Summary
Background/Objective
Maggot wound therapy (MWT) has been used in various wounds including diabetic foot ulcers, venous leg ulcers, pressure ulcers, and acute surgical wounds. However, the efficacy of MWT therapy has been controversial. We therefore conducted a cohort study and a meta-analysis to assess MWT effects.
Methods
A retrospective cohort study was performed in diabetic foot ulcer (DFU) patients who were treated with MWT or conventional wound therapy (CWT) in Thailand. The Kaplan-Meier curve was applied to estimate the healing probability. A meta-analysis was performed to pool our study with four previous cohort studies identified from Medline and Scopus.
Results
The estimated incidence of wound healing was 5.7/100 (95% CI: 4.49, 7.32) patients-week, and the median time to healing was 14 weeks. The hazard ratio (HR) of wound healing was 7.87 times significantly higher in the MWT than the CWT (p < 0.001) after adjusting for duration and size of ulcers, ankle brachial index (ABI), and glycated hemoglobin (HbA1c). Meta-analysis was applied and suggested that the treatment effects were moderately heterogeneous {Chi-square = 6.18 [degrees of freedom (d.f.) = 4]; p = 0.186; I2 = 35.2%}, with the pooled risk ratio (RR) of 1.77 [95% confidence intervals (CI) = 1.01, 3.11], i.e., the chance of wound healing was 20% significantly higher with MWT than CWT. The average costs of treatment in patients with DFU were lower in the MWT group than in the CWT group, with medians of US$292.82 and US$490, respectively.
Conclusion
Our evidence suggests that MWT is significantly better for wound healing and more cost-effective than CWT. An updated meta-analysis or large scale randomized controlled trial (RCT) is required to confirm this effect.
Keywords
chronic ulcer;chronic wound;maggot;maggot wound therapy;wound therapy
1. Background
One of the important aspects of wound management, especially in intractable wounds, is removal of necrotic tissue, which is known as debridement. The use of maggots for this purpose has been claimed to be an efficacious method,1 ; 2 that stimulates wound healing,3 ; 4 reduces the bacterial load,5 and eradicates methicillin resistant Staphylococcus aureus in vitro. 6 ; 7 Maggot wound therapy (MWT) has been applied in various settings, e.g., diabetic foot ulcers (DFU), 8 ; 9 peripheral arterial diseases,10 venous leg ulcers,11 pressure ulcers,12 and acute surgical wounds. 13 ; 14
The use of MWT is increasing as a reflection of the increasing acceptance since approval by the Food and Drug Administration. The recent enthusiasm for MWT is supported and calls for public attention. However, the results of MWT treatment are still controversial.10 A randomized controlled trial (RCT)15 could not demonstrate differences in the rate of wound healing and healing time between MWT and standard treatment in venous leg ulcers, although MWT did reduce the time to debridement by approximately 2 days, but with significantly higher pain scores. The cost-effectiveness of MWT has been reported in some studies with conflicting results3; 4 ; 5; MWT was initially shown to have lower costs of treatment and require fewer visits, but these could not be confirmed in a recent RCT study.16
We, therefore, performed a retrospective cohort study and follow-up meta-analysis of comparative studies to evaluate wound healing outcomes with MWT compared with conventional wound therapy (CWT).
2. Materials and methods
2.1. Cohort study
A retrospective cohort study was performed by reviewing the medical records of diabetic DFU patients who were treated at Bang Yai Hospital, Nonthaburi Province, Thailand from January 2008 to December 2009. Bang Yai hospital is a 30-bed hospital that provides primary care services. Patients who met the following criteria were included in the study: (1) presence of a single wound of the foot; (2) ability to walk without the use of a wheelchair or other assistive device; (3) data were available for at least 6 months of follow-up; and (4) no gangrenous wounds, necrotizing fasciitis, abscess, or osteomyelitis present. Patients were assigned by physicians who were well trained in chronic wound care, to receive MWT or CWT at the out-patient clinic or in-patient wards, based on physician judgment.
Maggots were prepared by Biomonde Thailand Co. Ltd., Bangkok, Thailand using a standard biological room. Briefly, adult flies were kept in a temperature-controlled room (25 °C) with constant light, air, and humidity (33%). After oviposition was documented, the eggs were transferred to a clean room and they were fed with sheep blood agar until they reached the post-feeding stage.17 ; 18 Then, maggots were applied to the wounds in a bio-bag, with an average of eight maggots/cm2 of wound surface.19 The wound was covered with wet light gauze, and the entire foot was loosely bandaged. The median number of applications of maggots was 8.25 [standard deviation (SD) = 5–13] times/patient. For the control group, the wound was dressed with normal saline or hydrogel and debridement was performed as judged by the treating physician. The wounds were debrided with a median of 8.79 (SD = 5–14) times/patient. The wound was evaluated once/week by nurse practitioners and evaluated using digital photographic images. Patients were classified as having wound healing if their wounds had ≥ 95% complete epithelial covering in the absence of a scab, and the wounds were suitable for split skin grafting, flap coverage, or self-healing.20 Healing time was defined as the time from treatment initiation to wound healing. Patients were followed up from treatment initiation until the end of December 2009. The study was approved by the Institutional Review Board prior to conducting the study and all participants had given informed consent.
