Highlights
- We studied the effect of in vivo mid-myocardial pacing in canines on TDR, based on our previous in vitro study.
- Under epicardial pacing, epicardium repolarizes first; longest TMid–Epi results in the longest TDR.
- In vivo TDR under mid-myocardial pacing remains the same level as that under endocardial pacing and keeps this advantage on the distant myocardium.
- Mid-myocardial pacing as compared to epicardial pacing significantly decreases TDR and remains this advantage on the distant myocardium.
Abstract
Objective
In our previous in vitro study mid-myocardial relative to epicardial pacing decreased transmural dispersion of depolarization (TDR) and prevented ventricular arrhythmia. We therefore hypothesized that in vivo mid-myocardial pacing in canines has a similar effect.
Methods and results
Using custom-made electrodes, monophasic action potentials were simultaneously recorded in vivo from left ventricular epicardial (Epi), mid-myocardial (Mid) and endocardial (Endo) layers of canines (n = 12). TDR was significantly increased at Epi (44.6 ± 6. 4 ms; 14.2 ± 5.1 ms; and 13.8 ± 5.4 ms for Epi, Mid and Endo pacing, respectively; P < 0.001), and similarly at Mid and Endo pacing (P = 0.855). This result was reproducible after ibutilide administration (n = 12). TDR was augmented at each layer pacing and significantly increased at Epi (78.1 ± 15.9 ms; 46.8 ± 16.0 ms; and 46.5 ± 15.2 ms for Epi, Mid, and Endo pacing, respectively; P < 0.001), but was similar at Endo and Mid pacing (P = 0.965). TDR at 3 cm from left ventricular apex pacing site was similar between Mid and Endo pacing, and still significantly increased at Epi pacing. At 3 cm distance, the first activation myocardium was still the epicardium at Epi, while sequence transformed from mid-myocardium to endocardium at Mid pacing.
Conclusion
Mid as compared to Epi pacing significantly decreases TDR and remains this advantage on the distant myocardium away from the pacing site.
Abbreviations
TDR, transmural dispersion of depolarization;Epi, epicardium;Mid, mid-myocardium;Endo, endocardium;HF, heart failure;CRT, cardiac resynchronization therapy;MAP, monophasic action potential;MAPD, monophasic action potential duration
Keywords
Transmural dispersion of repolarization;Mid-myocardial pacing;Ibutilide
1. Introduction
In symptomatic chronic heart failure (HF) patients on optimal medical treatment, severely depressed left ventricular ejection fraction (LVEF ≤ 35%) and complete left bundle branch block (LBBB), cardiac resynchronization therapy (CRT) reduces mortality and hospitalization rates, and improves cardiac function and structure [1]. However, CRT also is proarrhythmic secondary to epicardial pacing induced QT interval prolongation and increased transmural dispersion of repolarization (TDR) [2]; [3] ; [4]. Because our previous study [5] using the canine left ventricular wedge preparation in vitro model showed that mid-myocardial relative to epicardial pacing significantly decreased TDR and prevented ventricular arrhythmias, we hypothesized that pacing from the mid-myocardium in vivo in dogs also decreases TDR and arrhythmogenesis.
2. Methods
2.1. Animal preparation
Twelve adult mongrel canines, including both male and female, weighing 15–20 kg were anesthetized with sodium pentobarbital (30 mg/kg, intravenously). Additional smaller doses were given as needed to maintain deep anesthesia. The chest was opened via a left-thoracotomy, the pericardium was incised and the anterior wall of the left ventricle was exposed. A pacing lead was sewed at the right ventricle to prevent sinus pause or bradycardia.
