Abstract
Using the UVic Earth System Model, this study simulated the change of seawater chemistry and analyzed the chemical habitat surrounding shallow- and cold-water coral reefs from the year 1800 to 2300 employing RCP2.6, RCP4.5, RCP6.0, and RCP8.5 scenarios. The model results showed that the global ocean will continue to absorb atmospheric CO2 . Global mean surface ocean temperature will rise 1.1–2.8 K at the end of the 21st century across RCP scenarios. Meanwhile, the global mean surface ocean pH will drop 0.14–0.42 and the ocean surface mean concentration of carbonate will decrease 20%–51% across the RCP scenarios. The saturated state of sea water with respect to calcite carbonate minerals (Ω ) will decrease rapidly. During the pre-industrial period, 99% of the shallow-water coral reefs were surrounded by seawater with Ω > 3.5 and 87% of the deep-sea coral reefs were surrounded by seawater with aragonite supersaturation. Within the 21st century, except for the high mitigation scenario of RCP2.6, almost none shallow-water coral reefs will be surrounded by seawater with Ω > 3.5. Under the intensive emission scenario of RCP8.5, by the year 2100, the aragonite saturation horizon will rise to 308 m under the sea surface from 1138 m at the pre-industrial period, thus 73% of the cold-water coral reefs will be surrounded by seawater with aragonite undersaturation. By the year 2300, only 5% of the cold-water coral reefs will be surrounded by seawater with aragonite supersaturation.
Keywords
Simulation research ; Aragonite saturation state ; Ocean acidification ; Shallow-water coral reefs ; Cold-water coral reefs
1. Introduction
Since the beginning of the industrial revolution, the CO2 concentration in atmosphere has increased rapidly, because of human activities such as fossil fuel combustion and land use. Anthropogenic CO2 emissions have reached 545 Pg C from 1750 to 2011 (IPCC, 2013 ). Not all of CO2 emissions remain in the atmosphere, 26% of them are absorbed by the ocean and 28% are absorbed in terrestrial soil (Sabine et al., 2004 ). Recently, both observation and simulation studies have agreed that the ocean absorbs a large amount of CO2 , which mitigates global climate change. However, this absorption is not entirely harmless. The average pH of the sea surface has dropped from 8.2 to 8.1 since pre-industrial time, which means that the ocean hydrogen ion concentration has increased by 26% (Gattuso and Hansson, 2011 ). Doney et al. (2009) reported the ocean carbon chemical equilibrium formula as:
|
|
( 1) |
According to this equation, the increase of hydrogen ions (H+ ) will result in the decrease of carbonate ions concentration ( ), which will further reduce the calcium carbonate (CaCO3 ) saturation state. These changes in sea water will harm marine calcified organisms, especially the coral reefs (Doney et al., 2009 , Fine and Tchernov, 2007 , Fabry et al., 2008 and Guinotte and Fabry, 2008 ). Coral reefs are an important component of marine ecosystems and are composed of aragonite (a relatively soluble mineral form of calcium carbonate). Many laboratory experiments showed that the decline of the ocean aragonite saturation state would lead to the decrease in the shallow-water coral calcification rate, because it is difficult for corals to extract calcium ions and bicarbonate ions from the surrounding seawater to form bones and shells ( ). In addition, cold-water coral reefs also suffer from the effects of this change in the marine environment (Langdon et al., 2003 ). Guinotte et al. (2006) suggested that the global distribution of cold-water coral in the deep sea is limited by the surrounding seawater aragonite saturation state. Therefore, an increase in the CO2 concentration in the atmosphere can have important influences on the marine environment and coral reef systems.
Anthropogenic CO2 emissions will decrease the ocean surface pH and the concentration of carbonate ions (Caldeira and Wickett, 2005 , Orr et al., 2005 , Cao and Caldeira, 2008 and Steinacher et al., 2009 ). This development will directly affect the ocean aragonite saturation state. Aragonite saturation state (Ω ) is defined as ( Feely et al., 1988 ):
|
|
( 2) |
which is calculated from the calcium ion concentration ([Ca2+ ]), carbonate ion concentration ( ) and equilibrium thermodynamic solubility product ( ). The [Ca2+ ] and are calculated from the sea water salinity, total alkalinity (TAlk), and dissolved inorganic carbon (DIC). The solubility product is calculated based on seawater temperature, salinity, and pressure (Mucci, 1983 ), which is defined as the product of [Ca2+ ] and concentrations at the saturation state. When Ω < 1 the seawater is undersaturated with respect to aragonite, while Ω > 1 means it is supersaturated. The aragonite saturation horizon is defined as the depth which Ω = 1. In the ocean today, the marine aragonite saturation state decreases from the surface to the depth. Thus, the water above the saturation horizon is supersaturated and the water under the saturation horizon is undersaturated.
