Abstract
Background
Transthoracic Doppler-echocardiography (TTE) can estimate mean pulmonary arterial pressure (MPAP) and pulmonary capillary wedge pressure (PCWP) reliably, and cardiac magnetic resonance (CMR) is the best modality for non-invasive measurement of cardiac output (CO). We speculated that the combined use of TTE and CMR could provide a feasible method for non-invasive measurement of pulmonary vascular resistance (PVR) in pulmonary arterial hypertension (PAH).
Methods and results
Right heart catheterization (RHC) was undertaken in 77 patients (17M/60F) with PAH, and simultaneous TTE was carried out to evaluate MPAP, PCWP and CO. Within 2 days, CO was measured again with CMR in similar physiological status. Then, PVR was calculated with the integrated non-invasive method: TTE-derived (MPAP–PCWP)/CMR-derived CO and the isolated TTE method: TTE-derived (MPAP–PCWP)/TTE-derived CO, respectively. The PVR calculated with integrated non-invasive method correlated well with RHC-calculated PVR (r = 0.931, 95% confidence interval 0.893 to 0.956). Between the integrated non-invasive PVR and RHC-calculated PVR, the Bland–Altman analysis showed the satisfactory limits of agreement (mean value: − 0.89 ± 2.59). In comparison, the limits of agreement were less satisfactory between TTE-calculated PVR and RHC-calculated PVR (mean value: − 1.80 ± 3.33). Furthermore, there were excellent intra- and inter-observer correlations for the measurements of TTE and CMR (P < 0.001 for all).
Conclusions
The combined use of TTE and CMR provides a clinically reliable method to determine PVR non-invasively. In comparison with RHC, the integrated method shows good accuracy and repeatability, which suggests the potential for the evaluation and serial follow-up in patients with PAH.
Translational perspective
In PAH, the non-invasive measurement of PVR is very important in clinical practice. Up to now, however, the widely accepted non-invasive method is still unavailable. Since TTE can estimate (MPAP–PCWP) reliably and CMR is the best image modality for the measurement of CO, the combined use of two modalities has the potential to determine PVR non-invasively. In this research, the integrated non-invasive method showed good diagnostic accuracy and repeatability compared with RHC. Therefore, it might be a feasible method for non-invasive measurement of PVR in patients with PAH.
Keywords
Pulmonary arterial hypertension;Pulmonary vascular resistance;Doppler echocardiography;Cardiac magnetic resonance
1. Introduction
The measurement of pulmonary vascular resistance (PVR) is crucial in the diagnosis and management of pulmonary arterial hypertension (PAH). Right heart catheterization (RHC) is widely considered the “gold standard” to determine PVR, however, it is limited by the associated disadvantages [1]. Therefore, it is necessary to develop a feasible method for the non-invasive measurement of PVR. Recent reports have investigated the applicability of transthoracic Doppler-echocardiography (TTE) or cardiac magnetic resonance (CMR), and some novel methods have been proposed [2]; [3]; [4]; [5]; [6]; [7]; [8]; [9]; [10]; [11]; [12] ; [13]. However, the intrinsic limitations of each image modality compromised the accuracy and reproducibility in these methods. Up to now, with the isolated use of TTE or CMR, there was no widely accepted method for non-invasive evaluation of PVR.
Previous studies have demonstrated that TTE and CMR had complementary advantages. TTE had the potential to estimate mean pulmonary arterial pressure (MPAP) and pulmonary capillary wedge pressure (PCWP) reliably [14]; [15]; [16]; [17]; [18]; [19]; [20]; [21]; [22] ; [23]. In the non-invasive measurement of cardiac output (CO), however, CMR had more accuracy and reproducibility than TTE [24]; [25] ; [26]. Based on the formula (MPAP–PCWP)/CO, it became possible to calculate PVR directly with TTE-derived (MPAP–PCWP) and CMR-derived CO. Therefore, we hypothesized that the combined use of TTE and CMR might have the potential to estimate PVR non-invasively. This study was performed to determine whether the integrated modality formed a reliable non-invasive method to measure PVR in PAH.