2.2. Electrophysiology recording and different site pacing
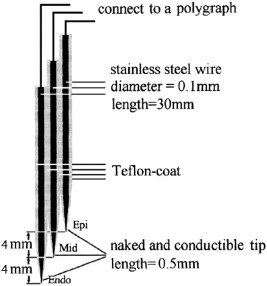
To obtain a slower controlled pacing heart rate, sinus rate was arrested by injection of 1.0–3.0 ml of formaldehyde (100 ml/l) into the region between the right atrial appendage and superior vena cava [6]. Right ventricular pacing was delivered at a cycle length (CL) of 1000 ms. Using the methods of Huang et al. [7], custom-made electrodes were used to record monophasic action potential (MAP) of the epicardium (Epi), mid-myocardium (Mid), and the endocardium (Endo). Because the thickness of the free wall of the canine left ventricle is approximately 8 mm, the distance between the tips of the 3 electrodes was 4 mm: the distal, middle and proximal electrodes were designated Endo, Mid and Epi, respectively (Fig. 1). Another custom-made electrode to pace the three layers was inserted at the left ventricular apex (LVa). The MAP electrodes penetrated the free wall of the canine left ventricle and the reference electrode was placed on the thorax. Leads I, II, III, aVR, aVL and aVF were attached to record surface electrocardiogram (sECG). All signals were recorded with a polygraph and were filtered to record frequencies between 0.05 Hz and 30 Hz for the MAP and between 30 Hz and 600 Hz for the sECG. The 90% of MAP duration (MAPD) of Endo (MAPD90Endo), Mid (MAPD90Mid) and Epi (MAPD90Epi), and the TDR at baseline and at pacing the three layers with basic stimulus (S1S1 = 1000 ms) were acquired. Ibutilide (0.2 ml/kg, intravenously) then was administered, and the MAPD90 of the three layers at baseline and at pacing were recorded. TDR was defined as the difference between longest and shortest repolarization time (RT), where RT = activation time + APD.
|
|
|
Fig. 1. Diagram of monophasic action potential recording electrodes. The distal, middle and proximal electrodes are the Endo, Mid and Epi electrodes, respectively [7]. |
2.3. Programmed electrical stimulation and ventricular arrhythmias
Arrhythmias were induced by programmed electrical stimulation. The basic stimulus (S1) was delivered to the Endo, Mid, or Epi. After every fifth S1 (S1S1 = 1000 ms), a premature stimulus (S2) was delivered. The S1–S2 coupling interval was progressively reduced until it reached the effective refractory period or induced ventricular arrhythmias (S2 stimuli were of 2–3 ms duration with intensity two-fold over baseline pacing threshold). The rates of induced ventricular arrhythmias at baseline and after ibutilide administration were recorded.
2.4. Ventricular conduction in different layers pacing
To observe the activation sequence of the three layers at a certain distance from the pacing site, two custom-made electrodes to record MAP were penetrated along a straight line at 1 cm and 3 cm from the pacing site at LVa. When pacing in the Endo, Mid, or Epi at the LVa, the activation sequence of the three layers and the TDR at 1- and 3-cm distance was acquired synchronously before and after ibutilide administration.
2.5. Statistical analysis
Continuous variables are expressed as mean ± standard deviation and discrete variables as percentages. One-way variance analysis was used to evaluate difference in electrophysiological parameters among Endo, Mid, and Epi pacing. Student–Newman–Keuls (SNK) was used as post hoc test in subsequent multiple comparative analyses. The paired t-test was used to compare electrophysiological parameters between basic and ibutilide groups. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Effect of endocardial, mid-myocardial, and epicardial pacing on transmural dispersion of repolarization
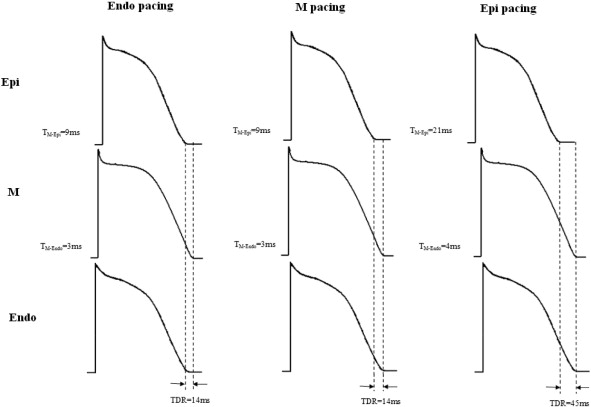
MAPD90, TMid–Epi and TDR results are shown in Table 1, Table 2 ; Table 3. At Endo pacing, the activation sequence was endocardium, mid-myocardium and epicardium; and longest and shortest MAPD90 were in the mid-myocardium (252.4 ± 29.5 ms) and epicardium (229.9 ± 26.0 ms), with conduction time between Mid and the Epi (TMid–Epi) of 8.6 ± 1.1 ms corresponding to the TDR of 13.8 ± 5.4 ms. At Mid pacing, the activation sequence was mid-myocardium, endocardium and epicardium; and longest and shortest MAPD90 were in the mid-myocardium (252.7 ± 29.5 ms) and epicardium (229.4 ± 26.4 ms), with TMid–Epi of 9.0 ± 0.9 ms corresponding to the TDR of 14.2 ± 5.1 ms. At Epi pacing, the activation sequence was epicardium, mid-myocardium and endocardium; and longest and shortest MAPD90 were in the mid-myocardium (252.9 ± 29.2 ms) and epicardium (230.1 ± 26.5 ms), with TMid–Epi of 21.8 ± 2.1 ms corresponding to the TDR of 44.6 ± 6.4 ms (Fig. 2).
