Abstract
Hairballs are a common problem in cats and may result in intestinal obstruction. Dietary fibre has been recommended to stimulate hair faecal excretion. The objective of this study was to assess the influence of psyllium and different levels of total dietary fibre (6% vs. 11% vs. 15% TdF) on hair faecal excretion in short and long-haired (LH) domestic cats. Twenty-one adult cats were divided into three panels: shedding panel, short-haired (SH) panel and LH panel. Shedding panel was used to assess shedding throughout the study with a normalized brushing. In parallel, SH panel and LH panel were used to evaluate the impact of the diets on faecal hair excretion. They were fed a low-fibre diet during phase 1 (diet 6, 6.0% TdF). In phase 2, panels were fed either diet 11 (11% TdF) or diet 15 (15% TdF). In phase 3, cats returned to diet 6 before a crossover on the other diet in phase 4. Those diets were fed to cats for 14 days. Faecal hair excretion was quantified daily. Data were analysed using a generalized linear model procedure. The assessment of shedding showed that the study did not occur during moulting season. In the LH panel, diet 11 and diet 15 increased (P < 0.001) faecal hair excretion by 81% and 113% respectively, compared to the control diet. In the SH panel, no influence of the diets was observed. This study supports that fibre affects faecal hair excretion in LH cats, suggesting that a diet with psyllium and 11% or 15% TdF might minimize hairball formation in 14 days and between shedding periods. In SH cats, 11% and 15% fibre levels had no effect on faecal hair excretion. This could be explained by the low quantity of hair ingested outside the shedding season or by the short duration of the study.
Introduction
A cat can spend 25% to 30% (Benjamin 1976; Panaman 1981) of its time on grooming. Grooming time may be greater than this for cats with limited outdoor access. Grooming behaviour may serve several functions for cats including cooling by coat moistening, removal of shed fur and ectoparasites and stress reduction (Willemse et al. 1994; Eckstein & Hart 2000a,b). Since the cats tongue is covered with keratinous papilla, a large amount of hair is ingested while licking. On average, a short-haired (SH) cat loses approximately 28 g of hair per kg of body weight per year, with two-thirds of this loss being ingested and found in excrement (Hendriks et al. 1998). Usually ingested hair is eliminated in faeces however large quantities can accumulate in the digestive tract forming trichobezoars (or hairballs) that can cause digestive problems.
There is no previously published data regarding the incidence of hairballs in pet cats. An epidemiological survey of a large number of cats is necessary to shed more light on the topic. With this aim, a survey of cat owners was recently conducted in order to estimate how common it is for domestic cats to bring up hairballs (Cannon 2013). The survey, based on 48 SH cats, suggested that there was an overall incidence of hairball vomiting of approximately 10% in healthy SH cats. Vomiting, abdominal pain and constipation are the most frequent clinical signs, but hairballs can also result in severe consequences such as anorexia or intestinal obstruction, leading to surgery. This phenomenon is particularly noticeable in long-haired (LH) cats living indoors and during moulting (spring and autumn) but any cat may be affected.
For cats that regularly or intermittently suffer from hairballs, preventive treatment is indicated. Common suggestions include regular brushings (Cannon 2013), the use of petroleum-based laxatives or lubricants (Ryan & Wolfer 1978; Barrs et al. 1999; Agnello & Kantrowitz 2003) or specific dry products (Dann et al. 2004; Beynen et al. 2011).
Diets formulated to reduce the incidence of hairballs generally contain increased levels of soluble and/or insoluble fibre with the aim of increasing the passage of hair through the gastrointestinal tract and in faeces without causing obstruction or constipation, as well as minimizing formation of hairballs in the digestive tract.
A recent study reported that a diet enriched with insoluble fibre (cellulose) reduced the severity of clinical signs in cats with existing hairballs (Beynen et al. 2011). A chew enriched in slippery elm and psyllium husk, a gel-forming and soluble fibre source, has also been shown to reduce the severity of hairball clinical signs in cats by delaying the gastric content (Dann et al. 2004). However, the efficacy of these diets has been evaluated from questionnaires or surveys and hairball elimination was subjectively assessed by cat owners. There are no published studies measuring objectively the efficacy of hairball products on faecal hair excretion in cats. This could be due to the difficulty of precisely quantifying faecal hair excretion.
