Abstract
Recently, the light-absorbing organic carbon, i.e., brown carbon (BrC), has received an increasing attention, because they could significantly absorb the solar radiation in the range of short wavelengths rather than the purely scattering effect. BrC is ubiquitous in the troposphere. It could undergo long range transport within the atmospheric circulation. After the deposition on the surface of snow or ice in the cryospheric region, as the major light absorbing impurities with black carbon and dust, BrC could reduce the snow albedo and accelerate the glacier melting. In this context, this paper summarized the current knowledge of BrC (in aerosols and snow) in the cryospheric regions including the Arctic, Antarctic, and Alpines. Although some works have been conducted in those region, the current dataset on the optical properties of BrC like Absorption Ångström Exponent (AAE ) and Mass Absorption Efficiency (MAE ) is still limited, which hampers stimulating an accurate evaluation of its climate effects. Especially in the Himalayas and Tibetan Plateau, where very limited information concerning BrC is available. Considering biomass burning as a dominant source of BrC, a large amount of emissions from biomass burning in South Asia could reach the Himalayas and Tibetan Plateau, where the climate effect of BrC merits more investigation in the future.
Keywords
Brown carbon ; Black carbon ; Atmospheric aerosol ; Snow ; Glacier
1. Introduction
Carbonaceous components in the atmospheric aerosols play an important role in the climate system, mainly due to their solar absorption and scattering properties (Seinfeld and Pandis, 2012 ). It is well established that black carbon (BC) could strongly absorb the solar radiation in visible bands (Ramanathan and Carmichael, 2008 ; Xu et al., 2009 ), resulting in a direct radiative forcing ranging from 0.17 to 1.48 W m−2 (Bond et al., 2013 ). In contrast to BC, organic carbon in the atmospheric aerosols is traditionally considered to be purely scattering by climate models. However, recent research demonstrated that substantial fraction of organic carbon in aerosols could absorb the light in the range of short wavelengths. Such kinds of organic substances with wavelength-dependent absorption are defined as brown carbon (BrC) (Andreae and Gelencser, 2006 ). BrC has multiple primary sources such as biomass burning (Saleh et al., 2014 ; Washenfelder et al., 2015 ), fossil fuel (e.g., coal) combustion (Bond, 2001 ; Yang et al., 2009 ), biogenic aerosols (e.g., plant debris and fungi) and soil humic matters. Meanwhile BrC could also be secondarily formed from anthropogenic or biogenic precursors (Lack et al., 2013 ; Zhang et al., 2011 ). Such precursors like isoprene (Limbeck et al., 2003 ) and, lignin pyrolysis products (Gelencser et al., 2003 ; Hoffer et al., 2004 ) could yield BrC through heterogeneous or multiphase reactions in the presence of sulfuric acid or hydroxyl radicals. Although the source of BrC may varied with different locations and environment (e.g., urban, rural, forest, ocean, mountain), globally, biomass burning was identified as the most important source of BrC (Chung et al., 2012 ; Lack et al., 2012 ; Laskin et al., 2015 ).
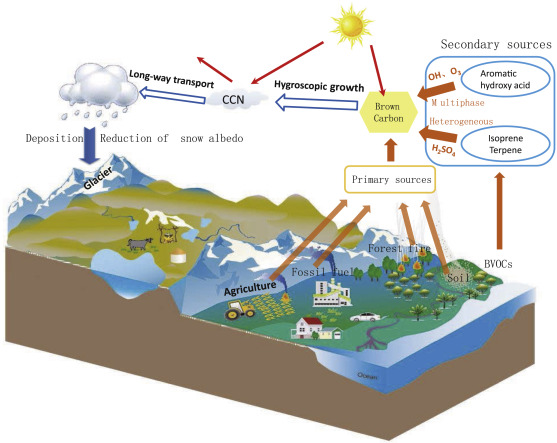
BC, BrC, and mineral dust are the dominant light-absorbing substances in atmospheric aerosols. After deposited on the surface of snow and glaciers through dry/wet deposition processes, those light-absorbing substances could efficiently reduce the snow albedo (surface darkening), decrease the upwelling radiation, thereby accelerating snow melting (Flanner et al., 2007 ; Kaspari et al., 2015 ; Yang et al., 2015 ). A brief illustration of the sources, transport and deposition of BrC, as well its effects on the climate system is presented in Fig. 1 .
|
|
|
Fig. 1. Schematic overview on the sources, transport, and radiative forcing of BrC, as well as its impact on the snow and glaciers after deposition. |
The cryosphere, comprising snow, river and lake ice, sea ice, glaciers, ice shelves and ice sheets, and frozen ground, is crucial in the Earths climate system (IPCC, 2014 ). The cryosphere is very sensitive to climate changes and anthropogenic activities. Currently, most efforts dedicated to the light-absorbing substances were focused on BC and dust, which were well documented in recent review literatures (Bond et al., 2013 ; Gertler et al., 2016 ; Wang et al., 2014 ). However, in this review, the state of art on the BrC in the cryosphere will be specifically summarized. Section 2 introduces the basic optical parameters of BrC, as well the current dataset available for the occurrence of BrC in aerosol and snow in the Artic, Antarctic, and mountain glaciers. Section 3 presents the current knowledge about the chemical speciation of BrC. Section 4 describes the radiative forcing of BrC in the atmosphere and snow, i.e., its impact on the cryosphere. Finally, research perspectives for the future research are proposed in Section 5 .