There are numerous simulation studies about ocean acidification under various CO2 emission scenarios. Using the ocean circulation model of the Lawrence Livermore National Laboratory, Caldeira and Wickett (2005) found that the global mean ocean surface pH would drop 0.3–0.5 at the end of the 21st century under the SRES scenario. Orr et al. (2005) commented that surface seawater would be undersaturated with respect to aragonite at the middle of the 21st century under the IS92a scenarios, which was based on the simulation results of 13 models, which would have a negative impact on polar shell plankton. In a recent research, it was found that 20% of the surface area of the Canadian Basin has been undersaturated with respect to aragonite (Robbins et al., 2013 ). This mismatch between observations and models is probably due to the low resolution of global models and the inability of the global models to accurately simulate sea ice. The actual speed of the decline of Arctic sea ice is faster than the forecast, because of global warming (Stroeve et al., 2007 ). On the basis of UVic ESCM simulation, Cao and Caldeira (2008) speculated that the ocean mean pH at high latitude would drop by more than 0.2 when the atmospheric CO2 concentration stabilized at 450 × 10−6 , and in this case, 7% of the area of the south ocean (south of 60°S) would be undersaturated in aragonite. Fine and Tchernov (2007) experimentally determined that Scleractinian coral would be huge, soft, and skeleton-free in acidified seawater (pH = 7.4).
Chinese scientists have conducted several studies using the global carbon cycle model simulation. For example, Xu and Li (2009) simulated the global ocean anthropogenic CO2 uptake. Cao et al. (2014a) and Wang et al. (2014) used UVic ESCM to simulate and analyze how climate sensitivity could affect the uptake of CO2 by the ocean under the RCP8.5 scenario. Cao et al. (2014b) analyzed the response of ocean acidification to a gradual increase and decrease of atmospheric CO2 and found that marine ecosystems would not respond well to recover to their natural chemical habitats even if the atmospheric CO2 content is lower than future predictions.
On the basis of the previous researches, our study used an intermediate complex earth system climate model to compare various ocean acidification rates from before the industrial revolution to the year 2300 under four CO2 Representative Concentration Pathway scenarios (RCPs). These scenarios have been widely employed in the IPCC AR5 report. At the same time, this study also analyzed the effect of ocean acidification on both shallow- and cold-water coral reefs. Besides being a supplement to the CMIP5 multi-models prediction in the AR5 report, the work also represents the first attempt to analyze the chemical habits of cold-water coral reefs under four RCPs.
2. Model and method
2.1. Model description
Developed at the University of Victoria at Canada, the UVic Earth System Climate Model is an intermediate complexity climate model, which consists of a 3D ocean general circulation model with a spherical grid resolution of 3.6° by longitude and 1.8° by latitude with 19 vertical layers in the ocean and one-layer in the atmosphere with an energy-moisture balance. It is coupled with a sea-ice model, an atmospheric model and a land-ice model (Weaver et al., 2001 ). The terrestrial carbon cycle model is based on the TRIFFID land surface and dynamic vegetation scheme of Hadley Centre Met Office (Meissner et al., 2003 ). In addition, the ocean carbon cycle consists of a CO2 air-sea interaction process, a marine inorganic carbon process (Orr et al., 1999 ) and a marine organic carbon process (Schmittner et al., 2008 ). The organic carbon process includes a simple marine ecosystems, which has nutrient (PO43− , NO3− ), interaction of phytoplankton and zooplankton and a feedback for the marine carbon cycle. This model has been widely used in numerous studies of climate change, ocean biochemistry cycles (Schmittner et al., 2008 ), climate feedback of ocean carbon cycles (Zickfeld et al., 2013 ) and ocean acidification (Cao and Caldeira, 2008 and Matthews et al., 2009 ).
2.2. Simulation experiments
We ran the UVic model for 10,000 model years with an atmospheric CO2 concentration of 280 × 10−6 as being the CO2 level of the pre-industrial time (Indermühle et al., 1999 ). Next, we set this state as the initial condition for the nominal year 1800 and simulated the climate change from 1800 to 2300. From 1800 to 2005, the model was driven by the historical concentration of atmospheric CO2 . After 2005, it was forced by the CO2 concentration in the atmosphere as prescribed by four RCP scenarios (RCP2.6, RCP4.5, RCP6.0, RCP8.5, the numbers behind RCP mean that global radiation forcing will reach 2.6, 4.5, 6.0, 8.5 W m2 at the year 2100, while atmosphere CO2 concentration will reach 412 × 10−6 , 538 × 10−6 , 670 × 10−6 , 936 × 10−6 , respectively).