The aim of this study was therefore to assess the impact of psyllium husk and the influence of different levels of total dietary fibre (6% vs. 11% vs. 15% TdF) on faecal hair excretion in short and LH cats by directly measuring hair in their faeces.
Materials and methods
The present study was performed in summer (from July to September).
Animals
Twenty-one healthy adult research cats (4.8 ± 1.1 kg) participated in the study. One panel (n = 7) was used to assess fur shedding throughout the study: this panel (called ‘shedding panel’) was composed of three LH cats (two Birmans and one Maine coon) and four SH cats (four European domestic SH cats). Two collective panels (n = 7) were used to evaluate the impact of the diets on faecal hair excretion: a SH panel composed of six Chartreux and one British Short Hair, and an LH panel composed of five European domestic LH cats, one Ragdoll and one Birman.
Ten cats were female and 11 cats were male, and were matched across groups: three females and four males were used in SH and shedding panels and four females and three males in LH panel. All cats were neutered and the median age was 4 years (range 2–11 years). All cats remained healthy throughout the study, based on physical examination. They were treated monthly with a topical flea – tick product (Frontline® Plus; Merial, Dultuh, GA, USA). They were housed in closed indoor (13 m2) and outdoor (14 m2) runs with unlimited outdoor access. Every morning, the cattery was cleaned. In order to prevent cats from being disturbed by external noises, music was played inside the cattery during the day. Temperature remained constant at 22–23°C in the cattery and cats were exposed to normal day length and natural lighting throughout the study.
Housing and management protocols adhered to European regulatory guidelines for animal welfare, whilst all experimental protocols complied with European Union guidelines on animal welfare and were approved by the Royal Canin Committee for Animal Ethics and Welfare.
Diet and food intake
Three experimental dry, expanded, complete and balanced maintenance diets designed for adult cats were used in the present study (Table 1). The diets varied in TdF and psyllium contents. The control diet (diet 6) contained a low-fibre level (6% TdF) and no psyllium. The first test diet (diet 11) presented moderate fibre content (11% TdF) and 0.5% psyllium husk. The second test diet (diet 15) contained a high fibre level (15% TdF) and 0.5% psyllium husk. The increase in TdF was obtained by supplementation of cellulose: 0% for diet 6, 5.17% for diet 11 and 9.68% for diet 15. During the study, all cats were fed ad libitum and the diet was given once a day, in the morning. All food consumptions were measured out using electronic scales (XS4001S; Mettler Toledo, Columbus OH, USA) accurate to within 0.1 g.
| Units | Diet 6 | Diet 11 | Diet 15 | |
|---|---|---|---|---|
| Dry matter | g 100 g−1* | 94.2 | 94.7 | 94.4 |
| Crude protein | g 100 g−1 | 33.8 | 34.7 | 34.8 |
| Fat | g 100 g−1 | 14.4 | 14.1 | 13.6 |
| Ash | g 100 g−1 | 7.3 | 7.3 | 7.3 |
| Crude fibre | g 100 g−1 | 1.2 | 4.6 | 7.2 |
| TdF | g 100 g−1 | 6.0 | 11.2 | 14.9 |
| Nitrogen free extract | g 100 g−1 | 43.2 | 39.3 | 37.1 |
| Metabolizable energy† | kJ kg−1 | 16 565 | 15 963 | 15 336 |
TdF, total dietary fibre. Ingredients: dehydrated poultry protein, maize, vegetable protein isolate, rice, wheat, cellulose (only for diet 11 and 15), animal fats, hydrolysed animal proteins, soya oil, beet pulp, minerals, fish oil, yeasts, fructo-oligosaccharides, psyllium (Plantago ovata) husks (>95%) and seeds (<5%) (only for diet 11 and 15), borage oil. *g 100 g−1 = grams per 100 g diet on an as fed basis; †Metabolizable energy is calculated with NRC 2006. NRC = National Research Council. | ||||
Protocol design
Study #1: assessment of shedding throughout the study in the shedding panel
Three times a week, each cat of the shedding panel received 24 brush strokes: six on the back, four on the neck, the head and the breast and two on the tail and the hind legs. For each cat, the hair collected was weighed carefully to quantify shedding over the study (a total of 8 weeks divided into four 14 day long phases designated phases 1–4).