3. Meta-analysis
Studies were identified using PubMed and Scopus search engines from January 1946 to September 15, 2011. The search strategy is described in Appendix 1.
3.1. Inclusion criteria
Comparative studies of MWT and CWT were included in the review if they met with the following criteria: patients aged ≥ 18 years and compared wound healing rate or wound healing time between groups. The reference lists of all relevant studies were also reviewed. If studies were duplicated, the one with the most complete data was chosen. For studies which reported insufficient data, the corresponding authors were contacted and invited to provide more information. Two attempts were made to contact authors and those who did not respond were excluded from the review.
3.2. Outcomes
The outcomes of interest were the rate of wound healing and/or healing time.
3.3. Data extraction
Two investigators (CW and NP) independently extracted the data using a standard data extraction form. Information extracted included general data (author, year of publication, journal), study characteristics (study design, setting), patient characteristics at baseline (age, type of wound, underlying disease, white blood cell count, ABI, percent amputation), and the outcomes as described above. Any disagreement was discussed and resolved by consensus with a third party (AT).
3.4. Risk of bias assessment
The quality of the cohort studies was independently assessed by CW, NP, and AT on the basis of representativeness of studied participants, information bias (i.e., ascertainment of outcome and treatment), and confounding bias (Appendix 2). For the RCT study, the assessment was done using established tools recommended by the Cochrane Library.21 Each item was graded as “no” for risk of bias, “yes” for risk of bias, and “unclear” if there was insufficient information to judge. Any disagreement between the reviewers was discussed and resolved by consensus.21
3.5. Statistical analysis
For the cohort study, the Kaplan-Meier method was applied to estimate the healing probability at 7 weeks, 14 weeks, 21 weeks, and 28 weeks after receiving treatments. The median healing times and 95% confidence intervals (CI) were estimated. The log-rank test was used to compare the time to healing between treatments and prognostic factors.
Factors with p < 0.1 were included in the multivariate Cox regression model. The likelihood ratio test with forward elimination was used to select variables in the Cox model. Hazard ratios (HR) and 95% CI were estimated.
For meta-analysis, the risk ratio (RR) of wound healing for each included study was estimated. The heterogeneity of RRs across studies was assessed using Cochrans Q test and the degree of heterogeneity was quantified using the I2 statistic. If the heterogeneity was significant or I2 > 25%, a random effects model using the Der-Simonian and Laird method was applied for pooling ORs, otherwise the fixed effects model was used.
For healing time, the mean difference of healing time of each study was estimated. The heterogeneity of the mean difference across studies was assessed as described above. An unstandardized mean difference was used for the summary estimate.
Meta-regression analysis was used to assess the source of heterogeneity by fitting age and type of wound in the meta-regression model. Funnel plot with or without contour-enhancement was applied to detect publication bias due to small study effects. Eggers test was used for assessing the asymmetry of the funnel plot.
All analyses were performed using STATA version 12.0 (StataCorp LP, College Station, TX). A p value < 0.05 was considered statistically significant, except for the heterogeneity test, for which p < 0.1 was used.
4. Results
4.1. Cohort study of diabetic wound healing
One hundred and eleven diabetic patients, with 1116 person-weeks of follow-up, were included in the cohort. Among them, 59 patients and 52 patients received MWT and CWT, respectively. Characteristics at baseline are described in Table 1. The mean ages were similar between two groups, i.e., 55.6 (SD = 12.20) years and 53.4 (SD = 11.42) years, respectively. Glycated hemoglobin (HbA1c) levels at baseline were also similar between groups, i.e., 7.0 (SD = 1.40) and 7.1 (SD = 1.87), respectively. However, the proportion with an abnormal ABI (< 0.9 or > 1.1) was much lower in the MWT group than in the CWT group (30.0% vs. 67.3%). In addition, patients in the MWT group had a smaller size (26.1 cm2 vs. 32.1 cm2) and a shorter duration (18.1 vs. 23.5) of ulcers than patients in the CWT group. The median number of applications of maggots was 8.25 (SD = 5–13) times/patient.
