controlled trials (RCTs). Pooled estimates were presented with 95% confidence intervals (CI).
Results
Six RCTs involving 1005 patients met the inclusion criteria. The incidence of PF [odds ratio (OR) 0.58, 95% CI, 0.42–0.81; p = 0.001], intra-abdominal abscess or collections (OR 0.43, 95% CI, 0.28–0.65; p < 0.001), and biliary fistula (OR 0.28, 95% CI, 0.11–0.74; p = 0.01) were found to be significantly lower in the PG group than in the PJ group. There was no significant difference in overall morbidity, other complications, hospital mortality, or length of hospital stay between the two groups.
Conclusion
The meta-analysis showed that PG following PD represents a safe procedure associated with fewer PFs compared with PJ.
Keywords
pancreatic fistula;pancreaticoduodenectomy;pancreaticogastrostomy;pancreaticojejunostomy
1. Introduction
As a result of recent improvements in surgical techniques and perioperative care, pancreaticoduodenectomy (PD) has been refined to be a safe operation with <5% perioperative mortality at high-volume centers, however, the morbidity rate remains as high as 20–50%.1 Pancreatic fistula (PF) is the most devastating postoperative complication occurring in 2.5–25% patients,2 and has become the main reason for increased morbidity and mortality, prolonged length of hospital stay, and increased medical costs.
After PD, pancreatic continuity can be restored by performing either a pancreaticojejunostomy (PJ) or a pancreaticogastrostomy (PG). A recent published meta-analysis3 of four randomized controlled trials (RCTs)4; 5; 6 ; 7 showed no significant difference between the two surgical modalities in terms of PF occurrence. By contrast, two more recent large-scale RCTs8 ; 9 reported that the PF rate with PG was lower than that with PJ. In the light of these conflicting findings, the present meta-analysis was attempted to provide a more updated evaluation of the effect of PG versus PJ with respect to the PF rate after PD.
2. Methods
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA).10
2.1. Study selection
Systematic literature searches in the Medline, EMBASE, OVID, and Cochrane databases were performed to identify published RCTs that compared the postoperative PF rate with PG versus PJ after PD from database inception to November 2013. The Medical Subject Heading (MeSH) search terms were “PD,” “pancreaticojejunostomy”, and “pancreaticogastrostomy”. Only studies on humans and in the English language were considered for inclusion. Reference lists of all retrieved articles were manually searched for additional studies.
2.2. Data extraction
Two reviewers (B.L. and L.W.) independently extracted the following parameters from each study: first author, year of publication, study population characteristics, number of patients randomized with each procedure, and endpoints. All relevant text, tables, and figures were reviewed for data extraction.
2.3. Criteria for inclusion and exclusion
RCTs that compared PG and PJ in patients undergoing PD for malignant and benign diseases of the pancreas and periampullary region were included in this review. Exclusion criteria were: animal studies, studies evaluating patients who underwent total pancreatectomy or central pancreatectomy, studies evaluating patients who underwent PD without immediate pancreatic anastomosis or duodenum-preserving pancreatectomy, and nonrandomized observational clinical studies.
2.4. Assessment of methodological quality
The RCTs were scored using the Jadad composite scale11 in which each study was evaluated by examining three factors: randomization, blinding, and withdrawals and drop-outs reported within the study period. The quality scale ranged from 0 points to 5 points, and a study with a score of ≥3 points was considered to be of high quality.
2.5. Endpoints
The primary endpoint was PF. PF was determined according to the consensus definition proposed by the International Study Group of Pancreatic Fistula (ISGPF),12 as the presence of amylase-rich fluid (>3 times the upper limit of normal in the serum) of any measurable volume on or after postoperative Day 3. The severity of PF was classified into three grades as follows: Grade A fistulas were transient and did not require any intervention; Grade B fistulas required adjustment to the clinical pathway but the patients were clinically well; and Grade C fistulas often required operative intervention and were associated with sepsis or death.
Secondary endpoints included overall morbidity, other complications, hospital mortality, and length of hospital stay.