2. Optical properties of BrC
The basic theory used to describe light absorption properties of BrC is the well-known power-law relationship. It can be defined as the following equation:
|
|
( 1) |
where Absλ (unit: Mm−1 or M−1 ) refers to the absorption coefficient of BrC at wavelength λ (unit: nm). K is a constant. AAE indicates the wavelength dependence of light absorption. It could be derived by fitting the power-law relationship Eq. (1) to the scanned spectra or calculated for a given range based on the following equation (Hoffer et al., 2006 ):
|
|
( 2) |
where Aλ is the absorbance of BrC at wavelength λ. It can be directly measured from the spectrophotometer as the attenuation of incident light intensity.
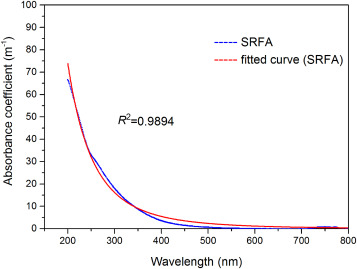
In the cryosphere, McNaughton et al. (2011) found that AAE of BrC in the western Arctic aerosols vary between 1.5 and 3, while Corr et al. (2012) reported much larger AAE of BrC (3–7) in the same location. The AAE of BrC is larger than that of BC (typically unity), which means the light absorptivity of BrC will increase more dramatically as the wavelength become shorter (an example is provided in Fig. 2 ). Furthermore, the magnitude of AAE is closely relevant to the particle size and composition ( Bahadur et al., 2012 ; Moosmueller et al., 2011 ), implying that the difference in AAE of BrC may be due to the various sources.
|
|
|
Fig. 2. The absorption spectra of a typical BrC compound (Suwannee River Fulvic Acid (SRFA) from International Humic Substance Society) measured with a Hitachi U-3900H. |
In addition to AAE , another crucial parameter for the definition of BrCs light absorption property is MAE (unit: m2 g−1 ), which demonstrate the light absorptivity for a specific mass unit of BrC. It could be derived from the following equation:
|
|
( 3) |
where C is the concentration of BrC. The MAE of BrC decreases sharply with an increasing wavelength, which means it has much stronger absorption in the shorter wavelength ( Alexander et al., 2008 ; Andreae and Gelencser, 2006 ). To our best knowledge, there are very limited studies available on the MAE of BrC in the cryosphere. The MAE and AAE of BrC reported or used in the published literatures are listed in Table 1 . McNaughton et al. (2011) reported that MAE of BrC in the western Arctic aerosols is (0.83 ± 0.5) m2 g−1 at 470 nm and (0.27 ± 0.08) m2 g−1 at 530 nm, which are comparable to the MAE of BrC in some rural or even urban aerosols, for instance, (0.7 ± 0.2) m2 g−1 at 365 nm in Gosan, Korea (Kirillova et al., 2014 ) and 0.60 m2 g−1 at 365 nm in DeKalb, USA (Hecobian et al., 2010 ). While the MAE of BC and dust in snow across Northern China were 6.3 m2 g−1 and 0.9 m2 g−1 , respectively (Wang et al., 2013 ). Similarly, the MAE of BC in Arctic snow was 6.0 m2 g−1 (Hegg et al., 2010 ). Although the MAE of BrC is lower than that of BC, the global burden of BrC aerosols was simulated to be 0.65 mg m−2 , nearly 3-fold of the BC concentration (0.19 mg m−2 ) in the atmosphere (Feng et al., 2013 ). Given the large abundance of BrC in the environment, a contribution of light absorption by BrC may not be ignored.
| Site | Sampling period | Sample type | MAE (W)a (m2 g−1 ) | AAE | Reference |
|---|---|---|---|---|---|
| Western Arctic | 31 March–19 April, 2008 | Aerosol | 0.83 ± 0.15 (470 nm); 0.27 ± 0.08 (530 nm) | 1.5–3 | McNaughton et al., 2011 |
| Western Arctic | Spring and summer of 2008 | Aerosol | 3–7 | Corr et al., 2012 | |
| Artic | 1998 and 2005–2009 | Snow | 5 | Doherty et al., 2010 | |
| Northern China | January and February of 2010 | Snow | 0.3 (550 nm) | 6 | Wang et al., 2013 |
| North America | 10 January and 28 January–21 March, 2013 | Snow | 5 | Doherty et al., 2014 |
a. W refers to the wavelength at which the MAE (mass absorption efficiency) is calculated; AAE (Absorption Ångström Exponent).
3. Chemical properties of BrC
The chemical composition of BrC is extremely complex. Nevertheless, in terms of chemical speciation, BrC could be generally divided into humic-like substances (HULIS) and tar ball (Chakrabarty et al., 2010 ; Cheng et al., 2016 ; Hoffer et al., 2016 ).
3.1. HULIS
HULIS are termed as their similarity with terrestrial/aquatic humic substances. They are mixtures of various macromolecular organic compounds and ubiquitous in atmospheric environment (Graber and Rudich, 2006 ; Lin et al., 2010a ; Mukai and Ambe, 1986 ; Zheng et al., 2013 ). HULIS mainly consist of aromatic and aliphatic structure bond with hydrocarbon side chains like hydroxyl, carbonyl, carboxyl and organosulfate functional groups (Chakrabarty et al., 2010 ; Graber and Rudich, 2006 ; Mukai and Ambe, 1986 ).