| Parameters | MWT | CWT | p |
|---|---|---|---|
| Number | 59 | 52 | |
| Age (y) | 55.5 ± 12.2 | 53.4 ± 11.4 | 0.4 |
| Male:Female (%) | 31:28 (1.1:1) | 30:22 (1.4:1) | 0.6 |
| HbA1c | 7.0 ± 1.4 | 7.1 ± 1.9 | 0.9 |
| ABI | |||
| < 0.40 | 6 (8.6) | 10 (7.4) | 0.47 |
| 0.50–0.89 | 15 (14.5) | 12 (12.5) | |
| 0.90–1.29 | 35 (32.1) | 25 (27.9) | |
| > 1.30 | 4 (4.8) | 5 (4.2) | |
| Underlying disease | |||
| Hypertension | 30 (30.5) | 27 (26.5) | 0.84 |
| Coronary arterial disease | 24 (24.1) | 21 (20.9) | 0.97 |
| Chronic renal failure | 6 (5.4) | 4 (4.6) | 0.67 |
| Size of ulcer (cm2) | 26.1 ± 12.8 | 32.1 ± 10.9 | 0.005 |
| Duration of ulcer (d) | 18.1 ± 4.9 | 23.5 ± 8.2 | < 0.001 |
| Wound closure | |||
| Self-healing | 44 (45) | 40 (39) | 0.89 |
| Skin graft | 10 (9.6) | 8 (8.4) | |
| Flap coverage | 6 (5.4) | 4 (4.6) | |
| Hospital cost, Baht, median (range) | 8784.7 (728.5, 8784.6) | 14,700 (1062.9, 14928) | < 0.001 |
Data are presented as n (%) or mean ± SD unless otherwise indicated.
CWT = conventional wound therapy; MWT = maggot wound therapy.
Among the cohort of 111 patients, there were 64 whose wounds were healed at the end of the study, with a median follow-up of 14 weeks (range = 0–45 weeks). The estimated incidence of wound healing was 5.7/100 patients-week, and the median time to healing was 14 weeks (95% CI: 10, 15), i.e., there were 50% of patients whose wounds were healed at approximately 14 weeks. The probability of healing at 7 weeks, 14 weeks, 21 weeks, and 28 weeks was 25.85% (95% CI: 19, 35), 56.24% (95% CI: 46, 67), 72.84% (95% CI: 62, 83), and 90.95% (95% CI: 70, 99), respectively.
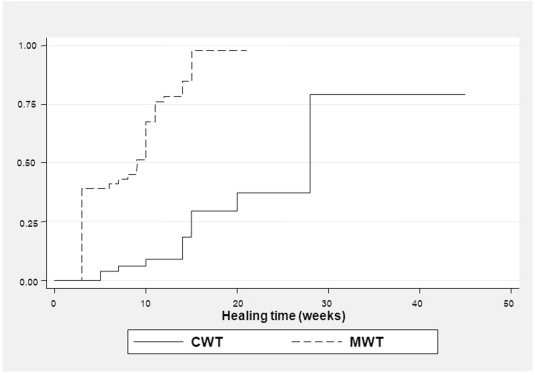
The probability of healing between treatment groups was estimated and the log-rank test was applied to compare the medians by treatment and prognostic variables (Table 2). As shown in Fig. 1, the median healing time was significantly shorter in the MWT group than in the CWT group (9 weeks vs. 28 weeks, Chi-square = 55.82, p < 0.001). In addition, ABI, duration of ulcer, and size of ulcer were significantly associated with wound healing. The median time to healing was shorter in patients whose duration of ulcer was < 20 days when compared with patients whose durations were ≥ 20 days (8 weeks vs. 15 weeks, Chi-square = 49.02, p < 0.001). Patients whose areas of ulcer were smaller than 28 cm2 took approximately 9 weeks to reach wound healing, whereas patients whose areas were 28 cm2 or larger took 15 weeks (Chi-square = 23.66, p < 0.001). The median healing time was significantly longer in patients with abnormal ABI than in those with normal ankle brachial index (15 weeks vs. 10 weeks, Chi-square = 6.73, p = 0.01).These significant variables were further included in the multivariate Cox model. There were non-significant differences according to age, sex, and HbA1c.