2.6. Statistical methods
Review Manager (RevMan) software 5.0 (Nordic Cochrane Centre, Copenhagen, Denmark) was used to conduct all analyses. Odds ratio (OR) and weighted mean differences (WMD) were used for the analysis of continuous and dichotomous variables, respectively. If the study provided medians and interquartile ranges instead of means and standard deviations (SDs), the means and SDs were imputed according to the methods described by Hozo et al.13 Pooled estimates were presented with 95% confidence intervals (CI). Pooled effect was calculated using either the fixed effects model or random effects model. Heterogeneity was evaluated using I2, with values >50% indicating considerable heterogeneity. Publication bias was assessed visually using a funnel plot, based on the results of PF.
3. Results
3.1. Eligible studies
We identified 1837 potentially relevant records. After excluding studies that did not fulfill our inclusion criteria, six articles were retrieved for inclusion (Fig. 1).4; 5; 6; 7; 8 ; 9 The two reviewers had 100% agreement in their reviews of the data extraction.
|
|
|
Figure 1. Study selection. RCT = randomized controlled trial. |
A total of 1005 patients were included in the meta-analysis: 503 in the PG group and 502 in the PJ group. Of the six included studies, two were conducted in Spain,7 ; 8 one in the USA,4 one in Italy,5 one in France,6 and one in Belgium.9 The sample size of each study varied from 108 patients to 329 patients. The characteristics of the included studies are shown in Table 1.4; 5; 6; 7; 8 ; 9
| Reference (Year) | Enrolment interval (Country) | Group | No. of patients (M/F) | Age (y) | Type of PD PPPD/CPD | Definition of PF | Quality score |
|---|---|---|---|---|---|---|---|
| Yeo et al4 (1995) | 1993–1995 (USA) | PG | 73 (33/40) | 61.5 ± 1.7 | 60/13 | Radiographically documented leak or >50 mL drainage of amylase rich fluid on or after POD10 | 4 |
| PJ | 72 (38/34) | 62.4 ± 1.4 | 59/13 | ||||
| Bassi et al5 (2005) | 2002–2004 (Italy) | PG | 69 (44/25) | 59.3 | 66/3 | Any clinical significant output of fluid, rich in amylase, confirmed by fistulography | 4 |
| PJ | 82 (51/31) | 55.5 | 70/12 | ||||
| Duffas et al6 (2005) | 1995–1999 (France) | PG | 81 (51/30) | 58.2 ± 11 | 18/63 | Radiologically documented leak or fluid rich in amylase (at least 4 times normal serum values) >3 d | 4 |
| PJ | 68 (35/33) | 58.6 ± 12 | 18/50 | ||||
| Fernández-Cruz et al7 (2008) | 2005–2007 (Spain) | PG | 53 (29/24) | 63 ± 13 | All PPPD | ISGPF | 4 |
| PJ | 55 (38/17) | 63 ± 14 | All PPPD | ||||
| Figueras et al8 (2013) | 2008–2012 (Spain) | PG | 65 (44/21) | 67 (35–80) | 30/35 | ISGPF | 4 |
| PJ | 58 (37/21) | 65.5 (42–80) | 28/30 | ||||
| Topal et al9 2013 | 2009–2012 (Belgium) | PG | 162 (100/62) | 67.0 (60.6–73.5) | 98/64 | ISGPF | 4 |
| PJ | 167 (91/76) | 66.1 (59.4–74.6) | 102/65 |
CPD = classical PD; ISGPF = International Study Group of Pancreatic Fistula; PD = pancreaticoduodenectomy; PG = pancreaticogastrostomy; PJ = pancreaticojejunostomy; POD = postoperative day; PPPD = pylorus-preserving pancreaticoduodenectomy.