2. Methods
2.1. Study patients
From January 2009 to July 2014, a total of 77 patients (age 32.42 ± 11.01 years, 17M/60F) with PAH were enrolled. Among these patients, there were 70 patients with idiopathic PAH and 7 patients with post-operative persistent PAH after surgical closure of ventricular septal defect (n = 4), patent ductus arteriosus (n = 2) and atrial septal defect (n = 1). All patients were in sinus rhythm. Patients (n = 16) with unstable clinical condition, inadequate image quality, arrhythmia (such as atrial fibrillation, frequent premature beats and so on), significant mitral or aortic regurgitation, variations of heart rate and blood pressure ≥ 10% (between RHC/TTE and CMR), and contraindications of CMR examination were all excluded from the study. For the patients aged ≥ 45 years (n = 11), coronary artery disease was excluded with selective coronary angiography (defined as ≥ 50% reduction in lumen diameter). The study was approved by our hospital research ethics committee, and informed consent was obtained from each patient.
2.2. Study design
In this study, PAH was defined as MPAP > 25 mm Hg and PCWP ≤ 15 mm Hg at rest (RHC) [27]. During RHC, simultaneous TTE examination was performed in patients with PAH. The invasive hemodynamic data, TTE-derived (MPAP–PCWP) and TTE-derived CO were obtained at rest. Within 2 days after RHC/TTE, CMR was performed without intervening therapy and the resting CO was calculated non-invasively in similar physiological status (variations of heart rate and blood pressure < 10%). According to the formula TTE-derived (MPAP–PCWP)/CMR-derived CO and TTE-derived (MPAP–PCWP)/TTE-derived CO, the non-invasive PVR was calculated, respectively. Furthermore, the results were compared with RHC-calculated PVR using the Bland–Altman analysis. Individuals in whom RHC, TTE and CMR variables were obtained were blinded to each others calculations. Furthermore, thirty-eight patients were randomly selected to validate the intra-observer and inter-observer variability, respectively. For intra-observer reproducibility, the examination was repeated twice by the same observer in a consecutive manner. For inter-observer reproducibility, CMR was validated by two independent observers based on the same imaging result (without any communication). And for TEE, two independent observers conducted the examination in turn also without communication.
2.3. RHC
Our procedure for cardiac catheterization has been described previously [28]. In brief, all patients underwent routine RHC (Swan-Ganz, Edwards Lifesciences) under local anesthesia. The complete hemodynamic data and blood samples were obtained at rest. The measurements included mean right atrial pressure, right ventricular pressure, systolic pulmonary arterial pressure (SPAP), diastolic pulmonary arterial pressure (DPAP), MPAP and PCWP. According to the oxymetric principle of Fick, CO and PVR [PVR = (MPAP–PCWP) / CO] were calculated.
2.4. TTE
In patients undergoing RHC, simultaneous TTE (Philips IE33, instrument equipped with a 3–5 MHz transducer) was performed. TTE-derived MPAP was calculated as TTE-derived SPAP × 0.61 + 2 mm Hg, according to Chemla et al. [16]. SPAP was estimated by TTE from the systolic right ventricular-to-right atrial pressure gradient using the modified Bernoulli equation, and the assessment of right atrial pressure was performed in accordance with previously described methods. Furthermore, isovolumic relaxation time (IVRT) and color M-mode Doppler flow propagation velocity (FPV) were measured. According to the equation: 4.5 (103 / [2 · VRT] + FPV) − 9, PCWP was estimated non-invasively [18]. Based on TTE-derived MPAP and PCWP, the trans-pulmonary pressure gradient (MPAP–PCWP) was calculated non-invasively. According to the recommendations in guideline, the measurements of stroke volume (SV) and CO (SV × heart rate) were made at the level of the left ventricular (LV) outflow tract [29].
2.5. CMR
All examinations were performed on a 1.5 Tesla MR scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) at rest, and the protocol has been described previously [30] ; [31]. In brief, scout images acquired by using half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence were used to analyze the morphology and structure of the heart. Retrospective electrocardiographic-gating cine images were acquired using a true fast imaging with steady-state precession (TrueFisp) sequence. For the volumetric and functional measurements, contiguous short-axis images through the entire ventricle (from base to apex, no gap) were obtained. The following parameters were used: FOV, 250 mm × 188 mm; slice thickness, 8 mm; matrix, 156 mm × 192 mm; TR, 2.8 ms; TE, 1.39 ms; flip angle, 70°; number of signal averages, 2–4. LV end-diastolic and end-systolic volumes, LV ejection fraction (LVEF), SV, and CO (SV × heart rate) were calculated. In addition, right ventricular (RV) end-diastolic and end-systolic volumes, RVEF were also calculated (the endocardial borders of all short-axis images at end-diastole and end-systole were manually traced with the inclusion of RV outflow to the pulmonary valve and the trabeculae in the RV volume).