| Factors | No. of healing | Person time (wk) | Healing rate/100 | Median healing time (wk) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | p | Hazard ratio | p | 95% CI | |||||
| Treatment | |||||||||
| Maggot | 51 | 682 | 11.75 | 9 | 7.66 | < 0.001 | 7.72 | < 0.001 | 3.83, 15.56 |
| Conventional | 13 | 434 | 1.91 | 28 | 1 | 1 | |||
| Duration of wound (d) | |||||||||
| < 20 | 38 | 349 | 10.89 | 8 | 7.93 | < 0.001 | 8.17 | < 0.001 | 3.43, 19.46 |
| ≥ 20 | 26 | 746 | 3.48 | 15 | |||||
| Size of wound (cm2) | |||||||||
| < 28 | 44 | 468 | 9.40 | 9 | 3.20 | < 0.001 | 2.09 | 0.027 | 1.09, 4.00 |
| ≥ 28 | 20 | 648 | 3.09 | 15 | |||||
| HbA1c | |||||||||
| ≤ 7 | 34 | 622 | 3.20 | 14 | 1.34 | 0.20 | 1.02 | 0.91 | 0.61, 1.73 |
| > 7 | 30 | 454 | 3.29 | 14 | 1 | 1 | |||
| ABI | |||||||||
| < 0.9 or > 1.3 | 23 | 577 | 3.99 | 15 | 0.54 | 0.01 | 1.03 | 0.92 | 0.59, 1.81 |
| 0.9–1.3 | 41 | 539 | 7.60 | 10 | 1 | 1 | |||
| Age (y) | |||||||||
| ≤ 55 | 38 | 656 | 3.60 | 14 | 0.98 | 0.92 | |||
| > 55 | 26 | 460 | 3.06 | 12 | 1 | ||||
| Sex | |||||||||
| Female | 28 | 623 | 3.66 | 12 | 0.90 | 0.66 | |||
| Male | 37 | 493 | 2.50 | 14 | 1 | ||||
ABI = Ankle brachial index; CI = confidence interval; HbA1c = glycated hemoglobin.
|
|
|
Figure 1. Kaplan-Meier estimates of healing between maggot wound therapy (MWT) and conventional wound therapy (CWT). |
The treatment effect was further estimated in the Cox model by adjusting for ankle brachial index, HbA1c, size of ulcer, and duration of ulcer (Table 2). This suggested that after adjusting for ABI, HbA1c, size of ulcer, and duration of ulcer, the chance of wound healing was approximately 7.7 (95% CI: 3.83, 15.56) times significantly higher in the MWT group than the CWT group.
In addition, duration and size of wound were significantly associated with wound healing, with HR of 8.17 (95% CI: 3.43, 19.46) and 2.09 (95% CI: 1.09, 4.00), respectively. The ABI was no longer significant in this model. A global Chi-square test based on Schoenfeld residuals was performed and suggested that the Cox proportional hazards assumption was not violated [Chi-square = 5.57, degrees of freedom (d.f.) = 5, p = 0.3498].
4.2. Meta-analysis
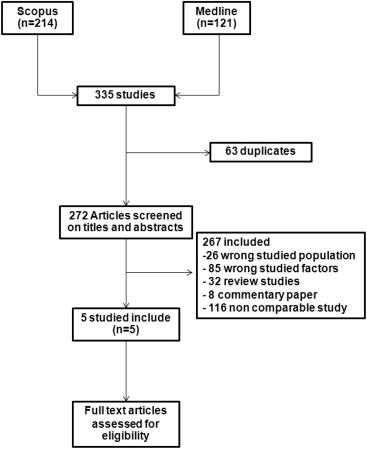
Among 272 studies identified from Medline and Scopus databases, 267 studies were ineligible, leaving five studies which were eligible to pool (Fig. 2). Characteristics of the six studies, including ours(Wilasrusmee et al, unpublished)20; 22; 23 ; 24 are described in Table 3; a total of 612 patients (345 vs. 267) were available for analysis. Among five studies, two were prospective cohort studies,20 ; 24 two were retrospective cohorts,22 ; 23 and one was a multicenter RCT.16 Three studies were based in the United States, one in the United Kingdom, and one in Malaysia. The mean patient age ranged from 54.6 years to 74.0 years. The types of wound were diabetic ulcers (4 studies; Wilasrusmee et al, unpublished),20; 22 ; 24 venous ulcers (1 study),16 and pressure ulcers (1 study).23
|
|
|
Figure 2. Identification of studies for inclusion. |
| Study | n | Mean age (y) | Type of wound | Country | Study design | No. of participants | Outcome | |
|---|---|---|---|---|---|---|---|---|
| MWT | CWT | |||||||
| Paul et al (2009)20 | 54 | 55.3 | Diabetic ulcer | Malaysia | Prospective | 25 | 29 | RH |
| Armstrong et al (2005)22 | 60 | 72.2 ± 6.8 | Diabetic ulcer | USA | Retrospective | 30 | 30 | RH, HT |
| Sherman (2002)23 | 92 | 64 ± 31.9 | Pressure ulcer | USA | Retrospective | 43 | 49 | RH, HT |
| Sherman (2003)24 | 28 | 65.5 ± 23.6 | Diabetic ulcer | USA | Prospective | 14 | 14 | RH, HT |
| Wilasrusmee et al (2011; unpublished) | 111 | 54.6 ± 11.8 | Diabetic ulcer | Thailand | Retrospective | 59 | 52 | RH, HT |
| Dumville et al (2009)16 | 267 | 74.0 ± 12.6 | Venous ulcer | UK | RCT | 180 | 87 | RH, HT |
CWT = conventional wound therapy; HT = healing time; MWT = maggot wound therapy; RH = rate of wound healing.