| Before ibutilide (n = 12) | After ibutilide (n = 12) | |||||
|---|---|---|---|---|---|---|
| Epi MAPD90 (ms) | Mid MAPD90 (ms) | Endo MAPD90 (ms) | Epi MAPD90 (ms) | Mid MAPD90 (ms) | Endo MAPD90 (ms) | |
| Endo pacing | 229.9 ± 26.0 | 252.4 ± 29.5 | 241.8 ± 28.5 | 249.3 ± 34.5⁎ | 304.6 ± 34.8⁎ | 264.8 ± 35.4⁎ |
| Mid pacing | 229.4 ± 26.4 | 252.7 ± 29.5 | 241.5 ± 28.1 | 249.3 ± 35.1⁎ | 304.6 ± 35.6⁎ | 264.7 ± 36.1⁎ |
| Epi pacing | 230.1 ± 26.5 | 252.9 ± 29.2 | 241.5 ± 28.4 | 249.3 ± 34.0⁎ | 304.2 ± 35.0⁎ | 264.9 ± 36.0⁎ |
| P value | 0.998 | 0.999 | 0.777 | 0.999 | 0.853 | 0.997 |
Endo indicates endocardial; Mid, mid-myocardial; Epi, epicardial; and MAPD90, monophasic action potential duration measured at 90% repolarization.
⁎. P < 0.05 (vs. after ibutilide).
| Endo pacing | Mid pacing | Epi pacing | P value | ||||
|---|---|---|---|---|---|---|---|
| ANOVA | Endo vs. Mid | Mid vs. Epi | Epi vs. Endo | ||||
| Before ibutilide | 8.6 ± 1.1 | 9.0 ± 0.9 | 21.8 ± 2.1 | < 0.001 | 0.552 | < 0.001 | < 0.001 |
| After ibutilide | 8.8 ± 0.8 | 8.4 ± 1.1 | 23.2 ± 2.2⁎ | < 0.001 | 0.561 | < 0.001 | < 0.001 |
Endo indicates endocardial; Mid, mid-myocardial; Epi, epicardial; and ANOVA, analysis of variance.
⁎. P < 0.05 (vs. after ibutilide).
| Endo pacing | Mid pacing | Epi pacing | P value | ||||
|---|---|---|---|---|---|---|---|
| ANOVA | Endo vs. Mid | Mid vs. Epi | Epi vs. Endo | ||||
| Before ibutilide | 13.8 ± 5.4 | 14.2 ± 5.1 | 44.6 ± 6.4 | < 0.001 | 0.855 | < 0.001 | < 0.001 |
| After ibutilide | 46.5 ± 15.2⁎ | 46.8 ± 16.0⁎ | 78.1 ± 15.9⁎ | < 0.001 | 0.965 | < 0.001 | < 0.001 |
Endo indicates endocardial; Mid, mid-myocardial; Epi, epicardial; and ANOVA, analysis of variance.
⁎. P < 0.05 (vs. after ibutilide).
|
|
|
Fig. 2. Effect of endocardial, mid-myocardial, and epicardial pacing on transmural activation sequence and ventricular repolarization before ibutilide. |
There was no significant difference among MAPD90 at Endo, Mid and Epi pacing (P > 0.05). However, TMid–Epi significantly increased when pacing site shifted from Mid to Epi (P < 0.05), but not from Endo to Mid. As a result, TDR at Epi pacing was significantly longer than those of pacing at Endo and Mid (P < 0.05).