2.6. Statistical analysis
Categorical variables were presented as counts with percentages and compared by Chi-square test or Fishers exact test. Continuous variables were presented as mean ± SD or median with IQR and compared by grouped t-test or Wilcoxon rank sum test. Pearson or spearman correlation coefficients were calculated first and linear regression models were constructed. Furthermore, Bland–Altman analysis was carried out for agreement assessments, the lower and upper limits of agreement were estimated, as the mean ± 2SDs with 95% confidence interval (CI). Pearsons or Spearmans correlation and Bland–Altman analysis were also used to assess the intra-observer and inter-observer reproducibility. A two-sided P < 0.05 was considered statistically significant. Statistical software used in this study was SPSS 16.0 and MedCalc 9.5.
3. Results
Clinical and demographic characteristics of the patients were listed in Table 1. The TTE-derived (MPAP–PCWP) and RHC-derived (MPAP–PCWP) were comparable (55.06 ± 17.67 mm Hg vs 58.45 ± 18.76 mm Hg, P = 0.25). The linear regression analysis revealed a good correlation (r = 0.934, 95% CI: 0.897–0.957) for all patients (Supplemental Fig. 1). Using the Bland–Altman analysis (Supplemental Fig. 2), TTE-derived (MPAP–PCWP) showed satisfactory limits of agreement with RHC-derived (MPAP–PCWP). The mean value was 3.39 ± 6.73 (SD), and TTE-derived (MPAP–PCWP) and RHC-derived (MPAP–PCWP) values were well within one standard deviation (17.67 and 18.76, respectively).
| Sex, M/F | 17/60 |
| Age, years | 32.42 ± 11.01 |
| BSA, m2 | 1.48 ± 0.14 |
| Diagnosis, n | 77 |
| Idiopathic PAH, n (%) | 70 (90.9%) |
| Post-operative persistent PAH, n (%) | 7 (9.1%) |
| RHC | |
| SPAP, mm Hg | 103.64 ± 31.38 |
| DPAP, mm Hg | 39.97 ± 16.31 |
| MPAP, mm Hg | 68.00 ± 19.28 |
| PCWP, mm Hg | 9.55 ± 1.96 |
| MPAP–PCWP, mm Hg | 58.45 ± 18.76 |
| CO, L/min | 4.83 ± 1.91 |
| Heart rate, bpm | 79.23 ± 10.05 |
| Invasive PVR, Wood | 13.86 ± 7.05 |
| TTE | |
| MPAP, mm Hg | 64.56 ± 18.05 |
| PCWP, mm Hg | 9.49 ± 1.99 |
| MPAP–PCWP, mm Hg | 55.06 ± 17.67 |
| CO, L/min | 3.76 ± 1.45 |
| TTE-calculated PVR, Wood | 16.55 ± 8.27 |
| Mild tricuspid regurgitation, n (%) | 42(54.5%) |
| Moderate tricuspid regurgitation, n (%) | 31(40.3%) |
| Severe tricuspid regurgitation, n (%) | 4(5.2%) |
| CMR | |
| LVEDV, ml | 88.90 ± 50.78 |
| LVESV, ml | 40.65 ± 32.09 |
| LVEF, % | 55.92 ± 9.91 |
| Heart rate, bpm | 77.97 ± 11.64 |
| CO, L/min | 4.18 ± 1.72 |
| RVEF, % | 25.15 ± 9.6 |
| Integrated non-invasive PVR, Wood | 14.75 ± 6.83 |
Notes: BSA, body surface area; Invasive PVR, PVR calculated with RHC; Integrated non-invasive PVR, PVR calculated with TTE-derived (MPAP–PCWP)/CMR-derived CO.