2.3. Correction and calculation
The UVic model was used to simulate ocean temperature, alkalinity (ALK), salinity, DIC and phosphate. On the basis of these data, other variables were calculated using the chemistry routine from the OCMIP-3 project (http://www.ipsl.jussieu.fr/OCMIP/phase3 ). These variables included, pH, the concentration of carbonate ions and saturated state of aragonite.
We estimated the aragonite saturation state for the shallow-water and deep-sea coral reef locations by assuming that each reef possessed the simulated seawater chemistry of the model grid cell where the reef was located. In this study, we used different assignment methods such as liner interpolation. We did not test the accuracy of each method, because of the uncertainty of the cold-water coral reefs' location and each methods own error. The information for the longitude and latitude of the shallow-water coral reef locations was obtained from the Reef Base Database (http:/www.reefbase.org ) and the information concerning the longitude, latitude, and depth of the cold-water coral locations was obtained from the Global Distribution of Cold-Water Coral Reefs (Freiwald et al., 2004 ).
We used the Global Ocean Data Analysis Project (GLODAP) data to correct the model output data. The GLODAP data was obtained in the 1990s and was subsequently gridded into 1° horizontal resolution with 33 vertical layers by Key et al. (2004) . Because the observation data is more accurate than the model data, we linearly interpolated the former into the grid of the UVic model.
3. Results
3.1. Comparison of the model and observed results
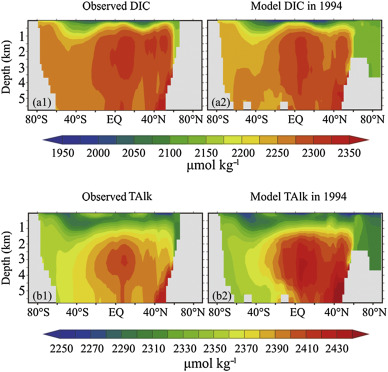
In this section, we compare the result of UVic model and the GLODAP observation data to test the reliability of the ocean simulation model. For example, the observed global mean DIC and TAlk were 2254 and 2363 μmol kg−1 , while during the same corresponding period, the model global mean DIC and TAlk were 2242 and 2368 μmol kg−1 . The model error of the DIC was 0.53% and the TAlk error was 0.21%. Furthermore, the model simulation distributions of the DIC and TAlk are similar to the observations (Fig. 1 ). This includes the simulation of the high value region and the data change of the upper ocean with latitude distribution.
|
|
|
Fig. 1. Latitude–depth distribution of ocean DIC and alkalinity from GLODAP observation and UVic model simulation. (a1) DIC of GLODAP data, (a2) DIC of model data in year 1994, (b1) total alkalinity of GLODAP data, (b2) total alkalinity of model data in year 1994. |
To reduce the error between model results and the observation data, we corrected the model data following a formula used in previous studies (Caldeira and Wickett, 2005 , Orr et al., 2005 and Cao and Caldeira, 2008 ):
|
|
( 3) |
Where Dco is the corrected data, Dm is the model data, Dm1994 is the model data of the year 1994, Dob is the observation data and n is the each year from 1800 to 2300. Here we assumed a simple linear error. The true error is more complex, so there is still some uncertainty.