3.2. Effect of endocardial, mid-myocardial, and epicardial pacing on transmural dispersion of repolarization after ibutilide administration
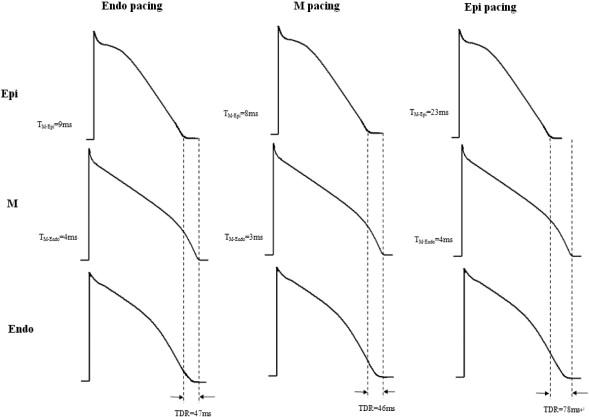
During ibutilide administration, activation and repolarization sequences were the same as those at baseline. Similarly, pacing at Epi induced significantly longer TMid–Epi and TDR (P < 0.05). Moreover, MAPD90 of the three layers at Endo, Mid and Epi pacing and TDR were significantly increased compared with those at baseline. In the presence of ibutilide, a shift from Endo or Mid to Epi pacing led to further TDR prolongation (Table 3, Fig. 3).
|
|
|
Fig. 3. Effect of endocardial, mid-myocardial, and epicardial pacing on transmural activation sequence and ventricular repolarization after ibutilide. |
3.3. Pacing site-dependent ventricular arrhythmia
At baseline conditions, although TDR increased from 13/14 ms to 44 ms when pacing sites shifted from Endo/Mid to Epi, no ventricular extrasystoles or tachycardia were induced by programmed electrical stimulation during pacing at the three layers. After ibutilide augmented the effect of Epi pacing to increase TDR, one or two ventricular extrasystoles were observed followed by an S2 interval from 300 ms to 210 ms in 9/12 (75%) canines at Epi pacing, and in 2/12 (16%) canines at Endo/Mid pacing. No ventricular tachycardias were induced at any coupling interval tested under any site pacing.
3.4. Remote transmural activation sequence and dispersion of repolarization at pacing different layers
Mid and Endo pacing had similar TDR, which is advantageous over Epi pacing with prolonged TDR. We determined if this advantage of Mid pacing applies to remote myocardial transmural activation sequence and dispersion of repolarization. At 1 and 3 cm from the LVa, TDR was similar between Mid and Endo pacing, and still significantly prolonged than that of Epi pacing (Table 4). It is interesting that the transmural activation sequence differed at 1 and 3 cm from the pacing site. When pacing at Endo or Epi, transmural activation sequence was Endo–Mid–Epi and Epi–Mid–Endo at 1 and 3 cm. However, for Mid pacing, transmural activation sequence differed at 1 cm (Mid–Endo–Epi) and 3 cm (Endo–Mid–Epi). Although the first place of remote myocardial transmural activation sequence changed from Mid at 1 cm to Endo at 3 cm, TDR at 1 and 3 cm remained shorter under Mid than Epi pacing.
| Endo pacing | Mid pacing | Epi pacing | P value | ||||
|---|---|---|---|---|---|---|---|
| ANOVA | Endo vs. Mid | Mid vs. Epi | Epi vs. Endo | ||||
| 1 cm | 14.5 ± 5.6 | 14.9 ± 5.4 | 46.7 ± 6.7 | < 0.001 | 0.856 | < 0.001 | < 0.001 |
| 3 cm | 14.4 ± 5.6 | 16.3 ± 7.3 | 46.4 ± 6.9 | < 0.001 | 0.501 | < 0.001 | < 0.001 |
Endo indicates endocardial; Mid, mid-myocardial; Epi, epicardial; and ANOVA, analysis of variance.