The CO from CMR and RHC were 4.18 ± 1.72 L/min and 4.83 ± 1.91 L/min, respectively. The linear regression analysis also showed a good correlation (r = 0.805, 95% CI: 0.709–0.872) (Supplemental Fig. 3). Using the Bland–Altman analysis (Supplemental Fig. 4), CMR-derived CO demonstrated the good limits of agreement with RHC-derived CO. The mean value was − 0.65 ± 0.85 (SD), and CMR-derived CO and RHC-derived CO values were well within one standard deviation (1.73 and 1.92, respectively).
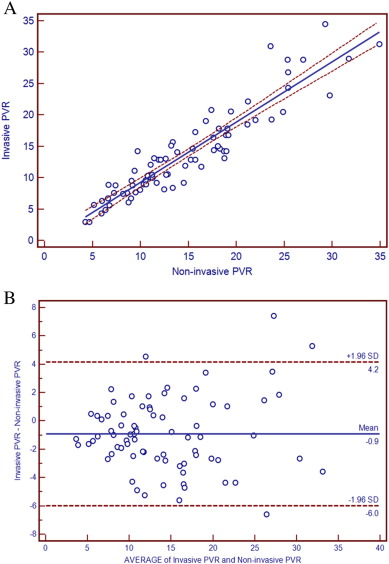
The heart rates were comparable between RHC/TTE and CMR (79.23 ± 10.05 bmp vs 77.97 ± 11.64 bmp, P = 0.474), and variation of heart rate was 1.26 ± 4.61. The integrated non-invasive PVR was 14.75 ± 6.83 Woods and invasive PVR was 13.86 ± 7.05 Woods. The good correlation (r = 0.931, 95% CI: 0.893–0.956) was confirmed with linear regression analysis (Fig. 1A). The Bland–Altman analysis showed the satisfactory limits of agreement between integrated non-invasive PVR and invasive PVR (Fig. 1B). The mean value was − 0.89 ± 2.59 (SD), and integrated non-invasive PVR and invasive PVR values were well within one standard deviation (6.84 and 7.06, respectively). While between TTE-calculated PVR and invasive PVR, linear regression analysis (Supplemental Fig. 5) showed the good correlation (r = 0.912, 95% CI: 0.865–0.943), and the Bland–Altman analysis (Supplemental Fig. 6) showed the less satisfactory limits of agreement (mean value: − 1.80 ± 3.33).
|
|
|
Fig. 1. A, Scatter diagram and regression line between integrated non-invasive PVR and invasive PVR (r = 0.931, 95% CI: 0.893–0.956) in all patients. B, Bland–Altman plot of the difference between integrated non-invasive PVR and invasive PVR against the mean of the non-invasive PVR and invasive PVR. |
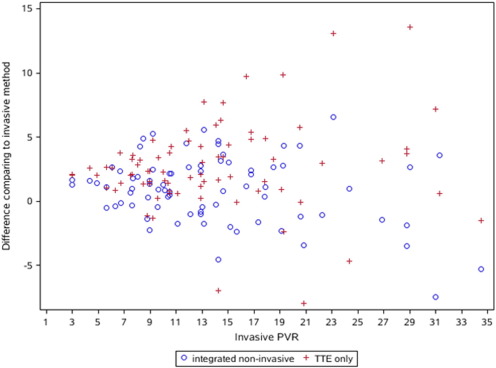
Furthermore, the difference between TTE-calculated PVR and invasive PVR was significantly larger than the difference between integrated non-invasive PVR and invasive PVR (Fig. 2, t = − 4.78, P < 0.001 by the paired t-test analysis). The mean value of the difference between them was − 1.79 ± 3.33 (SD). Based on the formula ABS ((X PVR - invasive PVR)/invasive PVR) for measuring the relative error, sd1 and sd2 were produced by non-invasive PVR and TTE-calculated PVR, respectively. And they all were divided into 4 categories by 0 to 0.1, 0.1 to 0.3, 0.3 to 0.5, and 0.5 to 1 (Table 2), respectively. Statistically significant difference was found between sd1 and sd2 for these categories by McNemar test (χ2 = − 19.371, P = 0.004).