3.2. Simulation of marine chemistry
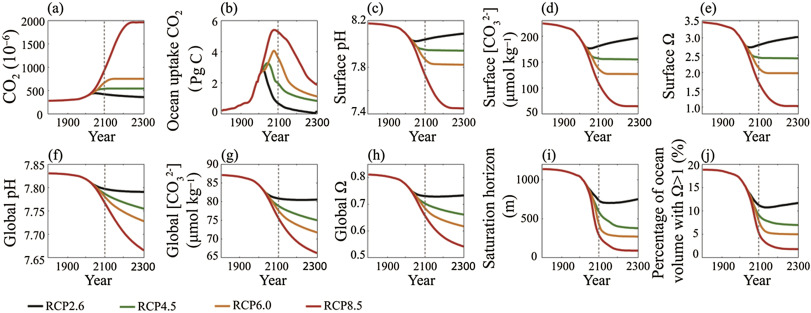
The atmospheric CO2 concentration increased at various rates under the RCP scenarios except RCP2.6 (Fig. 2 a), while the ocean continues to absorb CO2 from the atmosphere under all the scenarios (Fig. 2 b). Based on the model simulation results, the global oceans will absorb 162–411 Pg C from the year 2010 to 2100 across the RCP scenarios. By the year 2300, the global oceans will absorb 215−1135 Pg C. Compared with the pre-industrial period, the global mean ocean surface temperature will rise 1.1–2.8 K at the year 2100, which is in broad agreement with the prediction of CMIP5 (0.8–3.1 K) (IPCC, 2013 ). At the year 2300, the ocean temperature will increase 0.8–6.1 K. The increase of CO2 concentration is also important in the strength of the North Atlantic thermohaline circulation. The intensity of the North Atlantic Deep Water (NADW) was 21.5 Sv (1 Sv = 106 m3 s−1 ) at the year 1800. This will decrease to 16.6–19.6 Sv under RCP8.5 to RCP2.6 scenarios. After the CO2 concentration stabilizes, the NADW intensity will increase. Meanwhile, the ocean surface water warming will increase faster than that of the deep ocean so there will be a greater temperature gap between shallow-water and deep-water. Thus, ocean water stratification will be more stable and the convection currents will weaken.
|
|
|
Fig. 2. Time series under 4 RCP scenarios (a) atmospheric CO2 concentration, (b) CO2 uptake by ocean, (c) global mean ocean surface pH, (d) global mean ocean surface [CO32− ], (e) global mean ocean surface Ω , (f) global mean ocean pH, (g) global mean ocean [CO32− ], (h) global mean Ω , (i) global mean ocean saturation horizon of aragonite, (j) percent of ocean volume with Ω > 1.0. |
With respect to ocean chemistry, according to Equation (1) , the dissolved CO2 causes the seawater to produce more hydrogen ions, causing the pH to decrease. At the end of the 21st century, the global mean ocean surface pH will drop by 0.14–0.42 compared with the pre-industrial period (Fig. 2 c). The pH will rise 0.05 from the year 2100 to 2300 under the RCP2.6 scenario. Under the other three scenarios, the global mean pH will continue to decrease at different rates until the atmospheric CO2 concentration stabilizes. Under the intensive emission scenario of the RCP8.5 scenario, the global mean pH will drop 0.73 from the year 1800 to 2300, which means that the hydrogen ion concentration will increase by 437%. Between the period 1986–2005 and 2081–2100, the UVic model predicted a decrease in global mean surface pH by 0.067 units for RCP2.6, 0.144 for RCP4.5, 0.201 for RCP6.0, and 0.306 for RCP8.5. These projected pH changes are in close agreement with the CMIP5 Earth System Model results. For instance, between 1986–2005 and 2081–2100 the CMIP5 models project a model-mean decrease in the surface pH of 0.06–0.07 for RCP2.6, 0.14–0.15 for RCP4.5, 0.20–0.21 for RCP6.0, and 0.30–0.32 for RCP8.5. Due to the increase of the hydrogen ion concentration, the global mean ocean surface carbonate concentration will be reduced by 20%–51% by 2100 under the RCP scenarios. By 2300, the change will be 13%–71% (Fig. 2 d).
The entirety of the ocean will change more moderately than the ocean surface. The physical and chemical properties of the deep ocean lag behind the surface water (Cao et al., 2014b ). Even if the change of the surface water has stabilized following the atmospheric CO2 concentration stabilization, the deep ocean will still experience continued acidification (Fig. 2 f). Averaged over the whole ocean, the decrease in under the RCP scenarios ranges from 8% to 31%.
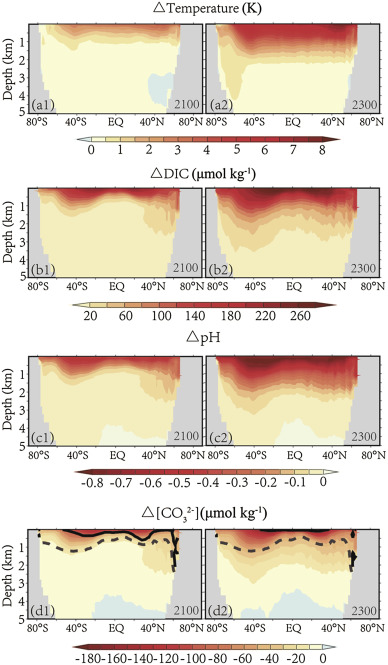
Fig. 3 shows the latitude–depth distribution of ocean temperature, DIC, pH and in years 2100 and 2300 relative to 1800. These changes extend from the surface to the deep sea. Temperature changes at different latitudes are relatively uniform and the area with the greatest warming appears on the surface ocean near 45°N latitude. The zonal average pH, DIC and exhibit greater changes in subtropical surface region than in the tropic and high-latitudes. In the vertical direction, these changes are delivered from subtropical area to mid-latitude regions. This is due to the subtropical water sinking and carrying anthropogenic CO2 into the deep ocean by these convection currents (Sabine et al., 2004 ).