There was a lack of uniformity in the definition of PF in three RCTs4; 5 ; 6 before the international consensus of PF definition was published. In the remaining three studies,7; 8 ; 9 PF was defined according to the ISGPF.12
3.2. Meta-analysis
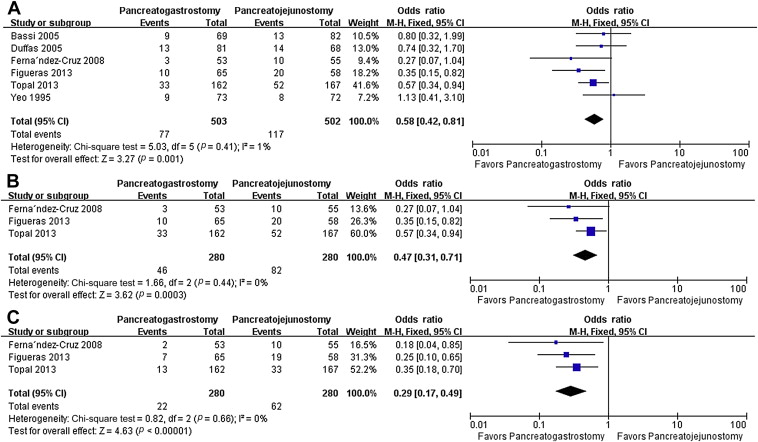
Table 2 shows the results for the outcomes. All studies provided information on the incidence of PF, which was found to be significantly lower in the PG group than that in the PJ group (OR 0.58, 95% CI, 0.42–0.81; p = 0.001). In three studies, 7; 8 ; 9 PF was defined and graded according to the recommendations of the ISGPF.13 Pooled analysis showed both overall PF (OR 0.47, 95% CI, 0.31–0.71; p = 0.0003) and clinically significant PF (Grade B or C) (OR 0.29, 95% CI, 0.17–0.49; p = 0.001) were less frequent in the PG group. No significant heterogeneity was found between studies regarding these outcomes ( Fig. 2).
| Outcome of interest | No. of studies | No. of patients | OR/WMD | 95% CI | p | I2 (%) |
|---|---|---|---|---|---|---|
| PF | 6 | 1005 | 0.58 | 0.42, 0.81 | 0.001 | 1 |
| ISGPF PF | 3 | 560 | 0.47 | 0.31, 0.71 | <0.001 | 0 |
| ISGPF B + C PF | 3 | 560 | 0.29 | 0.17, 0.49 | <0.001 | 0 |
| Overall morbidity | 6 | 1005 | 1.29 | 0.67, 2.48 | 0.49 | 32 |
| Delayed gastric emptying | 5 | 856 | 0.84 | 0.40, 1.78 | 0.66 | 66 |
| Intra-abdominal abscess or collection | 6 | 1005 | 0.43 | 0.28, 0.65 | <0.001 | 0 |
| Biliary fistula | 5 | 676 | 0.28 | 0.11, 0.74 | 0.01 | 9 |
| Hemorrhage | 5 | 860 | 1.27 | 0.81, 1.79 | 0.29 | 0 |
| Wound infection | 2 | 253 | 1.36 | 0.62, 2.98 | 0.44 | 0 |
| Reoperation | 4 | 737 | 0.87 | 0.53, 1.42 | 0.59 | 0 |
| Mortality | 6 | 1005 | 0.81 | 0.41, 1.60 | 0.55 | 0 |
| Length of hospital stay (d) | 5 | 854 | −1.46 | −3.43, 0.52 | 0.15 | 96 |
CI = confidence interval; ISGPF = International Study Group of Pancreatic Fistula; OR = odds ratio; PF = pancreatic fistula; WMD = weighted mean difference.