4.3. Risk of bias assessment
Among six studies, (Wilasrusmee et al, unpublished)20; 22; 23 ; 24 the risk of selection bias from the use of non-representative cases was present in four (80%) studies. The ascertainment of all outcomes was clearly described in three out of five (60%) studies,20; 22 ; 23 whereas the other two (40%) studies were unclear. The ascertainment of intervention was clear in five (80%) studies. Unclear ascertainment was found in one study, due to switching between CWT and MWT. Confounding bias was found likely to be present in three (60%) studies. In the RCT study,15 the quality was high except in the domain of blinding.
4.4. Rate of wound healing
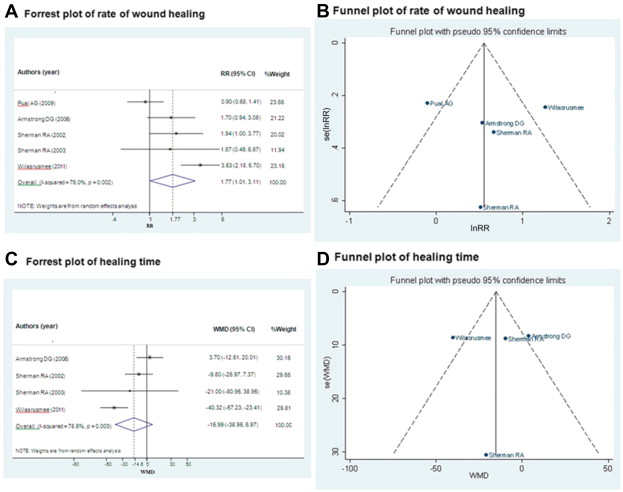
Pooling was performed based on four cohort studies20; 22; 23 ; 24 plus ours (Wilasrusmee et al, unpublished; n = 171 vs. 174). The RR was highly heterogeneous [Chi-square = 16.64 (d.f. = 4); p = 0.002; I2 = 76.04%) across the studies. The pooled RR with a random effect model was 1.77 (95% CI: 1.01, 3.11), suggesting that the chance of wound healing was 77% higher in the MWT group compared to the CWT group (Fig. 3A). Neither Eggers test nor the funnel plot suggested asymmetry (coefficient = 0.57, SE = 3.66, p = 0.89; see Fig. 3B).
|
|
|
Figure 3. Meta-analysis of rate of wound healing and healing time between maggot wound therapy (MWT) and conventional wound therapy (CWT). |
A sensitivity analysis was performed by adding the RCT176 to the pooling (n = 612) which resulted in the pooled RR of 1.53 (95% CI: 1.23, 1.90), indicating that the chance of wound healing was 53% significantly higher in the MWT group compared to the CWT group. Subgroup analyses of four diabetic studies and three studies with good ascertainment of outcome resulted in the pooled RR of 1.74 (95% CI: 0.85, 3.53) and 1.40 (95% CI: 1.23, 1.58), respectively.
4.5. Healing time
Four studies (Wilasrusmee et al, unpublished)22; 23 ; 24 (n = 146 vs. 145) were included in pooling of the healing time. The pooled effect was highly heterogeneous [Chi-square = 14.05 (d.f. = 3); p = 0.003; I2 = 78.6%] across studies with an unstandardized mean difference of –15.99 (95% CI: –38.95, 6.97) days (Fig. 3C). This suggested that the healing time was 15.99 days shorter in the MWT group than the CWT group, but this was not statistically significant. Neither Eggers test nor a funnel plot suggested asymmetry (coefficient = –0.42, SE = 3.71, p = 0.92; see Fig. 3D).
Adding the RCT study16 (n = 585) did not change the results, with an unstandardized mean difference of –14.56 (95% CI: –32.91, 3.80) days.
4.6. Cost-effectiveness analysis
Among 111 patients, the total direct medical cost of treatments was estimated for each patient, including the costs for nursing care, admission, wound dressing, and material used for treatments.