|
|
|
Fig. 3. Latitude–depth distribution of ocean variables in 2100 and 2300 differ from 1800 (Dashed lines are latitude mean aragonite saturation horizon in the year 1800; solid lines are latitude mean aragonite saturation horizon in the year 2100 and 2300). |
3.3. Chemical habits of coral reefs
Aragonite is a relatively soluble mineral form of calcium carbonate, which is an important component of coral reefs. In the aragonite supersaturated water, corals can easily extract calcium and carbonate ion from the surrounding sea to build the reef. With a volume weighted average, 19% of the ocean was supersaturated in aragonite during the pre-industrial period (Fig. 2 j). This will drop to 5%–11% under RCP8.5 to RCP2.6 scenarios. By 2300, over 98% of the ocean will be undersaturated under the RCP8.5 scenario, which could be lethal to coral reefs.
The increasing atmosphere CO2 concentration leads to higher seawater temperature and a lower aragonite saturation state, so that corals will suffer the combined effects of global warming and ocean acidification (Pandolfi et al., 2011 and Reynaud et al., 2003 ). The surface seawater will be undersaturated in the Arctic and the south ocean by the mid-21st century under the RCP8.5 scenario. This undersaturation will be delayed 20 years and 60 years under the RCP6.0 and RCP4.5 scenarios and undersaturation will not occur under the high mitigation scenario of RCP2.6. However, a relative observation shows that the Canadian basin surface appears as an aragonite undersaturated area. However, that is due to the limited resolution of the UVic model. Although UVic model has a coupled sea-ice model, the present sea-ice model cannot simulate and predict the future sea-ice variation trend. The real melt rate of sea-ice is faster than the model estimation (Stroeve et al., 2007 ). Averaged over the whole ocean, the aragonite saturation horizon will increase from a depth of 1138 m in the pre-industrial period to 308 m at the year 2100 under RCP8.5 scenario. In each part of the ocean, it will lift from 1967 m–530 m in Atlantic–Arctic and 805 m–196 m in Pacific–India.
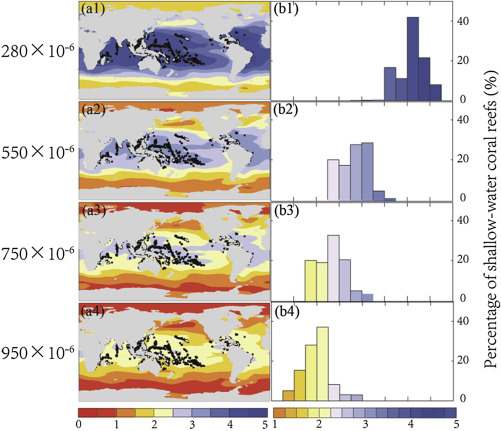
The aragonite saturation states around both shallow- and cold-water coral reefs will rapidly decline. Maps were generated using UV-CDAT (http://uvcdat.llnl.gov/ ). In the pre-industrial period, the water surrounding shallow-water coral reefs had a mean Ω value of 4.1. Over 99% of these areas are surrounded by seawater with Ω > 3.5 ( Fig. 4 ). Under the RCP scenarios, the average Ω of the seawater surrounding the shallow-water coral reefs will decrease to 2.2–3.0. At the year 2055, there will be less than 1% of these areas surrounded by seawater with Ω > 3.5. This situation will be delayed for 20 years and 35 years under the RCP6.0 and RCP4.5 scenarios, respectively. Even if CO2 emissions follow the RCP2.6 scenario, there will be only 27% of shallow-water coral reefs surrounded by seawater with Ω > 3.5.