3.1.1. Experimental methods for extraction and determination of HULIS
Given the unclear physical-chemical characteristics of HULIS, there is no uniform extract method applicable in all conditions. It is even hard to evaluate whether an extract method valid to isolate HULIS from atmospheric media (Graber and Rudich, 2006 ). Table 2 summarizes the isolation and quantification method applied in published literatures. It is obvious that the solid phase extraction (SPE) method is the most frequently employed in isolating HULIS from aerosols and snow in cryospheric regions for its simple operation and high extract efficiency (Facchini et al., 2000 ; Fan et al., 2012 ). Specifically, there are four different SPE methods, owing to the diverse sorbents: ENVI-18 (Fan et al., 2012 ), HLB (Varga et al., 2001 ), XAD (Duarte and Duarte, 2005 ) and DEAE (diethylaminoethyl) (Baduel et al., 2009 ). They are competent to the exaction of HULIS with low limit of detection and high recovery yield. However, they also have their particular preference and disadvantage in the isolation of HULIS (Fan et al., 2012 ; Fan et al., 2013 ). HLB method favors polar organic matters with high recovery. It also can preserve the nature of samples while not change the properties of isolated HULIS. XAD tends to isolate hydrophobic and aromatic components due to its sorbent (non-ionic macro porous resin) used. Acidizing of solute is not necessary for DEAE and it prefers the compounds containing more aromatic signatures. However, large amount of salts could present in the eluents with DEAE extraction, which will interfere the characterization of HULIS.
| Site | Sample type | Isolat. Meth. | Quantif. Meth. | HULIS concentration (μgC L−1 ) | Reference | ||
|---|---|---|---|---|---|---|---|
| Min | Max | Average | |||||
| Station Nord, Arctic | Aerosol (PM10 ) | HLB | TOC | 0.02a | Nguyen et al., 2014 | ||
| Barrow, Alaska, Arctic | Snowpack | DEAE | TOC | 1 | 16 | Voisin et al., 2012 | |
| Alaska, Arctic | Snowpack | UV–Vis | 1200b | 1500b | France et al., 2012 | ||
| Col du DÔme, Alpine | Ice core | UV–Vis | 50 | 400 | Legrand et al., 2007 | ||
| Col du DÔme, Alpine | Ice core | DEAE | TOC | 4 | 250 | Guilhermet et al., 2013 | |
| Antarctic | Snow, coast | XAD-8 | Gravimetry | 16c | 397c | Calace et al., 2005 | |
| Antarctic | Snow, inland | XAD-8 | Gravimetry | 25c | 146c | Calace et al., 2005 | |
a. in the unit of μgC m−3 .
b. the concentration of non-BC light-absorbers in snowpack.
c. in the unit of μg L−1 .
For the quantification of HULIS followed by its isolation, there are total organic carbon (TOC) measurement (Kiss et al., 2002 ; Krivacsy et al., 2008 ; Salma et al., 2007 ), UV–Vis detection (Krivacsy et al., 2008 ; Samburova et al., 2005 ), evaporative light-scattering detection (Emmenegger et al., 2007 ; Lin et al., 2010b ) and gravimetric method (Kiss et al., 2002 ). TOC method is an easy and common approach to analyze the amount of HULIS-C. Hoffer et al. (2006) assumed the conversion factor is 1.90 between HULIS and HULIS-C for the aerosol samples collected in the Amazon basin, while 1.81 was used by Salma et al. (2007) for the quantity of HULIS at Budapest, Hungary. The choosing of proper conversion factor is critical during such calculation, which may arise large uncertainties.
3.1.2. Abundance of HULIS in aerosols and snow
We focus on the concentration of atmospheric HULIS at the regions where there are a wide range of distributions of glacier and ice sheets (i.e., Arctic, Alps). Considering the albedo modification, glaciers are very sensitive to the increase in light-absorbing impurities like HULIS. Meanwhile, the analysis of the HULIS trapped and accumulated in polar ice sheets and mid-latitude mountain glaciers provides a unique chance to reconstruct the changing atmospheric composition in the past (Legrand et al., 2013 ).
Nguyen et al. (2014) found the annual mass concentration of HULIS in Arctic aerosols is (0.02 ± 0.01) μg m−3 and the concentration of HULIS showed an obvious seasonal variation with the highest value occurred in biomass burning influenced periods. Guilhermet et al. (2013) estimated the concentration of water-soluble atmospheric HULIS was 0.07 μg m−3 in summer, basing on the analysis of HULIS trapped in the ice core at Col du Dôme (4250 m a.s.l.), Alpine.
Legrand et al. (2007) determined HULIS in an Alpine ice core by measuring the absorbance with a UV–VIS spectrophotometer at the wavelengths of 250 and 350 nm. They found that summertime mass concentrations of HULIS were generally below 150 × 10−9 before 1945, while the contributions tended to increase after World War II (ranging from 200 × 10−9 to 900 × 10−9 ). The increased HULIS in Alpine glaciers could be attributed to the growing emission of anthropogenic precursors as well as the enhanced atmospheric oxidants (Legrand et al., 2007 ).