|
|
|
Fig. 2. Scatter of the difference between integrated non-invasive PVR and invasive PVR and the difference between TTE–PVR and invasive PVR against the invasive PVR. The latter was significantly larger than the former (t = − 4.78, P < 0.001) by the paired t-test analysis. |
| Difference comparing to RHC (integrated non-invasive) | Difference comparing to RHC (TTE only) | Total | |||
|---|---|---|---|---|---|
| 0% to 10% | 10% to 30% | 30% to 50% | 50% to 100% | ||
| 0% to 10% | 8(10.39) | 16(20.78) | 3(3.90) | 1(1.30) | 28(36.36) |
| 10% to 30% | 5(6.49) | 15(19.48) | 12(15.58) | 4(5.19) | 36(46.75) |
| 30% to 50% | 0(0.00) | 2(2.60) | 3(3.90) | 4(5.19) | 9(11.69) |
| 50% to 100% | 0(0.00) | 2(2.60) | 1(1.30) | 1(1.30) | 4(5.19) |
| Total | 13(16.88) | 35(45.45) | 19(24.68) | 10(12.99) | 77(100.00) |
For TTE-derived (MPAP–PCWP), the intra-observer and inter-observer reproducibility was high, and the average differences were − 1.13 and − 1.87, respectively. The Pearson correlation coefficient was 0.977 and 0.955, respectively (P < 0.001 for both). For CMR-derived CO, the intra-observer and inter-observer variability were − 0.076 and − 0.186, respectively. The Pearson correlation coefficient was 0.995 and 0.993, respectively (P < 0.001 for both). Both the intra-observer (Supplemental Fig. 7) and inter-observer (Supplemental Fig. 8) have the respectively satisfactory reproducibility for TTE-derived (MPAP–PCWP) by using the Bland–Altman analysis, and the intra-observer (Supplemental Fig. 9) and inter-observer (Supplemental Fig. 10) for CMR-derived CO do the same as it.
4. Discussions
In this research, PVR was calculated directly with the combined use of TTE-derived (MPAP–PCWP) and CMR-derived CO. In comparison to RHC, PVR from this integrated non-invasive method showed the good accuracy and reproducibility in patients with PAH. To our knowledge, it is the first report to determine PVR with the combined use of TTE and CMR, and our findings suggested that this integrated method was clinically feasible for non-invasive calculation of PVR in PAH.
In patients with PAH, PVR is an important prognostic factor and is frequently repeated for serial follow-up. Though RHC is the “gold standard” for the evaluation of PVR, its invasive nature makes it unsuitable for frequent and repeated use [1] ; [27]. In addition, the cost and ionizing radiation also become the important disadvantages of RHC. Therefore, it is urgent to develop the reliable and accurate methods for non-invasive estimation of PVR. In preliminary studies, the significant relationships have been identified between some parameters of TTE/CMR and PVR, and several composite numerical models have been established to evaluate PVR non-invasively [2]; [3]; [4]; [5]; [6]; [7]; [8]; [9]; [10]; [11]; [12] ; [13]. Limited by the intrinsic nature of each image modality, these methods were usually indirect, and the widely accepted method was still unavailable.
Based on the complementary advantages of TTE and CMR, a new method was proposed for the non-invasive measurement of PVR in this study. Since PVR is directly related to trans-pulmonary pressure gradient (MPAP–PCWP) and inversely related to CO, the non-invasive PVR can be achieved by non-invasive estimation of (MPAP–PCWP) and CO, respectively. It has been previously demonstrated that TTE estimated MPAP and PCWP reliably, but its ability to calculate CO was not as accurate and reproductive as CMR [25] ; [26]. In this study, our findings also proved the flaw of 2D-TTE in the measurement of CO. On the contrary, CMR was the best non-invasive modality to measure CO but its ability to determine MPAP/PCWP was limited [13] ; [32]. Therefore, TTE and CMR can complement each other, and the combined use of two image modalities made it possible to determine PVR non-invasively. In this study, our findings confirmed the potential of this integrated method. Furthermore, the attempt was also made to estimate PVR directly with the isolated use of 2D-TTE in this study. In comparison with RHC, however, TTE-calculated PVR was less satisfactory, which disagreed with recent research [33].