Study #2: impact of the diets on faecal hair excretion in SH and LH cats
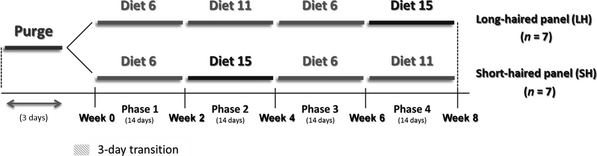
One week before starting the study, SH and LH panels underwent a purge for 3 days to eliminate accumulated hair in the digestive tract (Savorial, MSD Santé animale, Beaucouzé, France). Then they were fed diet 6 for 14 days during phase 1. In phase 2 (14 days), both panels were fed either diet 11 or diet 15. In phase 3, all cats returned to diet 6 for 14 days before crossing over to the other experimental diet (diet 15 or diet 11) in phase 4 (14 days). In order to avoid digestive disorders, 3 days of gradual food transition were made at the beginning of each phase (Fig. 1).
|
|
|
Figure 1. Study design 2: impact of the diets on faecal hair excretion. |
Faecal hair excretion was quantified daily for each collective panel fed each diet according to the modified method of Hendriks et al. (1998) and Tournier et al. (2005). All faeces excreted were collected every morning, pooled and stored at −20°C. Pooled faeces were then dried in an oven (70°C, 24 h) then ground carefully in a blender (GM200; Retsch Grindomix, Haan, Germany) for 12 s at medium speed. Through a sieve (1 mm), hair was then collected and washed using a solution of 20 mL of detergent (IDOS 2000; Elcopharma, Villeneuve s/Yonne, France), and 2 L of boiling water for 30 min. A second stage of sieving and washing (15 min) was then performed. After a third sieving process, hair samples were isolated by vacuum filtration, and then hair moisture was evaporated in an oven (110°C, 15 h). The hair sample obtained was finally weighed by a precision balance (XP504 DR; Mettler Toledo, OH, USA).
Statistical analysis
Statistical analyses were performed with the SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA). Results were expressed as mean ± SEM.
Study #1
A mixed model was performed to evaluate the impact of the following fixed effects: hair length (long or short), phase (from 1–4) and the interaction hair length × phase on the quantity of hair brushed. The cat was defined as a random term considering that each cat was its own control through weekly measurements. False discovery rate method was used to avoid alpha risk inflation due to multiple comparisons. In this respect, P-values were adjusted to compare phase within hair length and hair length within phase.
Study #2
A generalized linear model was used to assess the effects of panel (SH/LH), diet (diet 6/diet 11/diet 15) and the interaction panel × diet on faecal hair excretion and energy consumption. For faecal hair excretion, food intake was added as covariate to this two-way analysis of variance.
Residuals of each model were checked for fit with normal distribution according to Kolmogorov–Smirnov, Cramer-von Mises and Anderson-Darling tests. Results are expressed as least square means ± SEM. Differences in Least-squares (LS) means were assessed using the Scheffe test to correct for the multiple comparisons. Significance level was set at 5%.
Results
Study #1
The quantity of hair brushed in the shedding panel was impacted by hair length (P = 0.013) but not by phase (P = 0.409). Significant interaction was found (hair length × phase: P < 0.001). The quantities of hair collected were higher in LH cats than in SH cats in the second phase (519.3 ± 20.7 mg vs. 311.2 ± 30.2 mg, P = 0.0155), the third phase (552.5 ± 23.0 mg vs. 312.6 ± 33.4 mg, P = 0.0055) and the fourth phase (552.9 ± 23.5 mg vs. 316.7 ± 31.6 mg, P = 0.006). This effect was not significant during the first phase (338.2 ± 17.8 mg vs. 484.1 ± 65.2 mg, P = 0.088). No significant individual effect was observed on the quantity of hair brushed (P = 0.061).