Using a DEAE resin, Legrand et al. (2013) extracted water-soluble HULIS in Antarctic snow samples, and then quantified it with a Shimadzu TOC analyzer. Water-soluble HULIS in central Antarctica snow was determined to be 2 × 10−9 (annual average), much lower than those in Alpine snow of 30 × 10−9 . In Arctic (Barrow, Alaska) snowpack, Beine et al. (2011) estimated that HULIS and other unknown chromophores could account for nearly half of the total absorption measured between 300 and 500 nm. Voisin et al. (2012) further calculated the MAE of HULIS in the snow from the same region as (26 ± 11) cm2 mg−1 at 250 nm. And AAE varied from 4.5 (between 300 and 350 nm) to 7.7 (between 450 and 500 nm). The high values of AAE were the typical characteristics of BrC ( Andreae and Gelencser, 2006 ), i.e., the HULIS in snow has strong absorption in the short wavelengths.
3.2. Tar balls
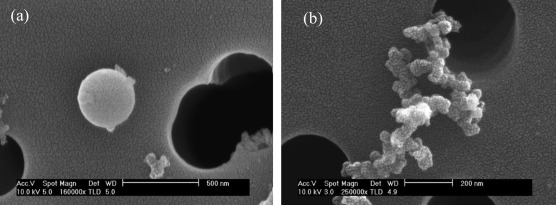
Tar ball, emitted from biomass burning (especially in smoldering condition), is an important type of BrC (Chakrabarty et al., 2010 ; Hoffer et al., 2016 ). They are spherical (30–500 nm in diameter), amorphous, and typically not aggregated with other particles (Hand et al., 2005 ; Posfai et al., 2004 ), which are distinct from soot in terms of morphology (i.e., BC) (Fig. 3 ). According to Alexander et al. (2008) , the light absorption of tar ball (carbon sphere) in East Asian outflow may be comparable to or even larger than soot at the individual particle level. The derived mean MAE of tar balls range from ∼3.6 m2 g−1 to 4.1 m2 g−1 at 550 nm, approximating to that of BC (Alexander et al., 2008 ). For the laboratory-generated tar balls, their MAE values were determined to be 0.8–3.0 m2 g−1 (λ = 550 nm), with average AAE of 2.9 in the wavelength band of 467–652 nm ( Hoffer et al., 2016 ).
|
|
|
Fig. 3. Secondary electron images of (a) tar ball and (b) soot in the Himalayan aerosols (6500 m a.s.l.) observed by scanning electron microscopy (Cong et al., 2010 ). |
Tar ball is ubiquitous in the troposphere, especially in the regions receiving strong influence of biomass burning. According to our previous study in the Himalayas and Tibetan Plateau aerosols, tar balls accounted for 3%–27% of the particle number concentrations (Cong et al., 2009 ). Given the abundance and their optical properties, tar balls impart considerable effects in radiative forcing. However, few studies on the tar balls have been carried out in the snow surface and ice core. At the same time, the degree to which tar balls contribute to the solar absorption of BrC, especially relative to HULIS, is still unclear. Their hygroscopic properties, mixing states and aging processes urgently require more attentions to achieve accurate modeling of the radiative forcing.
4. Radiative forcing of BrC in the atmosphere and snow
Globally, the radiative forcing by BrC was estimated to be in the range of 0.1–0.25 W m−2 , close to 25% of that by BC (Feng et al., 2013 ). Chung et al. (2012) also highlighted the global significance of BrC. They reported that it could be responsible for 20% of carbonaceous aerosol solar absorption at 550 nm. Moreover, Lin et al. (2014) calculated the global absorption of BrC in the atmosphere ranging from +0.22 W m−2 to + 0.57 W m−2 , which is equal to 27%–70% of the BC absorption. Nevertheless, regarding the cryospheric area, such assessment in the regional scale is still absent.
BrC in the atmosphere could be transported to the surface of snow by dry or wet deposition, which will further lead to the enhancement of snow melting. For the radiative forcing of BrC in snow, most current studies focus on their contribution to the total light absorption relative to BC and dust, rather than providing an absolute value (in the unit of W m−2 ). Dang and Hegg (2014) proposed that HULIS and polar organic carbon from the western North American snow samples account for 13% of the total light absorption. While for the Arctic snow samples, Doherty et al. (2010) proportioned about 40% of the visible and ultraviolet absorption to BrC. However, it should be kept in mind that this approach must base on a given AAE values of BrC, which might be variable with different sources and environment. Apart from these regional analysis, Lin et al. (2014) applied the IMPACT (Integrated Massively Parallel Atmospheric Chemical Transport) model to assess the global radiative forcing of organic aerosols deposited over in-land snow with the range of 1.1 × 10−3 W m−2 and 3.1 × 10−3 W m−2 . These values could account for as large as 24% of radiative forcing by BC. However, they can not separate the radiative forcing of BrC from total organics yet.
Moreover, BrC, BC and dust as the major light-absorbing matters in the cryosphere, they tend to mix with each other during the transport or aging process (Kaspari et al., 2014 ; Lack and Cappa, 2010 ; Liu et al., 2015 ), leading to another form of radiative forcing by acting as a lens, i.e., lensing effect. Lack et al. (2012) found that the absorption enhancement ascribes to internal mixing can be 1.4 times at 532 nm in biomass burning particles. The enhancement strongly dependent on the coating thickness, mixing state (Liu et al., 2015 ). Similar with BrC, dust also might mixed with BC to change its albedo reduction in snow (Kaspari et al., 2014 ). Thus BrC in the cryosphere not only absorbs solar radiation directly but also can leads to an extra radiative forcing by interaction with BC. If the lensing effect was incorporated into the modeling, the current results on the radiative forcing of BrC may be modified considerably.