|
|
|
Figure 2. Results of the meta-analysis on primary endpoints. (A) Pancreatic fistula; (B) ISGPF pancreatic fistula; and (C) clinically significant pancreatic fistula (ISGPF grade B +C). CI = confidence interval; df = degrees of freedom, M-H = Mantel-Haenszel; ISGPF = International Study Group of Pancreatic Fistula. |
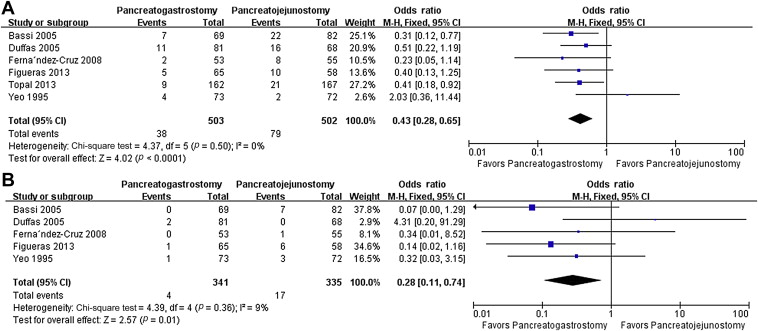
Regarding secondary endpoints, intra-abdominal abscess or collections (OR 0.43, 95% CI, 0.28–0.65; p < 0.001) and biliary fistula (OR 0.28, 95% CI, 0.11–0.74; p = 0.01) were found to be significantly lower in the PG group than in the PJ group ( Fig. 3). By contrast, overall morbidity (OR 1.29, 95% CI, 0.67–2.48; p = 0.49), delayed gastric emptying (DGE) (OR 0.84, 95% CI, 0.40–1.78; p = 0.66), hemorrhage (OR 1.27, 95% CI, 0.81–1.79; p = 0.29), wound infection (OR 1.36, 95% CI, 0.62–2.98; p = 0.44), reoperation (OR 0.87, 95% CI, 0.53–1.42; p = 0.59), hospital mortality (OR 0.81, 95% CI, 0.41–1.60; p = 0.55), and length of hospital stay (WMD −1.46, 95% CI, −3.43–0.52; p = 0.15), were comparable between the two groups. No significant heterogeneity was found between studies regarding these outcomes, except for DGE and length of hospital stay.
|
|
|
Figure 3. Results of the meta-analysis on secondary endpoints. (A) Intra-abdominal abscess or collections; and (B) biliary fistula. CI = confidence interval; df = degrees of freedom, M-H = Mantel-Haenszel. |
3.3. Publication bias
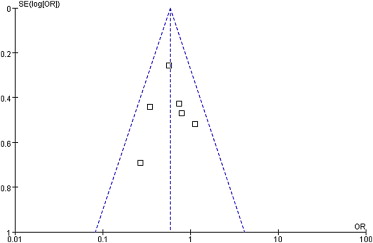
The funnel plot for the primary outcome (PF) was symmetric, indicating the absence of publication bias (Fig. 4).
|
|
|
Figure 4. Funnel plot analysis of publication bias. The outcome was the pancreatic fistula. OR = odds ratio; SE(log[OR]) = standard error (log[OR]). |
4. Discussion
PJ is the conventional predominant surgical modality for restoring pancreaticoenteric continuity following PD. Despite the many technical variations tested including the anastomosis site of the jejunum used (end vs. side), type of anastomosis (duct-to-mucosa vs. invagination), Pengs binding technique, using an isolated Roux-en-Y limb, and use of fibrin glue and internal or external pancreatic duct stenting,14 no universal consensus has been reached regarding the safest technique of PJ that is less prone to fistula formation.
PG was initially described by Waugh and Clagett15 in 1946 as an alternative to PJ anastomosis, and has gained favor in recent years. A previous meta-analysis3 of four RCTs4; 5; 6 ; 7 reported that there was no significant difference between PG and PJ in terms of PF, probably because of the small number of patients (n = 553) enrolled in that meta-analysis. It is generally believed that the power of a meta-analysis to detect a possible treatment effect increases with the increase in the number of patients enrolled. The present updated meta-analysis that pooled six RCTs involving a total of 1005 participants seems to conclude that PG is associated with less PF compared with PJ. The major strength of the present meta-analysis is that all the included studies had a Jadad score of 4. It has been suggested that inadequate generation of the allocation sequence, allocation concealment, and double blinding lead to exaggerated estimates of the intervention benefit and may contribute to discrepancies between the results of RCTs in meta-analyses. 16 Another strength is that there is no significant heterogeneity or publication bias regarding the primary outcome (PF).