|
|
|
Fig. 4. Model-simulated ocean surface aragonite saturation state with shallow-water coral reefs under RCP8.5 scenario, (a1–a4) surface aragonite saturation state overlaid with shallow-water coral reef locations (black dots) at CO2 levels of 280 × 10−6 (around the year 1800), 550 × 10−6 (around the year 2050), 750 × 10−6 (around the year 2080), and 950 × 10−6 (around the year 2100), (b1–b4) percentage distribution of shallow-water coral reefs surrounded by seawater at each aragonite saturation bin. |
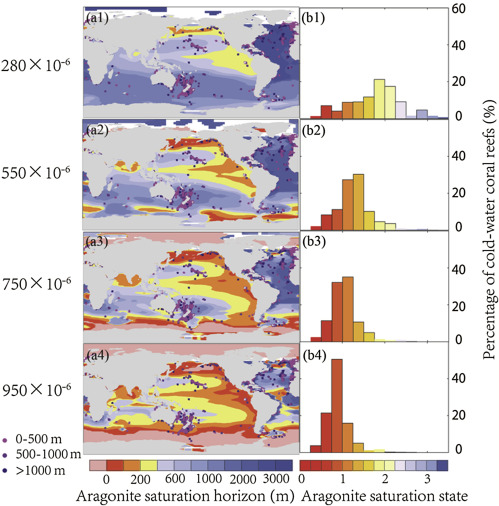
With respect to the cold-water coral reefs, the pre-industrial aragonite saturation state of the seawater surrounding deep-sea coral reefs was 1.8 (Fig. 5 ). By the year 2100, the average saturation state of the water around cold-water coral reefs will decline to 0.9–1.4 (across RCP8.5 to RCP2.6). Meanwhile, there is only 27%–72% of the cold-water coral reefs that will be surrounded by seawater with Ω > 1. By the year 2300, more than 96% of these will be surrounded by seawater with aragonite undersaturation according to the RCP8.5 scenario.
|
|
|
Fig. 5. Model-simulated ocean aragonite saturation horizon with cold-water coral reefs under RCP8.5 scenario, (a1–a4) aragonite saturation horizon overlaid with deep-sea coral reef locations. Coral reef locations at different depth range are represented by different colored dots. Areas in pink represent regions where aragonite saturation horizon has reached the surface. (b1–b4) percentage distribution of cold-water coral reefs surrounded by seawater at each aragonite saturation bin. |
Based on the experiments of shallow-water coral reefs, the calcification rate of reef-building corals would be significantly reduced when aragonite saturation decreased slightly under the situation of supersaturated (Fabry et al., 2008 and Langdon et al., 2003 ). Whats more, recent observations show that the calcification rate of corals has been declining in recent decades (De'ath et al., 2013 and Su, 2012 ). Therefore, there are reasons to believe that ocean acidification would have significant influence on coral reefs before the seawater becomes undersaturated in aragonite.
4. Conclusions and discussion
(1)The ocean will continuously absorb CO2 from the atmosphere under four RCP scenarios. Thus, the global mean ocean surface temperature will rise by 1.1–2.8 K while the global mean ocean surface pH and [CO32− ] will drop 0.14–0.42 and 20%–51% across the RCPs scenarios. The physical and chemical changes of the deep sea lag behind the ocean surface. The effects will last longer than the time under which the atmospheric CO2 concentration is stabilized. It is credible that a large amount of anthropogenic CO2 emissions will continue to impact the whole ocean.
(2)The aragonite saturation state of sea water surrounding coral reefs will decrease rapidly. During the pre-industrial period, over 99% of shallow-water coral reefs were surrounded by seawater with Ω > 3.5 and 87% of cold-water coral reefs were surrounded by seawater with Ω > 1. On the basis of the model-simulations, by the end of the 21st century, less than 1% of shallow-water coral reefs will be surrounded by seawater with Ω > 3.5 with the exception of the RCP2.6 scenario, while 73% of cold-water coral reefs will suffer from undersaturated aragonite seawater under the RCP8.5 scenario. The calcification rate of coral reefs will decrease because of the lowering of the aragonite saturation state. Thus, both shallow- and cold-water coral reefs will suffer from ocean acidification.
(3)The increasing CO2 concentration will lead to higher temperatures and lower aragonite saturation state in the ocean. This means that coral reefs will be synergistically impacted by global warming and ocean acidification. Analyzing the situation using four RCP scenarios, reducing CO2 emissions can effectively slow the process of ocean physical and chemical changes.