The average cost of treatments was lower in the MWT group than the CWT group, with a median of 8784.66 baht (range = 728.59, 8784.66) and 14,700 baht (range = 1062.95, 14928.00; p < 0.001), which is approximately equivalent to US$292.82 and US$490, respectively. As described in Table 2, the median healing times were 9 weeks and 28 weeks for the MWT and the CWT groups, respectively. The corresponding healing rates were 12/100 patients-week and 2/100 patients-week, respectively. As a result, the incremental cost-effectiveness ratios were –311.3 and –591.53 for healing time and healing rate, respectively.
5. Discussion
We performed a cohort study and a meta-analysis to investigate the effect of MWT compared with CWT. Our findings from the cohort study suggest that MWT has ∼7 times higher chance of wound healing than CWT. The time to healing was also ∼19 weeks shorter in the MWT group than in the CWT group. In addition, the duration and size of wounds were also associated with wound healing. The MWT effects were, however, diluted and highly heterogeneous when we combined our findings with other cohort studies, with a pooled RR of 1.77 and time to healing of 2 weeks. The incremental cost-effectiveness ratios for healing rate and healing time were 591.5 baht (US$ ∼20) and 311.3 baht (US$ ∼10) lower in the MWT group than in the CWT group.
Various new technologies, pharmaceuticals, and devices have been proposed in an effort to promote wound healing.25 It is difficult to make strong recommendations in wound management, due to a paucity of high quality evidence, inadequate sample size, limited follow-up period, non-random allocation of treatment and control, concurrent intervention, and blinding assessment of outcomes.26
The wound healing properties of MWT have been demonstrated in many studies.27; 28 ; 29 In addition to directly ingesting necrotic tissue, maggots appear to indirectly promote healing by inducing and amplifying interleukin-6, carboxypeptidase, leucine aminopeptidase, collagenase, serine proteases, and epidermal growth factor.27 ; 30 They also stimulated the proliferation of human fibroblasts and chondrocytes, as well as the synthesis of cartilage-specific collagen type II and allantoin, which has a soothing effect on the skin.29 The United States Food and Drug Administration (FDA) approved MWT for the debridement of non-healing necrotic skin and soft tissue wounds, such as pressure ulcers, venous stasis ulcers, neuropathic foot wounds, and postoperative wounds.27
The cost of management for MWT in our patients was less than that for CWT, due mainly to a more rapid decrease in wound size, an increase in granulation tissue, and better wound preparation for surgical closure.31 ; 32 Laboratory evidence strongly supports the beneficial effects of MWT in removing pathogenic bacteria both in vitro and in vivo.5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26; 27; 28; 29; 30; 31; 32 ; 33 MWT has been reported as a cost-effective method in debridement and treatment of DFU,2 venous ulcer,34 and various difficult-to-treat wounds.31 However, economic evaluation in the single RCT16 demonstrated small or similar health benefits of MWT over CWT (hydrogel), as measured by quality adjusted life years (QALY) and time to healing. The cost effectiveness analysis in our study has shown that MWT is a cost saving method when compared to CWT. However, our study analysis was based on the retrospective cohort study of patients in Thailand, which has different cost structures than Western countries. It should also be kept in mind that patients with less severe ulcers were more likely to assign to MWT than CWT. As a result, cost analysis might be bias.
Although the ABI was not significantly associated with wound healing in the multivariate Cox model, time to healing between ABI groups were different; i.e., 14 weeks, 14 weeks, 11 weeks, and 12 weeks for ABI ≤ 0.4, 0.5–0.9, 0.9–1.3, and > 1.3,35 respectively. Because our sample size was small, ABI of ≤ 0.4, 0.5–0.9, and > 1.3 were thus combined as abnormal groups when fitting in the Cox model in order to prevent the model from over fitting.
The benefits of MWT of improved wound healing were confirmed in our cohort study, which demonstrated a better wound healing rate and shorter time to healing. Our meta-analysis, the first in this area, showed that MWT was at least as effective as CWT, if not better; the 95% CI was borderline significant (1.01, 3.11) with a heterogeneous RR of 1.77 from five comparative studies and 345 patients. The only RCT study16 in 267 patients indicated a slightly increased likelihood of healing, but did not find any significant differences; the HR was 1.13 (95% CI: 0.76, 1.68). The wound healing time was 16 days shorter in the meta-analysis of four studies with 393 patients, but this did not reach statistical significance, whereas it was 9 days non-significantly shorter than reported in the RCT.16 Important limitations include the fact that our retrospective cohort and the included studies were mainly observational studies, which were more likely to face selection and confounding biases; thus cause and effect relationships cannot be inferred from our main finding. There was high heterogeneity in the pooled estimates; generalization of maggot effects was less likely. Performing a subgroup analysis in DFU patients could not reduce a degree of heterogeneity and resulted in non-significant maggot effects, due to the power of the test. However, the RCT study results were largely similar, but suffered from poor precision. More RCT studies are needed to better define this potential benefit.