The description of PF varies greatly in the pancreatic surgical literature. In 2005, the ISGPF proposed a standardized definition of DGE.12 Three of the six RCTs used the ISGPF criteria and consistently found that the rate and severity of PF was significantly lower with the PG technique compared with that following PJ.7; 8 ; 9 The pooled data are also in concordance with these RCTs.
There are several theoretical advantages that apparently contribute to the low PF rate of PG. Pancreatic enzymes can be inactivated by gastric acid. However, as the stomach does not contain enterokinase, it needs to be converted from trypsinogen to trypsin to activate other proteolytic enzymes. This lack of enzyme activation may help prevent autodigestion of the anastomosis. In addition, alkaline pancreatic secretions may help prevent marginal ulceration. As the pancreas is in close proximity to the posterior wall of the stomach, potentially less tension is produced on the anastomosis. The abundant blood supply to the stomach wall facilitates anastomotic healing, and the thick stomach wall can hold sutures securely. Nasogastric decompression allows for continuous emptying of the stomach, thus producing less tension on the anastomosis. This benefit with PG is unmatched by PJ anastomosis.17
Our meta-analysis also showed that the occurrence of biliary fistula was significantly lower in the PG group. This difference might be related to the fact that reconstruction by PG can shunt the flow of pancreatic juice and bile, thus decreasing the pressure of tension at biliary anastomosis. It is also possible that the lower occurrence of pancreatic and biliary fistulas in the PG group is associated with the lower incidence of intra-abdominal abscess or collection.
There are concerns about the long-term outcome with the PG procedure. Lemaire et al18 reported that patients who underwent PD using the PG technique remained free from diabetes but developed marked pancreatic exocrine insufficiency during a median follow-up period of 32 months. Similarly, Pessaux et al19 reported the presence of exocrine pancreatic insufficiency in 95% (18/19) of their patients with PG during a mean follow-up period of 40.3 months. The possible explanation is that pancreatic enzymatic secretions can be neutralized by gastric acid. However, an RCT by Konishi et al20 demonstrated no difference between PG and PJ. Regarding endocrine function, no difference was identified between these two reconstructions in several reports.8; 20 ; 21 Ishikawa et al22 concluded that the decline in glucose tolerance after PD seemed to be associated with the low reserve of endocrine function rather than the type of pancreatic anastomosis or associated complications.
The current analysis showed no significant difference in DGE between PJ and PG. Although some researchers reported that DGE developed more frequently in patients with postoperative complications such as PF, biliary fistula, and intra-abdominal collections or abscesses,23 the results are not confirmed by other investigators.24 ; 25
There are some limitations with our study. Only one of the six RCTs included in our meta-analysis focused on patients at high risk of PF (with soft pancreas and a diameter of the main duct <5 mm).5 Therefore, the advantage of PG in such subgroup patients could not be reliably assessed. Another limitation is that all six RCTs were conducted in Western countries. This may raise a question regarding the validity of the results and applicability to other areas.
In conclusion, our meta-analysis has demonstrated that PG following PD represents a safe procedure that is associated with lower occurrences of PF compared with PJ.
Acknowledgments
We thank Dr Yanfang Zhao (Department of Health Statistics, Second Military Medical University, Shanghai, China) for her critical revision of the meta-analysis section.