Compared with RHC, TTE can estimate trans-pulmonary pressure gradient (MPAP–PCWP) reliably in this study. In the estimation of MPAP, several studies have identified a strong relationship between TTE and RHC [14]; [15] ; [16]. The incremental value of TTE for non-invasive measurement of MPAP has been proven in routine clinical practice [34]. Several TTE methods have been proposed to estimate MPAP, and the simple Chemla' formula (MPAP = 0.61 × SPAP + 2) was adopted in this study [16]. Furthermore, it has previously been demonstrated that PCWP can be estimated reliably with different TTE methods [17]; [18]; [19]; [20]; [21]; [22] ; [23]. In this study, PCWP was determined non-invasively based on the combined use of pulsed and color M-mode Doppler echocardiography [18]. Therefore, it became possible to estimate trans-pulmonary pressure gradient (MPAP–PCWP) non-invasively with TTE, which was confirmed in this study.
In this research, CO was measured non-invasively with volumetric cine CMR and agreed closely with that from RHC in similar physiological status. There were two methods available in CMR-calculated CO (volumetric cine and velocity-encoded cine), and they had excellent agreement [26]. CMR can overcome the limitations of TTE and provides the best non-invasive method for measurements of SV and CO. Previous reports have showed that CMR-derived CO had good accuracy and reproducibility compared with RHC-derived CO [24]; [25] ; [26]. In this study, our findings also showed the reasonable agreement between CMR and RHC, and further corroborated the previous studies.
5. Study limitations
In patients with PAH, both physiologic and imaging factors were contributors to variations in the measurement of PVR. To minimize these influences, the related factors were controlled strictly between RHC/TTE and CMR. In addition, PAH was severe and PVR was high in this study. Therefore, the patients were selected highly in this study. Given the difficulty in the estimation of PAP, the normal controls were absent in this research. CO was measured non-invasively with volumetric cine CMR in this study. Though there was excellent agreement between volumetric and velocity-encoded cine CMR, the latter was used more frequently and might permit inclusion of more patients. In this research, 30% or greater difference was observed in more than 10% of cases, which added more insight to the influence if the integrated method was used in clinical practice. Compared with the benefit of non-invasive procedure, however, the diagnostic value of integrated method might be acceptable. Furthermore, TTE-derived CO was based on conventional 2D-TTE, not 3D-TTE, in this research. Recent reports suggested that 3D-TTE might be more accurate than 2D-TTE in the measurement of SV [35] ; [36]. Therefore, further research was required to evaluate the possibility of non-invasive PVR based on 3D-TTE.
6. Conclusions
In this study, our findings suggest that PVR can be calculated directly using TTE-derived (MPAP–PCWP) and CMR-derived CO. Furthermore, the results from this integrated non-invasive method showed good diagnostic accuracy and repeatability compared with RHC. Therefore, the combined use of TTE and CMR can provide a clinically feasible method for non-invasive measurement of PVR in patients with PAH.
Sources of funding
This work was supported by the Peking Union Medical College Youth Fund (33320140038) and National Natural Science Foundation of China (81341045).
Disclosures
None.
Appendix A. Supplementary data
Supplementary figures
References
- [1] M.M. Hoeper, H.J. Bogaard, R. Condliffe, R. Frantz, D. Khanna, M. Kurzyna, et al.; Definitions and diagnosis of pulmonary hypertension; J. Am. Coll. Cardiol., 62 (2013), pp. D42–D50
- [2] A.E. Abbas, F.D. Fortuin, N.B. Schiller, C.P. Appleton, C.A. Moreno, S.J. Lester; A simple method for noninvasive estimation of pulmonary vascular resistance; J. Am. Coll. Cardiol., 41 (2003), pp. 1021–1027