5. Perspective
As discussed above, unlike BC, the chemical and optical properties of BrC remains poorly understood especially in the cryospheric regions. The measurements of BrC still suffer from some problems. The light absorption characteristics of BrC were commonly determined in the water extracts. However, the remaining fraction of BrC (water insoluble) is usually ignored. In particular, the water-insoluble BrC may have higher mass-specific absorption than the water-soluble fraction. Another problem is that BrC were extracted by different chemical solvents like acetone and methanol besides water in different studies. Therefore, their abundances obtained by different pretreatment and determination methods are difficult to compare with each other. For many researches, BrC is defined as the light-absorbing carbon measured by optical instruments such as Aethalometer. The relationship between the specific chemical composition of BrC and optical properties also needs more research. Another uncertainty is associated with the determination of MAE of BrC in the mixing state. In order to derive reliable result from this method, more precise optical knowledge of BrC is needed. The hydroscopic properties of BrC need more attention, since it determines the role as cloud condensation nuclei for the formation of clouds.
Discrepancy between observations and model results in a substantial uncertainties in quantifying the effect of BrC. Accurate model simulation of BrC depends on our comprehensive knowledge regarding its sources, chemical composition, optical properties, and so on. More BrC measurements are needed not only in laboratory characterization, but also in field campaigns being carried out in surface air, total atmospheric column and snow. The mixing state, aging and transformation during wet scavenging and, dry deposition on the glacier surface are also important issues.
In terms of geographic distribution of research site, although there are several works have been conducted in Alpine, Arctic and Antarctic, the knowledge about the spatial distributions of BrC is still in deficit especially in the Himalayas and Tibetan Plateau. High levels of carbonaceous aerosol from biomass/biofuel burning exist over South Asia (Bonasoni et al., 2010 ; Gustafsson et al., 2009 ; Luthi et al., 2015 ). Increasing evidences have demonstrated that those air pollutants could reach the high altitude of Himalayas and even transport into the inland of the Tibetan Plateau (Cong et al., 2015 ). Whats more, the strong solar radiation on the Himalayas and Tibetan Plateau may also accelerates the formation of BrC through the photochemical reactions. With the vast snow and ice cover (more than 100,000 km2 (Yao et al., 2012 )), the Himalayas and Tibetan Plateau have been proved to be very sensitive to climate change. Since biomass burning and secondary formation are the dominant sources of BrC, climate effects of BrC in the Himalayas and Tibetan Plateau (not only the atmospheric warming but also the reduction of snow albedo) merit more in-depth investigation.
Acknowledgments
This work is supported by National Science Foundation of China (41522103 , 41501082 and 41225002 ) and Strategic Priority Research Program-Climate Change: Carbon Budget and Relevant Issues (XDA05100105 ).
References
- Alexander et al., 2008 D.T.L. Alexander, P.A. Crozier, J.R. Anderson; Brown carbon spheres in East Asian outflow and their optical properties; Science, 321 (2008), pp. 833–836
- Andreae and Gelencser, 2006 M.O. Andreae, A. Gelencser; Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols; Atmos. Chem. Phys., 6 (2006), pp. 3131–3148
- Baduel et al., 2009 C. Baduel, D. Voisin, J.L. Jaffrezo; Comparison of analytical methods for humic like substances (HULIS) measurements in atmospheric particles; Atmos. Chem. Phys., 9 (2009), pp. 5949–5962
- Bahadur et al., 2012 R. Bahadur, P.S. Praveen, Y. Xu, et al.; Solar absorption by elemental and brown carbon determined from spectral observations; Proc. Natl. Acad. Sci. U. S. A., 109 (2012), pp. 17366–17371
- Beine et al., 2011 H. Beine, C. Anastasio, G. Esposito, et al.; Soluble, light-absorbing species in snow at Barrow, Alaska; J. Geophys. Res. Atmos., 116 (2011)
- Bonasoni et al., 2010 P. Bonasoni, P. Laj, A. Marinoni, et al.; Atmospheric brown clouds in the Himalayas: first two years of continuous observations at the Nepal climate observatory-pyramid (5079 m); Atmos. Chem. Phys., 10 (2010), pp. 7515–7531
- Bond, 2001 T.C. Bond; Spectral dependence of visible light absorption by carbonaceous particles emitted from coal combustion; Geophys. Res. Lett., 28 (2001), pp. 4075–4078