References
- 1 M. Kawai, H. Yamaue; Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery; Surg Today, 40 (2010), pp. 1011–1017
- 2 Y. Zhou, C. Yang, S. Wang, J. Chen, B. Li; Does external pancreatic duct stent decrease pancreatic fistula rate after pancreatic resection?: a meta-analysis; Pancreatology, 11 (2011), pp. 362–370
- 3 T. He, Y. Zhao, Q. Chen, X. Wang, H. Lin, W. Han; Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a systematic review and meta-analysis; Dig Surg, 30 (2013), pp. 56–69
- 4 C.J. Yeo, J.L. Cameron, M.M. Maher, et al.; A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy; Ann Surg, 222 (1995), pp. 580–588
- 5 C. Bassi, M. Falconi, E. Molinari, et al.; Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study; Ann Surg, 242 (2005), pp. 767–771
- 6 J.P. Duffas, B. Suc, S. Msika, et al.; A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy; Am J Surg, 189 (2005), pp. 720–729
- 7 L. Fernández-Cruz, R. Cosa, L. Blanco, M.A. López-Boado, E. Astudillo; Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study; Ann Surg, 248 (2008), pp. 930–938
- 8 J. Figueras, L. Sabater, P. Planellas, et al.; Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy; Br J Surg, 100 (2013), pp. 1597–1605
- 9 B. Topal, S. Fieuws, R. Aerts, et al.; Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial; Lancet Oncol, 14 (2013), pp. 655–662
- 10 D. Moher, A. Liberati, J. Tetzlaff, PRISMA Group; Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement; PLoS Med, 6 (2009), p. e1000097
- 11 A.R. Jadad, R.A. Moore, D. Carroll, et al.; Assessing the quality of reports of randomized clinical trials: is blinding necessary?; Control Clin Trials, 17 (1996), pp. 1–12
- 12 C. Bassi, C. Dervenis, G. Butturini, et al.; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition; Surgery, 138 (2005), pp. 8–13
- 13 S.P. Hozo, B. Djulbegovic, I. Hozo; Estimating the mean and variance from the median, range, and the size of a sample; BMC Med Res Methodo, 5 (2005), p. 13
- 14 R.T. Poon, S.H. Lo, D. Fong, S.T. Fan, J. Wong; Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy; Am J Surg, 183 (2002), pp. 42–52
- 15 J.M. Waugh, O.T. Clagett; Resection of the duodenum and head of the pancreas for carcinoma; Surgery, 20 (1946), pp. 224–232
- 16 L.L. Kjaergard, J. Villumsen, C. Gluud; Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses; Ann Intern Med, 135 (2001), pp. 982–989
- 17 G.V. Aranha, P. Hodul, E. Golts, D. Oh, J. Pickleman, S. Creech; A comparison of pancreaticogastrostomy and pancreaticojejunostomy following pancreaticoduodenectomy; J Gastrointest Surg, 7 (2003), pp. 672–682
- 18 E. Lemaire, D. O'Toole, A. Sauvanet, P. Hammel, J. Belghiti, P. Ruszniewski; Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis; Br J Surg, 87 (2000), pp. 434–438
- 19 P. Pessaux, C. Aube, J. Lebigot, et al.; Permeability and functionality of pancreaticogastrostomy after pancreaticoduodenectomy with dynamic magnetic resonance pancreatography after secretin stimulation; J Am Coll Surg, 194 (2002), pp. 454–462
- 20 M. Konishi, M. Ryu, T. Kinoshita, K. Inoue; Pathophysiology after pylorus-preserving pancreatoduodenectomy: a comparative study of pancreatogastrostomy and pancreatojejunostomy; Hepatogastroenterology, 46 (1999), pp. 1181–1186
- 21 W.L. Fang, C.H. Su, Y.M. Shyr, et al.; Functional and morphological changes in pancreatic remnant after pancreaticoduodenectomy; Pancreas, 35 (2007), pp. 361–365
- 22 O. Ishikawa, H. Ohigashi, H. Eguchi, et al.; Long-term follow-up of glucose tolerance function after pancreaticoduodenectomy: comparison between pancreaticogastrostomy and pancreaticojejunostomy; Surgery, 136 (2004), pp. 617–623
- 23 G. Malleo, S. Crippa, G. Butturini, et al.; Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors; HPB (Oxford), 12 (2010), pp. 610–618
- 24 E. Akizuki, Y. Kimura, T. Nobuoka, et al.; Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy: prospective consecutive analysis of delayed gastric emptying according to the ISGPS definition and the amount of dietary intake; Ann Surg, 249 (2009), pp. 986–994
- 25 M. Nikfarjam, E.T. Kimchi, N.J. Gusani, et al.; A reduction in delayed gastric emptying by classic pancreaticoduodenectomy with an antecolic gastrojejunal anastomosis and a retrogastric omental patch; J Gastrointest Surg, 13 (2009), pp. 1674–1682
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?