- [3] F. Scapellato, P.L. Temporelli, E. Eleuteri, U. Corrà, A. Imparato, P. Giannuzzi; Accurate noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with chronic failure heart failure; J. Am. Coll. Cardiol., 37 (2001), pp. 1813–1819
- [4] F. Haddad, R. Zamanian, A.S. Beraud, I. Schnittger, J. Feinstein, T. Peterson, et al.; A novel non-invasive method of estimating pulmonary vascular resistance in patients with pulmonary arterial hypertension; J. Am. Soc. Echocardiogr., 22 (2009), pp. 523–529
- [5] H. Kouzu, S. Nakatani, S. Kyotani, H. Kanzaki, N. Nakanishi, M. Kitakaze; Noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension; Am. J. Cardiol., 103 (2009), pp. 872–876
- [6] S.V. Gurudevan, P.J. Malouf, A.M. Kahn, W.R. Auger, T.J. Waltman, M. Madani, et al.; Noninvasive assessment of pulmonary vascular resistance using Doppler tissue imaging of the tricuspid annulus; J. Am. Soc. Echocardiogr., 20 (2007), pp. 1167–1171
- [7] A.E. Abbas, L.M. Franey, T. Marwick, M.T. Maeder, D.M. Kaye, V. APS, et al.; Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography; J. Am. Soc. Echocardiogr., 26 (2013), pp. 1170–1177
- [8] A.R. Opotowsky, M. Santos, B.A. Maron, J. Afilalo, A.B. Waxman, M.J. Landzberg, et al.; Towards widespread noninvasive assessment of pulmonary vascular resistance in clinical practice; J. Am. Soc. Echocardiogr., 27 (2014), pp. 108–109
- [9] E. Mousseaux, J.P. Tasu, O. Jolivet, G. Simonneau, J. Bittoun, J.C. Gaux; Pulmonary arterial resistance: noninvasive measurement with indexes of pulmonary flow estimated at velocity-encoded MR imaging—preliminary experience; Radiology, 212 (1999), pp. 896–902
- [10] A. García-Alvarez, L. Fernández-Friera, J.G. Mirelis, S. Sawit, A. Nair, J. Kallman, et al.; Non-invasive estimation of pulmonary vascular resistance with cardiac magnetic resonance; Eur. Heart J., 32 (2011), pp. 2438–2445
- [11] A. García-Álvarez, L. Fernández-Friera, J.M. García-Ruiz, M. Nuño-Ayala, D. Pereda, R. Fernández-Jiménez, et al.; Noninvasive monitoring of serial changes in pulmonary vascular resistance and acute vasodilator testing using cardiac magnetic resonance; J. Am. Coll. Cardiol., 62 (2013), pp. 1621–1631
- [12] A. Bell, P. Beerbaum, G. Greil, S. Hegde, A.M. Toschke, T. Schaeffter, et al.; Noninvasive assessment of pulmonary artery flow and resistance by cardiac magnetic resonance in congenital heart diseases with unrestricted left-to-right shunt; JACC Cardiovasc. Imaging, 2 (2009), pp. 1285–1291
- [13] A.J. Swift, S. Rajaram, J. Hurdman, C. Hill, C. Davies, T.W. Sproson, et al.; Noninvasive estimation of PA pressure, flow, and resistance with CMR imaging: derivation and prospective validation study from the ASPIRE registry; JACC Cardiovasc. Imaging, 6 (2013), pp. 1036–1047
- [14] T. Masuyama, K. Kodama, A. Kitabatake, H. Sato, S. Nanto, M. Inoue; Continuous-wave Doppler echocardiographic detection of pulmonary regurgitation and its application to noninvasive estimation of pulmonary artery pressure; Circulation, 74 (1986), pp. 484–492
- [15] K.L. Chan, P.J. Currie, J.B. Seward, D.J. Hagler, D.D. Mair, A.J. Tajik; Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure; J. Am. Coll. Cardiol., 9 (1987), pp. 549–554
- [16] D. Chemla, V. Castelain, M. Humbert, J.L. Hébert, G. Simonneau, Y. Lecarpentier, et al.; New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure; Chest, 126 (2004), pp. 1313–1317
- [17] P. Giannuzzi, A. Imparato, P.L. Temporelli, F. de Vito, P.L. Silva, F. Scapellato, et al.; Doppler-derived mitral deceleration time of early filling as a strong predictor of pulmonary capillary wedge pressure in post infarction patients with left ventricular systolic dysfunction; J. Am. Coll. Cardiol., 23 (1994), pp. 1630–1637
- [18] F. Gonzalez-Vilchez, M. Ares, J. Ayuela, L. Alonso; Combined use of pulsed and color M-mode Doppler echocardiography for the estimation of pulmonary capillary wedge pressure: an empirical approach based on an analytical relation; J. Am. Coll. Cardiol., 34 (1999), pp. 515–523