4. Discussion
In our previous study [5] on in vitro effects on TDR of pacing at the three layers, Epi pacing altered transmural activation sequence of the intrinsically heterogeneous ventricular myocardium and augmented TDR favoring arrhythmia development, while Mid pacing did not change transmural activation sequence from Mid to Epi and consequently maintained roughly the same TDR as Endo pacing. Therefore, Mid as compared to Epi pacing effectively reduced TDR and reentry-related arrhythmia. However, in vitro and in vivo conditions differ, the latter being influenced by anatomy, and autonomic nerve and humoral coordination, among other factors. To render our in vitro observations more clinically relevant to CRT device implantation, we conducted the present study to ascertain if Mid pacing retained its advantages in vivo and, consistent with in vitro experiment results, found that: 1. Under Epi as compared with Endo or Mid pacing, epicardium activates and repolarizes first; longest TMid–Epi results in further delay of mid-myocardium activation; and these two aspects lead to the longest TDR; while 2. Under Mid pacing, mid-myocardium is activated first; epicardium is activated last and repolarizes first as in normal ventricular activation and repolarization; TMid–Epi remains similar to that under Endo pacing, and therefore, TDR under Mid pacing remains at about the same level as that under Endo pacing and normal physiological state, rendering Mid pacing more advantageous than Epi pacing.
Previous studies illustrated that ventricular arrhythmias, especially Torsade de Pointes (Tdp), is easily induced only when TDR exceeds a critical point (usually 80–90 ms) [8]; [9] ; [10], and pacing any of the three layers in the normal heart yields much lower TDRs. In the presence of ibutilide, the rapid component of the delayed rectifier potassium current (Ikr) that is evenly distributed among the three layers is blocked, and the repolarizing current mainly depends on the slow component of the delayed rectifier potassium current (Iks). Because the density of Iks in the mid-myocardium is lower than that in the epicardium or endocardium, the outward current of the mid-myocardium is significantly weaker than that in the other layers [11] thereby increasing mid-myocardial relative to endocardial and epicardial MAPD90 and prolonging TDR in the presence of ibutilide. In this context, the effect of increasing TDR by Epi pacing can be more obvious. Moreover, studies have shown that TDR is augmented in pathological conditions, such as ischemic and dilated cardiomyopathy [12] ; [13]. In addition, previous studies have clearly shown the effect of sympathetic stimulation on TDR and after depolarization. When these conditions are concurrent in one patient, the superimposition effect of increasing TDR by drugs, pathological myocardium and Epi pacing could easily augment TDR to reach the critical proarrhythmic point placing the patient at greater risk for malignant ventricular arrhythmia. Therefore, in these patients, caution should be exercised with Epi pacing or CRT implantation, and Mid pacing might allow to reduce the risk of malignant ventricular arrhythmia.
Although the present study did not include a three-dimensional assessment of ventricular transmural activation sequence under pacing of the three layers, the myocardium at linear distances from the pacing site was used to gain insight into transmural activation sequence of remote areas. At 3 cm distance under Epi pacing, the epicardium still activated first and the TDR remained the longest suggesting that the disadvantages of Epi pacing applies to distant myocardium, which might lead to repolarization heterogeneity of the entire ventricle. However, at 3 cm distance under Mid pacing, mid-myocardium does not activate first, and first activation layer transforms from mid-myocardium to endocardium and not epicardium, which could be explained by the faster electrical conduction speed of Purkinjes fibers under the endocardium than under the mid-myocardium. The latter difference in conduction speed was not present at 1 cm distance, however, at 3 cm distance, endocardial activation is apparently earlier than that of mid-myocardium. Electrical conduction and transmural activation sequence under Mid pacing therefore might have two potential advantages: 1. Avoidance of disadvantages of Epi pacing that might include non-physiological transmural activation sequence and TDR increase in the entire ventricle; and, 2. The activation sequence transformation from mid-myocardium to endocardium contributes to ventricular activation synchronization through rapid conduction and physiological transmural activation sequence.