- Bond et al., 2013 T.C. Bond, S.J. Doherty, D.W. Fahey, et al.; Bounding the role of black carbon in the climate system: a scientific assessment; J. Geophys. Res. Atmos., 118 (2013), pp. 5380–5552
- Calace et al., 2005 N. Calace, E. Cantafora, S. Mirante, et al.; Transport and modification of humic substances present in Antarctic snow and ancient ice; J. Environ. Monit., 7 (2005), pp. 1320–1325
- Chakrabarty et al., 2010 R.K. Chakrabarty, H. Moosmueller, L.W.A. Chen, et al.; Brown carbon in tar balls from smoldering biomass combustion; Atmos. Chem. Phys., 10 (2010), pp. 6363–6370
- Cheng et al., 2016 Y. Cheng, K.-b. He, Z.-y. Du, et al.; The characteristics of brown carbon aerosol during winter in Beijing; Atmos. Environ., 127 (2016), pp. 355–364
- Chung et al., 2012 C.E. Chung, V. Ramanathan, D. Decremer; Observationally constrained estimates of carbonaceous aerosol radiative forcing; Proc. Natl. Acad. Sci. U. S. A., 109 (2012), pp. 11624–11629
- Cong et al., 2009 Z. Cong, S. Kang, S. Dong, et al.; Individual particle analysis of atmospheric aerosols at Nam Co, Tibetan Plateau; Aerosol Air Qual. Res., 9 (2009), pp. 323–331
- Cong et al., 2010 Z. Cong, S. Kang, S. Dong, et al.; Elemental and individual particle analysis of atmospheric aerosols from high Himalayas; Environ. Monit. Assess., 160 (2010), pp. 323–335
- Cong et al., 2015 Z. Cong, K. Kawamura, S. Kang, et al.; Penetration of biomass-burning emissions from South Asia through the Himalayas: new insights from atmospheric organic acids; Sci. Rep., 5 (2015)
- Corr et al., 2012 C.A. Corr, S.R. Hall, K. Ullmann, et al.; Spectral absorption of biomass burning aerosol determined from retrieved single scattering albedo during ARCTAS; Atmos. Chem. Phys., 12 (2012), pp. 10505–10518
- Dang and Hegg, 2014 C. Dang, D.A. Hegg; Quantifying light absorption by organic carbon in Western North American snow by serial chemical extractions; J. Geophys. Res. Atmos., 119 (2014)
- Doherty et al., 2010 S.J. Doherty, S.G. Warren, T.C. Grenfell, et al.; Light-absorbing impurities in Arctic snow; Atmos. Chem. Phys., 10 (2010), pp. 11647–11680
- Doherty et al., 2014 S.J. Doherty, C. Dang, D.A. Hegg, et al.; Black carbon and other light-absorbing particles in snow of central North America; J. Geophys. Res. Atmos., 119 (2014), pp. 12807–12831
- Duarte and Duarte, 2005 R. Duarte, A.C. Duarte; Application of non-ionic solid sorbents (XAD resins) for the isolation and fractionation of water-soluble organic compounds from atmospheric aerosols; J. Atmos. Chem., 51 (2005), pp. 79–93
- Emmenegger et al., 2007 C. Emmenegger, A. Reinhardt, C. Hueglin, et al.; Evaporative light scattering: a novel detection method for the quantitative analysis of humic-like substances in aerosols; Environ. Sci. Technol., 41 (2007), pp. 2473–2478
- Facchini et al., 2000 M.C. Facchini, S. Decesari, M. Mircea, et al.; Surface tension of atmospheric wet aerosol and cloud/fog droplets in relation to their organic carbon content and chemical composition; Atmos. Environ., 34 (2000), pp. 4853–4857
- Fan et al., 2012 X. Fan, J. Song, P.A. Peng; Comparison of isolation and quantification methods to measure humic-like substances (HULIS) in atmospheric particles; Atmos. Environ., 60 (2012), pp. 366–374
- Fan et al., 2013 X. Fan, J. Song, P.A. Peng; Comparative study for separation of atmospheric humic-like substance (HULIS) by ENVI-18, HLB, XAD-8 and DEAE sorbents: elemental composition, FT-IR, H-1 NMR and off-line thermochemolysis with tetramethylammonium hydroxide (TMAH); Chemosphere, 93 (2013), pp. 1710–1719
- Feng et al., 2013 Y. Feng, V. Ramanathan, V. Kotamarthi; Brown carbon: a significant atmospheric absorber of solar radiation?; Atmos. Chem. Phys., 13 (2013), pp. 8607–8621
- Flanner et al., 2007 M.G. Flanner, C.S. Zender, J.T. Randerson, et al.; Present-day climate forcing and response from black carbon in snow; J. Geophys. Res. Atmos., 112 (2007) http://dx.doi.org/10.1029/2006JD008003
- France et al., 2012 J.L. France, H.J. Reay, M.D. King, et al.; Hydroxyl radical and NOx production rates, black carbon concentrations and light-absorbing impurities in snow from field measurements of light penetration and nadir reflectivity of onshore and offshore coastal Alaskan snow; J. Geophys. Res. Atmos., 117 (2012)
- Gelencser et al., 2003 A. Gelencser, A. Hoffer, G. Kiss, et al.; In-situ formation of light-absorbing organic matter in cloud water; J. Atmos. Chem., 45 (2003), pp. 25–33
- Gertler et al., 2016 C.G. Gertler, S.P. Puppala, A. Panday, et al.; Black carbon and the Himalayan cryosphere: a review; Atmos. Environ., 125 (Part B) (2016), pp. 404–417
- Graber and Rudich, 2006 E.R. Graber, Y. Rudich; Atmospheric HULIS: how humic-like are they? A comprehensive and critical review; Atmos. Chem. Phys., 6 (2006), pp. 729–753