- [19] H.F. Kuecherer, I.A. Muhiudeen, F.M. Kusumoto, E. Lee, L.E. Moulinier, M.K. Cahalan, et al.; Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow; Circulation, 82 (1990), pp. 1127–1139
- [20] O. Rossvol, L.K. Hatle; Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures; J. Am. Coll. Cardiol., 21 (1993), pp. 1687–1696
- [21] S.F. Nagueh, H.A. Kopelen, W.A. Zoghbi; Feasibility and accuracy of Doppler echocardiography estimation of pulmonary artery occlusive pressure in the intensive care unit; Am. J. Cardiol., 75 (1995), pp. 1256–1262
- [22] R.E. Falicov, L. Resnekov; Relationship of the pulmonary artery end diastolic pressure to the left ventricular end-diastolic and mean filling pressures in patients with and without left ventricular dysfunction; Circulation, 42 (1970), pp. 65–73
- [23] S. Mulvagh, M.A. Quinones, N.S. Kleiman, J. Cheirif, W.A. Zoghbi; Estimation of left ventricular end-diastolic pressure from Doppler transmitral flow velocity in cardiac patients independent of systolic performance; J. Am. Coll. Cardiol., 20 (1992), pp. 112–119
- [24] V. Muthurangu, A. Taylor, R. Andriantsimiavona, S. Hegde, M.E. Miquel, R. Tulloh, et al.; Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase-contrast magnetic resonance flow; Circulation, 110 (2004), pp. 826–834
- [25] H.Y. Lin, D. Freed, T.W. Lee, R.C. Arora, A. Ali, W. Almoustadi, et al.; Quantitative assessment of cardiac output and left ventricular function by noninvasive phase-contrast and cine MRI: validation study with invasive pressure–volume loop analysis in a swine model; J. Magn. Reson. Imaging, 34 (2011), pp. 203–210
- [26] P. Omvik; Magnetic resonance imaging and cine computerized tomography as future tools for cardiac output measurement; Eur. Heart J., 11 (Suppl. I) (1990), pp. 141–143
- [27] V.V. McLaughlin, S.L. Archer, D.B. Badesch, R.J. Barst, H.W. Farber, J.R. Lindner, et al.; ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association; J. Am. Coll. Cardiol., 53 (2009), pp. 1573–1619
- [28] C. Yan, S. Zhao, S. Jiang, Z. Xu, L. Huang, H. Zheng, et al.; Transcatheter closure of patent ductus arteriosus with severe pulmonary arterial hypertension in adults; Heart, 93 (2007), pp. 514–518
- [29] M.A. Quiñones, C.M. Otto, M. Stoddard, A. Waggoner, W.A. Zoghbi; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography; J. Am. Soc. Echocardiogr., 15 (2002), pp. 167–184
- [30] Y. Chaowu, Z. Shihua, L. Jian, L. Li, F. Wei; Cardiovascular magnetic resonance characteristics in children with hypertrophic cardiomyopathy; Circ. Heart Fail., 6 (2013), pp. 1013–1020
- [31] L. Wang, Y. Zhang, C. Yan, J. He, C. Xiong, S. Zhao, et al.; Evaluation of right ventricular volume and ejection fraction by gated (18)F-FDG PET in patients with pulmonary hypertension: comparison with cardiac MRI and CT; J. Nucl. Cardiol., 20 (2013), pp. 242–252
- [32] G. Reiter, U. Reiter, G. Kovacs, B. Kainz, K. Schmidt, R. Maier, et al.; Magnetic resonance-derived 3-dimensional blood flow patterns in the main pulmonary artery as a marker of pulmonary hypertension and a measure of elevated mean pulmonary arterial pressure; Circ Cardiovasc. Imaging, 1 (2008), pp. 23–30
- [33] P1. Lindqvist, S. Söderberg, M.C. Gonzalez, E. Tossavainen, M.Y. Henein; Echocardiography based estimation of pulmonary vascular resistance in patients with pulmonary hypertension: a simultaneous Doppler echocardiography and cardiac catheterization study; Eur. J. Echocardiogr., 12 (2011), pp. 961–966
- [34] B. Ristow, N.B. Schiller; Stepping away from ritual right heart catheterization into the era of noninvasively measured pulmonary artery pressure; J. Am. Soc. Echocardiogr., 22 (2009), pp. 820–822
- [35] C. Jenkins, K. Bricknell, L. Hanekom, T.H. Marwick; Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography; J. Am. Coll. Cardiol., 44 (2004), pp. 878–886
- [36] C. Jenkins, J. Chan, L. Hanekom, T.H. Marwick; Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters; J. Am. Soc. Echocardiogr., 19 (2006), pp. 1119–1128
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?