The predictions of the deep ocean acidification have greater uncertainty in comparison to those of the ocean surface. This is mainly due to the ocean currents simulation and physical transmission process between models (Cao et al., 2009 ). The model prediction and simulation of deep ocean acidification and its influence on the cold-water coral reefs need to be quantified using the simulations from other earth system models. This study focuses on the open ocean chemistry of the aragonite saturation state of seawater surrounding coral reefs, but the carbonate chemistry within the coral reef system might be substantially different from that of the surrounding seawater (McCulloch et al., 2012 and Andersson et al., 2014 ). Furthermore, changes in a number of other environmental factors, many of which are associated with human activities, such as heat stress, light, salinity, the abundance of food and nutrients, overfishing, and pollution could all influence the fate of coral reefs (Pandolfi et al., 2011 ).
Acknowledgements
This work was supported by National Natural Science Foundation of China (41276073 , 41422503 ), National Key Basic Research Program of China (2015CB953601 ), Zhejiang University K.P. Chaos High Technology Development Foundation and the Fundamental Research Funds for the Central Universities .
References
- Andersson et al., 2014 A.J. Andersson, K.L. Yeakel, N.R. Bates, et al.; Partial offsets in ocean acidification from changing coral reef biogeochemistry; Nat. Clim. Change, 4 (2014), pp. 56–61
- Caldeira and Wickett, 2005 K. Caldeira, M.E. Wickett; Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean; J. Geophys. Res. Oceans, 110 (2005), p. C09S04 http://dx.doi.org/10.1029/2004JC002671
- Cao and Caldeira, 2008 L. Cao, K. Caldeira; Atmospheric CO2 stabilization and ocean acidification ; Geophys. Res. Lett., 35 (19) (2008), p. L19609
- Cao et al., 2009 L. Cao, M. Eby, A. Ridgwell, et al.; The role of ocean transport in the uptake of anthropogenic CO2; J. Biogeosci., 6 (2009), pp. 375–390
- Cao et al., 2014a L. Cao, S.-J. Wang, M.-D. Zheng, et al.; Sensitivity of ocean acidification and oxygen to the uncertainty in climate change; J. Environ. Res. Lett., 9 (6) (2014), p. 0640 http://dx.doi.org/10.1088/1748-9326/9/6/064005
- Cao et al., 2014b L. Cao, H. Zhang, M.-D. Zheng, et al.; Response of ocean acidification to a gradual increase and decrease of atmospheric CO2; Environ. Res. Lett., 9 (2) (2014), pp. 239–246
- De'ath et al., 2013 G. De'ath, K. Fabricius, J. Lough; Yes — Coral calcification rates have decreased in the last twenty-five years!; Mar. Geol., 346 (2013), pp. 400–402
- Doney et al., 2009 S.C. Doney, V.J. Fabry, R.A. Feely, et al.; Ocean acidification: the other CO2 problem ; Ann. Rev. Mar. Sci., 1 (2009), pp. 169–192
- Fabry et al., 2008 V.J. Fabry, B.A. Seibel, R.A. Feely, et al.; Impacts of ocean acidification on marine fauna and ecosystem processes; Ices J. Mar. Sci., 65 (2008), pp. 414–432
- Feely et al., 1988 R.A. Feely, R.H. Byrne, J.G. Acker, et al.; Winter-summer variations of calcite and aragonite saturation in the Northeast Pacific; Mar. Chem., 25 (88) (1988), pp. 227–241
- Fine and Tchernov, 2007 M. Fine, D. Tchernov; Scleractinian coral species survive and recover from decalcification; Science, 315 (2007), p. 1811
- Freiwald et al., 2004 A. Freiwald, J.H. Fosså, A. Grehan, et al.; Cold-water Coral Reefs; UNEP World Conservation Monitoring Centre, Cambridge UK (2004)
- Gattuso and Hansson, 2011 J.P. Gattuso, L. Hansson; Ocean Acidification: Background and History; Oxford University Press, Oxford (2011)
- Guinotte and Fabry, 2008 J.M. Guinotte, V.J. Fabry; Ocean acidification and its potential effects on marine ecosystems; Ann. N. Y. Acad. Sci., 1134 (2008), pp. 320–342
- Guinotte et al., 2006 J. Guinotte, J. Orr, S. Cairns, et al.; Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals?; Front. Ecol. Environ., 3 (2006), pp. 141–146
- Indermühle et al., 1999 A. Indermühle, T.F. Stocker, F. Joos, et al.; Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica ; Nature, 398 (6723) (1999), pp. 121–126