- Guilhermet et al., 2013 J. Guilhermet, S. Preunkert, D. Voisin, et al.; Major 20th century changes of water-soluble humic-like substances (HULISWS) aerosol over Europe inferred from Alpine ice cores; J. Geophys. Res. Atmos., 118 (2013), pp. 3869–3878
- Gustafsson et al., 2009 O. Gustafsson, M. Krusa, Z. Zencak, et al.; Brown clouds over South Asia: biomass or fossil fuel combustion?; Science, 323 (2009), pp. 495–498
- Hand et al., 2005 J.L. Hand, W. Malm, A. Laskin, et al.; Optical, physical, and chemical properties of tar balls observed during the Yosemite Aerosol Characterization Study; J. Geophys. Res. Atmos., 110 (2005)
- Hecobian et al., 2010 A. Hecobian, X. Zhang, M. Zheng, et al.; Water-soluble organic aerosol material and the light-absorption characteristics of aqueous extracts measured over the Southeastern United States; Atmos. Chem. Phys., 10 (2010), pp. 5965–5977
- Hegg et al., 2010 D.A. Hegg, S.G. Warren, T.C. Grenfell, et al.; Sources of light-absorbing aerosol in arctic snow and their seasonal variation; Atmos. Chem. Phys., 10 (2010), pp. 10923–10938
- Hoffer et al., 2004 A. Hoffer, G. Kiss, M. Blazso, et al.; Chemical characterization of humic-like substances (HULIS) formed from a lignin-type precursor in model cloud water; Geophys. Res. Lett., 31 (2004)
- Hoffer et al., 2006 A. Hoffer, A. Gelencser, P. Guyon, et al.; Optical properties of humic-like substances (HULIS) in biomass-burning aerosols; Atmos. Chem. Phys., 6 (2006), pp. 3563–3570
- Hoffer et al., 2016 A. Hoffer, A. Tóth, I. Nyirő-Kósa, et al.; Light absorption properties of laboratory-generated tar ball particles; Atmos. Chem. Phys., 16 (2016), pp. 239–246
- IPCC, 2014 IPCC; Climate Change 2013: the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press, Cambridge (2014)
- Kaspari et al., 2014 S. Kaspari, T.H. Painter, M. Gysel, et al.; Seasonal and elevational variations of black carbon and dust in snow and ice in the Solu-Khumbu, Nepal and estimated radiative forcings; Atmos. Chem. Phys., 14 (2014), pp. 8089–8103
- Kaspari et al., 2015 S. Kaspari, S.M. Skiles, I. Delaney, et al.; Accelerated glacier melt on Snow Dome, Mount Olympus, Washington, USA, due to deposition of black carbon and mineral dust from wildfire; J. Geophys. Res. Atmos., 120 (2015), pp. 2793–2807
- Kirillova et al., 2014 E.N. Kirillova, A. Andersson, J. Han, et al.; Sources and light absorption of water-soluble organic carbon aerosols in the outflow from northern China; Atmos. Chem. Phys., 14 (2014), pp. 1413–1422
- Kiss et al., 2002 G. Kiss, B. Varga, I. Galambos, et al.; Characterization of water-soluble organic matter isolated from atmospheric fine aerosol; J. Geophys. Res. Atmos., 107 (2002)
- Krivacsy et al., 2008 Z. Krivacsy, G. Kiss, D. Ceburnis, et al.; Study of water-soluble atmospheric humic matter in urban and marine environments; Atmos. Res., 87 (2008), pp. 1–12
- Lack and Cappa, 2010 D.A. Lack, C.D. Cappa; Impact of brown and clear carbon on light absorption enhancement, single scatter albedo and absorption wavelength dependence of black carbon; Atmos. Chem. Phys., 10 (2010), pp. 4207–4220
- Lack et al., 2012 D.A. Lack, J.M. Langridge, R. Bahreini, et al.; Brown carbon and internal mixing in biomass burning particles; Proc. Natl. Acad. Sci. U. S. A., 109 (2012), pp. 14802–14807
- Lack et al., 2013 D.A. Lack, R. Bahreini, J.M. Langridge, et al.; Brown carbon absorption linked to organic mass tracers in biomass burning particles; Atmos. Chem. Phys., 13 (2013), pp. 2415–2422
- Laskin et al., 2015 A. Laskin, J. Laskin, S.A. Nizkorodov; Chemistry of atmospheric brown carbon; Chem. Rev., 115 (2015), pp. 4335–4382
- Legrand et al., 2007 M. Legrand, S. Preunkert, M. Schock, et al.; Major 20th century changes of carbonaceous aerosol components (EC, WinOC, DOC, HULIS, carboxylic acids, and cellulose) derived from Alpine ice cores; J. Geophys. Res. Atmos., 112 (2007)
- Legrand et al., 2013 M. Legrand, S. Preunkert, B. Jourdain, et al.; Water-soluble organic carbon in snow and ice deposited at Alpine, Greenland, and Antarctic sites: a critical review of available data and their atmospheric relevance; Clim. Past, 9 (2013), pp. 2195–2211
- Limbeck et al., 2003 A. Limbeck, M. Kulmala, H. Puxbaum; Secondary organic aerosol formation in the atmosphere via heterogeneous reaction of gaseous isoprene on acidic particles; Geophys. Res. Lett., 30 (2003)
- Lin et al., 2014 G. Lin, J.E. Penner, M.G. Flanner, et al.; Radiative forcing of organic aerosol in the atmosphere and on snow: effects of SOA and brown carbon; J. Geophys. Res. Atmos., 119 (2014), pp. 7453–7476
- Lin et al., 2010a P. Lin, G. Engling, J.Z. Yu; Humic-like substances in fresh emissions of rice straw burning and in ambient aerosols in the Pearl River Delta Region, China; Atmos. Chem. Phys., 10 (2010), pp. 6487–6500