The results of this study should help physicians decide on the choice of appropriate interventions for their patients. Our evidence suggests that MWT is significantly better in wound healing than CWT, and at less cost. This, however, needs to be confirmed by a large scale RCT or a meta-analysis of RCTs.
Financial disclosures
None.
Appendix 1. Search strategy for Medline and Scopus
- Arterial occlusion
- Chronic wound
- Diabetes
- Diabetic
- Diabetic foot ulcer
- Diabetic foot wound
- Diabetic wound
- Foot ulcer
- Gangrene
- Ischemic ulcer
- Leg ulcer
- Peripheral arterial disease
- Peripheral vascular disease
- Venous ulcer
- Unhealed wound
- Maggot
- Maggot debridement
- Maggot healing
- Maggot therapy
- Larval therapy
- Lucillia sericata
- Conventional debridement
- Conventional wound care
- Debridement
- Hydrogel
- Wet dressing
- Wound care
- Wound therapy
- Amputation
- Curing time
- Healing time
- Limb amputation
- Ulcer healing
- Wound curing
- Wound healing
- Wound infection
- (1 or 2 or 3………..or 15)
- (16 or 17 or 18………..or 21)
- (22 or 23 or 24………..or 28)
- (29 or 30 or 31……………or 36)
- (37 and 38 and 39 and 40)
Appendix 2. Risk of bias assessment form.
For cohort study
| Author | Domain | Item | Low risk of bias |
|---|---|---|---|
| Selection bias | Representative of cohorts A. Consecutive/randomly selected from cases population with extensive inclusion criteria B. Spectrum of diseases: same type of wound C. Did not mention | ||
| Information bias | Ascertainment of outcome measurement A. Cleary describe definition of outcomes B. Did not describe | ||
| Ascertainment of interventions A. No switching of treatment between groups B. Not described | |||
| Confounding bias | Confounding bias A. Adjusting confounding factors in analysis B. Did not adjust confounding factors |
For RCT study
| Author | Adequate sequence generation | Adequate allocation concealment | Blinding | Address incomplete outcome data | Selective outcome report | Free of other bias | Description of other bias |
|---|---|---|---|---|---|---|---|
References
- 1 F. Gottrup, B. Jorgensen; Maggot debridement: an alternative method for debridement; Eplasty, 11 (2011), p. e33
- 2 E.P. Cherniack; Bugs as drugs, part 1: Insects. The “new” alternative medicine for the 21st century?; Altern Med Rev, 15 (2010), pp. 124–135
- 3 L. Davydov; Maggot therapy in wound management in modern era and a review of published literature; J Pharm Pract, 24 (2011), pp. 89–93
- 4 R.A. Sherman, M.J. Hall, S. Thomas; Medicinal maggots: an ancient remedy for some contemporary afflictions; Annu Rev Entomol, 45 (2000), pp. 55–81
- 5 F.A. Blake, N. Abromeit, M. Bubenheim, L. Li, R. Schmelzle; The biosurgical wound debridement: experimental investigation of efficiency and practicability; Wound Repair Regen, 15 (2007), pp. 756–761
- 6 S. Arora, C. Baptista, C.S. Lim; Maggot metabolites and their combinatory effects with antibiotic on Staphylococcus aureus; Ann Clin Microbiol Antimicrob (2011), p. 10 http://dx.doi.org/10.1186/1476-0711-10-6
- 7 D. Jaklic, A. Lapanje, K. Zupancic, D. Smrke, N. Gunde-Cimerman; Selective antimicrobial activity of maggots against pathogenic bacteria; J Med Microbiol, 57 (2008), pp. 617–625
- 8 J. Edwards, S. Stapley; Debridement of diabetic foot ulcers; Cochrane Database Syst Rev (Online) (1) (2010), p. CD003556 http://dx.doi.org/10.1002/14651858.CD003556.pub2
- 9 R.A. Sherman, E.A. Pechter; Maggot therapy: a review of the therapeutic applications of fly larvae in human medicine, especially for treating osteomyelitis; Med Vet Entomol, 2 (1988), pp. 225–230
- 10 R.A. Sherman; Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century; J Diabetes Sci Technol, 3 (2009), pp. 336–344
- 11 J.C. Dumville, G. Worthy, M.O. Soares, et al.; VenUS II: A randomised controlled trial of larval therapy in the management of leg ulcers; Health Technol Assess, 13 (2009), pp. 1–182