Undoubtedly, Endo pacing is much closer to physiological pacing. However, Endo pacing in LV may be associated with intractable complications such as mitral regurgitation, thrombosis, and pericardial tamponade, among others [5]. Mid pacing appears promising as an alternative method by avoiding adverse effects of Epi pacing including non-physiological transmural activation sequence and TDR increase, and by improving intraventricular conduction through rapid conduction and physiological transmural activation sequence originating from activation sequence transformation from mid-myocardium to endocardium at a certain distance. Although there are no Mid electrode products and matched applications available at present, the results of the experiments in vitro and vivo provide a basis for the development of said products and applications.
5. Study limitations
First, MAP recorded by custom-made electrodes reflects the potential of a group cells, not a single cell. Second, the present study did not include a three-dimensional assessment of ventricular transmural activation sequence under pacing of the three layers. Third, the whole study was conducted in the state of pentobarbital anesthesia state, trauma, arrest of sinus rate, and other factors, which could influence the results.
6. Conclusion
In the present study, Mid pacing retained its advantages in vivo and found that: 1. Mid pacing remains a normal sequence of ventricular repolarization; 2. Under Mid pacing, TDR remains at about the same level as that under Endo pacing and normal physiological state, rendering Mid pacing more advantageous than Epi pacing; and 3. The advantages of sequence of ventricular repolarization and TDR remains on the distant myocardium away from the pacing site.
Conflict of interest
None declared.
Funding
The study was supported by a grant from the National Natural Science Foundation of China (No. 30971230).
References
- [1] European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA), M. Brignole, A. Auricchio, G. Baron-Esquivias, P. Bordachar, et al.; ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA); Europace, 15 (2013), pp. 1070–1118
- [2] M. Rivero-Ayerza, M. Vanderheyden, S. Verstreken, M. de Zutter, P. Geelen, P. Brugada; Images in cardiovascular medicine. Polymorphic ventricular tachycardia induced by left ventricular pacing; Circulation, 109 (2004), pp. 2924–2925
- [3] B.K. Kantharia, J.A. Patel, B.S. Nagra, G.S. Ledley; Electrical storm of monomorphic ventricular tachycardia after a cardiac-resynchronization-therapy-defibrillator upgrade; Europace, 8 (2006), pp. 625–628
- [4] V.A. Medina-Ravell, R.S. Lankipalli, G.X. Yan, C. Antzelevitch, N.A. Medina-Malpica, O.A. Medina-Malpica, et al.; Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes; Circulation, 107 (2003), pp. 740–746
- [5] T. Xu, H. Wang, J.Y. Zhang, Y. Zhang, R. Zhang, L.Q. Jiang, et al.; Effects of mid-myocardial pacing on transmural dispersion of repolarization and arrhythmogenesis; Europace, 14 (2012), pp. 1363–1368
- [6] H. Jiang, Z. Lu, Y. Yu, D. Zhao, B. Yang, C. Huang; Relationship between sympathetic nerve sprouting and repolarization dispersion at peri-infarct zone after myocardial infarction; Auton Neurosci, 134 (2007), pp. 18–25
- [7] C. Huang, M. Bao, H. Jiang, J. Liu, B. Yang, T. Wang; Differences in the changing trends of monophasic action potential duration and effective refractory period of the ventricular myocardium after myocardial infarction in vivo; Circ J, 68 (2004), pp. 1205–1209
- [8] W. Shimizu, C. Antzelevitch; Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome; J Am Coll Cardiol, 35 (2000), pp. 778–786
- [9] W. Shimizu, C. Antzelevitch; Effects of a K(+) channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome; Circulation, 102 (2000), pp. 706–712
- [10] W. Shimizu, C. Antzelevitch; Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes; Circulation, 98 (1998), pp. 2314–2322
- [11] D.W. Liu, C. Antzelevitch; Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell; Circ Res, 76 (1995), pp. 351–365
- [12] A. Lukas, C. Antzelevitch; Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current; Circulation, 88 (1993), pp. 2903–2915
- [13] R. Bai, J. Lü, J. Pu, N. Liu, Q. Zhou, Y. Ruan, et al.; Left ventricular epicardial activation increases transmural dispersion of repolarization in healthy, long QT, and dilated cardiomyopathy dogs; Pacing Clin Electrophysiol, 28 (2005), pp. 1098–1106
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?