- Lin et al., 2010b P. Lin, X.-F. Huang, L.-Y. He, et al.; Abundance and size distribution of HULIS in ambient aerosols at a rural site in South China; J. Aerosol Sci., 41 (2010), pp. 74–87
- Liu et al., 2015 S. Liu, A.C. Aiken, K. Gorkowski, et al.; Enhanced light absorption by mixed source black and brown carbon particles in UK winter; Nat. Commun., 6 (2015)
- Luthi et al., 2015 Z.L. Luthi, B. Skerlak, S.W. Kim, et al.; Atmospheric brown clouds reach the Tibetan Plateau by crossing the Himalayas; Atmos. Chem. Phys., 15 (2015), pp. 6007–6021
- McNaughton et al., 2011 C.S. McNaughton, A.D. Clarke, S. Freitag, et al.; Absorbing aerosol in the troposphere of the Western Arctic during the 2008 ARCTAS/ARCPAC airborne field campaigns; Atmos. Chem. Phys., 11 (2011), pp. 7561–7582
- Moosmueller et al., 2011 H. Moosmueller, R.K. Chakrabarty, K.M. Ehlers, et al.; Absorption Angstrom coefficient, brown carbon, and aerosols: basic concepts, bulk matter, and spherical particles; Atmos. Chem. Phys., 11 (2011), pp. 1217–1225
- Mukai and Ambe, 1986 H. Mukai, Y. Ambe; Characterization of a humic acid-like brown substance in airborne particulate matter and tentative identification of its origin; Atmos. Environ., 20 (1986), pp. 813–819
- Nguyen et al., 2014 Q.T. Nguyen, T.B. Kristensen, A.M.K. Hansen, et al.; Characterization of humic-like substances in Arctic aerosols; J. Geophys. Res. Atmos., 119 (2014), pp. 5011–5027
- Posfai et al., 2004 M. Posfai, A. Gelencser, R. Simonics, et al.; Atmospheric tar balls: particles from biomass and biofuel burning; J. Geophys. Res. Atmos., 109 (2004) http://dx.doi.org/10.1029/2003JD004169
- Ramanathan and Carmichael, 2008 V. Ramanathan, G. Carmichael; Global and regional climate changes due to black carbon; Nat. Geosci., 1 (2008), pp. 221–227
- Saleh et al., 2014 R. Saleh, E.S. Robinson, D.S. Tkacik, et al.; Brownness of organics in aerosols from biomass burning linked to their black carbon content; Nat. Geosci., 7 (2014), pp. 647–650
- Salma et al., 2007 I. Salma, R. Ocskay, X. Chi, et al.; Sampling artefacts, concentration and chemical composition of fine water-soluble organic carbon and humic-like substances in a continental urban atmospheric environment; Atmos. Environ., 41 (2007), pp. 4106–4118
- Samburova et al., 2005 V. Samburova, S. Szidat, C. Hueglin, et al.; Seasonal variation of high-molecular-weight compounds in the water-soluble fraction of organic urban aerosols; J. Geophys. Res. Atmos., 110 (2005)
- Seinfeld and Pandis, 2012 J.H. Seinfeld, S.N. Pandis; Atmospheric Chemistry and Physics: from Air Pollution to Climate Change; John Wiley & Sons (2012)
- Varga et al., 2001 B. Varga, G. Kiss, I. Ganszky, et al.; Isolation of water-soluble organic matter from atmospheric aerosol; Talanta, 55 (2001), pp. 561–572
- Voisin et al., 2012 D. Voisin, J.-L. Jaffrezo, S. Houdier, et al.; Carbonaceous species and humic like substances (HULIS) in Arctic snowpack during OASIS field campaign in Barrow; J. Geophys. Res. Atmos., 117 (2012)
- Wang et al., 2013 X. Wang, S.J. Doherty, J. Huang; Black carbon and other light-absorbing impurities in snow across Northern China; J. Geophys. Res. Atmos., 118 (2013), pp. 1471–1492
- Wang et al., 2014 X. Wang, B. Xu, J. Ming; An overview of the studies on black carbon and mineral dust deposition in snow and ice cores in East Asia; J. Meteorological Res., 28 (2014), pp. 354–370
- Washenfelder et al., 2015 R. Washenfelder, A. Attwood, C. Brock, et al.; Biomass burning dominates brown carbon absorption in the rural southeastern United States; Geophys. Res. Lett., 42 (2015), pp. 653–664
- Xu et al., 2009 B. Xu, J. Cao, J. Hansen, et al.; Black soot and the survival of Tibetan glaciers; Proc. Natl. Acad. Sci. U. S. A., 106 (2009), pp. 22114–22118
- Yang et al., 2009 M. Yang, S.G. Howell, J. Zhuang, et al.; Attribution of aerosol light absorption to black carbon, brown carbon, and dust in China: interpretations of atmospheric measurements during EAST-AIRE; Atmos. Chem. Phys., 9 (2009), pp. 2035–2050
- Yang et al., 2015 S. Yang, B. Xu, J. Cao, et al.; Climate effect of black carbon aerosol in a Tibetan Plateau glacier; Atmos. Environ., 111 (2015), pp. 71–78
- Yao et al., 2012 T. Yao, L.G. Thompson, V. Mosbrugger, et al.; Third pole environment (TPE); Environ. Dev., 3 (2012), pp. 52–64
- Zhang et al., 2011 X. Zhang, Y.H. Lin, J.D. Surratt, et al.; Light-absorbing soluble organic aerosol in Los Angeles and Atlanta: a contrast in secondary organic aerosol; Geophys. Res. Lett., 38 (2011)
- Zheng et al., 2013 G. Zheng, K. He, F. Duan, et al.; Measurement of humic-like substances in aerosols: a review; Environ. Pollut., 181 (2013), pp. 301–314
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?