- 12 R.A. Sherman; Maggot debridement in modern medicine; Infect Med, 15 (1998), pp. 651–656
- 13 R.A. Sherman, C.E. Shapiro, R.M. Yang; Maggot therapy for problematic wounds: uncommon and off-label applications; Adv Skin Wound Care, 20 (2007), pp. 602–610
- 14 Q. Li, R. Lu, R. Huo, H. Fu; Maggots of Musca domestica in treatment of acute intractable wound; Surgery, 145 (2009), pp. 122–123
- 15 J.C. Dumville, G. Worthy, J.M. Bland, et al.; Larval therapy for leg ulcers (VenUS II): randomised controlled trial; BMJ, 338 (2009), pp. 1047–1049
- 16 M.O. Soares, C.P. Iglesias, J.M. Bland, et al.; Cost effectiveness analysis of larval therapy for leg ulcers; BMJ, 338 (2009), pp. 1050–1053
- 17 F. Firoozfar, S.H. Mosa Kazemi, K. Shemshad, et al.; Laboratory colonization of Lucilia sericata Meigen (Diptera: Caliphoridae) strain from Hashtgerd, Iran; J Vector Borne Dis, 49 (2012), pp. 23–26
- 18 M. Mai, J. Amendt; Effect of different post-feeding intervals on the total time of development of the blowfly Lucilia sericata (Diptera: Caliphoridae); Forensic Sci Int, 221 (2012), pp. 65–69
- 19 Co.KG. BG Biomonde Fly larva handbook (2004) Barsuttel: BioMonde GmbH. (Mimeographed)
- 20 A.G. Paul, N.W. Ahmad, H. Lee, et al.; Maggot debridement therapy with Lucilia cuprina: A comparison with conventional debridement in diabetic foot ulcers; Int Wound J, 6 (2009), pp. 39–46
- 21 M. Davoli, L. Amato; Do Cochrane reviews provide useful information to guide policy and practice? The experience of the Cochrane drugs and alcohol group; Epidemiol Psychiatr Sci, 20 (2011), pp. 219–223
- 22 D.G. Armstrong, P. Salas, B. Short, et al.; Maggot therapy in “lower-extremity hospice” wound care: fewer amputations and more antibiotic-free days; J Am Podiatr Med Assoc, 95 (2005), pp. 254–257
- 23 R.A. Sherman; Maggot versus conservative debridement therapy for the treatment of pressure ulcers; Wound Repair Regen, 10 (2002), pp. 208–214
- 24 R.A. Sherman; Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy; Diabetes Care, 26 (2003), pp. 446–451
- 25 C.E. Attinger, J.E. Janis, J. Steinberg, J. Schwartz, A. Al-Attar, K. Couch; Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants; Plast Reconstr Surg, 117 (7 Suppl) (2006), pp. 72S–109S
- 26 R. Collier; New interest in maggot therapy; CMAJ, 182 (2010), pp. E121–E122
- 27 G.F.J.G. Cazander; Maggot therapy for wound healing: clinical relevance, mechanisms of action and future prospects; J Wound Tech, 5 (2009), pp. 18–23
- 28 A.G. Smith, R.A. Powis, D.I. Pritchard, S.T. Britland; Greenbottle (Lucilia sericata) larval secretions delivered from a prototype hydrogel wound dressing accelerate the closure of model wounds; Biotechnol Prog, 22 (2006), pp. 1690–1696
- 29 A. Gupta; A review of the use of maggots in wound therapy; Ann Plast Surg, 60 (2008), pp. 224–227
- 30 M.J. Cuevas, J. Tieppo, N.P. Marroni, M.J. Tunon, J. Gonzalez-Gallego; Suppression of amphiregulin/epidermal growth factor receptor signals contributes to the protective effects of quercetin in cirrhotic rats; J Nutr, 141 (2011), pp. 1299–1305
- 31 A. Turkmen, K. Graham, D.A. McGrouther; Therapeutic applications of the larvae for wound debridement; J Plast Reconstr Aesthet Surg, 63 (2010), pp. 184–188
- 32 F.L. Bowling, E.V. Salgami, A.J. Boulton; Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers; Diabetes Care, 30 (2007), pp. 370–371
- 33 J. Wayman, V. Nirojogi, A. Walker, A. Sowinski, M.A. Walker; The cost effectiveness of larval therapy in venous ulcers; J Tissue Viability, 10 (2000), pp. 91–94
- 34 D.C. Chan, D.H. Fong, J.Y. Leung, N.G. Patil, G.K. Leung; Maggot debridement therapy in chronic wound care; Hong Kong Med J, 13 (2007), pp. 382–386
- 35 B.S. Ferket, S. Spronk, E.B. Colkesen, M.G. Hunink; Systematic review of guidelines on peripheral artery disease screening; Am J Med, 125 (2012), pp. 198–208 e3
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?