- IPCC, 2013 IPCC; Climate Change 2013: the Physical Science Basis; Cambridge University Press, Cambridge, UK (2013)
- Key et al., 2004 M.R. Key, A.L. Kozyr, C. Sabine, et al.; A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP); Glob. Biogeochem. Cycles, 18 (4) (2004), pp. 357–370
- Langdon et al., 2003 C. Langdon, W.S. Broecker, D.E. Hammond, et al.; Effect of elevated CO2 on the community metabolism of an experimental coral reef ; Glob. Biogeochem. Cycles, 17 (1) (2003), p. 11
- Matthews et al., 2009 H.D. Matthews, L. Cao, K. Caldeira; Sensitivity of ocean acidification to geoengineered climate stabilization; Geophys. Res. Lett., 36 (10) (2009), p. L10706
- McCulloch et al., 2012 M.J. McCulloch, J. Falter, J. Trotter, et al.; Coral resilience to ocean acidification and global warming through pH upregulation; Nat. Clim. Change, 2 (2012), pp. 623–627
- Meissner et al., 2003 K.J. Meissner, A.J. Weaver, H.D. Matthews, et al.; The role of land surface dynamics in glacial inception: a study with the UVic Earth System model; Clim. Dyn., 21 (2003), pp. 515–537
- Mucci, 1983 A. Mucci; The solubility of calcite and aragonite in seawater at various salinities, temperatures and one atmosphere total pressure; Am. J. Sci., 283 (7) (1983), pp. 780–799
- Orr et al., 1999 J.C. Orr, R. Najjar, C. Sabine, et al.; Abiotic-HOWTO; Internal OCMIP Report LSCE/CEA, Gifsur-Yvette, Saclay, France (1999)
- Orr et al., 2005 J.C. Orr, V.J. Fabry, O. Aumont, et al.; Anthropogenic ocean acidification over the twenty first century and its impact on calcifying organisms; Nature, 437 (2005), pp. 681–686
- Pandolfi et al., 2011 J.M. Pandolfi, S.R. Connolly, D.J. Marshall, et al.; Projecting coral reef futures under global warming and ocean acidification; Science, 333 (6041) (2011), pp. 418–422
- Reynaud et al., 2003 S. Reynaud, N. Leclercq, S. Romainelioud, et al.; Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral ; Glob. Change Biol., 9 (2003), pp. 1660–1668
- Robbins et al., 2013 L.L. Robbins, J.G. Wynn, J.T. Lisle, et al.; Baseline monitoring of the western Arctic Ocean estimates 20% of Canadian basin surface waters are undersaturated with respect to aragonite; PLoS One, 8 (9) (2013), p. 274
- Sabine et al., 2004 C.L. Sabine, R.A. Feely, N. Gruber, et al.; The oceanic sink for anthropogenic CO2; Science, 305 (5682) (2004), pp. 367–371
- Schmittner et al., 2008 A. Schmittner, A. Oschlies, H.D. Matthews, et al.; Future changes in climate, ocean circulation, ecosystems, and biogeochemical cycling simulated for a business-as-usual CO2 emission scenario until year 4000 AD ; Glob. Biogeochem. Cycles, 22 (2008) http://dx.doi.org/10.1029/2007GB002953
- Steinacher et al., 2009 M. Steinacher, F. Joos, T.L. Frölicher, et al.; Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model; Biogeosciences, 6 (4) (2009), pp. 515–533
- Stroeve et al., 2007 J. Stroeve, M.M. Holland, W. Meier, et al.; Arctic sea ice decline: faster than forecast; Geophys. Res. Lett., 34 (9) (2007), pp. 529–536
- Su, 2012 R.-X. Su; Coral calcification under increasing atmospheric CO2 concentration and global warming in the southern South China Sea ; Quat. Int., 279–280 (2012), p. 474 (in Chinese)
- Wang et al., 2014 S.-J. Wang, L. Cao, N. Li; Responses of the ocean carbon cycle to climate change: results from an earth system climate model simulation; Adv. Clim. Change Res., 5 (2014), pp. 123–130
- Weaver et al., 2001 A.J. Weaver, M. Eby, E.C. Wiebe, et al.; The UVic Earth system climate model: model description, climatology, and applications to past, present and future climate; Atmos. Ocean, 39 (4) (2001), pp. 361–428
- Xu and Li, 2009 Y.-F. Xu, Y.-C. Li; Estimates of anthropogenic CO2 uptake in a global ocean model ; Adv. Atmos. Sci., 26 (2) (2009), pp. 265–274
- Zickfeld et al., 2013 K. Zickfeld, M. Eby, K. Alexander, et al.; Long-term climate change commitment and reversibility: an EMIC intercomparison; J. Clim., 26 (16) (2013), pp. 5782–5809
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?