Study #2
Energy consumption
Energy consumption results expressed in kJ day−1 are shown in Table 2. Energy consumption was influenced by diet and panel effects (diet effect: P < 0.001; panel effect P < 0.001; panel × diet: P < 0.001). Indeed diet effect depended on the panel considered. For LH panel, energy consumption did not vary between the different diets. For SH panel, no difference was found in energy intake during the phases 2, 3 and 4. However, significantly higher energy consumption was observed when cats were fed diet 6 in phase 1 (P < 0.001).
| LH panel | Diet 6 | Diet 11 | Diet 6 | Diet 15 |
|---|---|---|---|---|
| Energy consumption (kJ day−1) | 812.2 ± 91.3 | 824.4 ± 17.6 | 862.9 ± 15.1 | 826.5 ± 14.7 |
| Faecal hair excretion (mg day−1) | 1512 ± 185a | 2735 ± 254b | 1610 ± 177a | 3435 ± 164b |
| SH panel | Diet 6 | Diet 15 | Diet 6 | Diet 11 |
|---|---|---|---|---|
| Energy consumption (kJ day−1) | 1062.6 ± 18.0a | 916.5 ± 25.1b | 897.2 ± 13.4b | 903.5 ± 18.8b |
| Faecal hair excretion (mg day−1) | 1627 ± 156 | 1631 ± 105 | 1084 ± 107 | 1431 ± 109 |
| LH, long-haired; SH, short-haired. Different superscript letters (a, b) in the same line are significantly different (P < 0.05). | ||||
Faecal hair excretion
On the day after the 3 days of purge, 2.45 and 6.31 g of hair were recovered in faeces of SH and LH panels respectively.
Faecal hair excretion data for LH and SH cats fed different diets are given in Table 2. Faecal hair excretion was influenced by diet only in LH panel (diet effect: P < 0.001; panel effect P < 0.001; panel × diet: P < 0.001). Indeed in LH cats, faecal hair excretion significantly increased by 81% in diet 11 and by 113% in diet 15 compared to diet 6 (P < 0.001). The cumulative amounts of hair excreted during the 14-day period were 40.5 and 48.1 g when LH panels were fed diet 11 and diet 15 respectively but only 22 g when fed diet 6. No significant differences in faecal hair excretion were observed between phases 1 and 3 when the cats were fed diet 6, and between diet 11 and 15 fed in phases 2 and 4. In SH cats, no difference was observed in faecal hair excretion when fed diet 6, 11 or 15.
Discussion
The objective of the present study was to assess the influence of psyllium and different levels of TdF (6% vs. 11% vs. 15%) on faecal hair excretion in short and LH cats.
The predisposition of cats to forming hairballs following hair ingestion could be due to their digestive physiological particularities. For example, it has been reported that cats lack interdigestive migrating myoelectric complexes (IMMCs). In other species, these motor complexes begin in the stomach during periods of fasting and result in powerful peristaltic waves which facilitate transportation of indigestible substances such as bone, fibre and hair from the stomach through the small intestine (De Vos 1993). In cats, activity resembling IMMCs has only been identified in the distal small intestine (Roche et al. 1982). This slow peristalsis in cats could thus explain both the accumulation of hair in their digestive tract and their slow total gastrointestinal transit time (around 35–40 h) (Fucci et al. 1995; Peachey et al. 2000) compared to small breed dogs (24 h) (Hernot et al. 2005).
Very few studies have been conducted to evaluate the efficacy of diets on the incidence of hairballs in cats (Cannon 2013; German & German 2013). This is likely due to the difficulty of precisely quantifying hairballs. Among existing studies, most used questionnaires or surveys and the frequency of hairball vomiting (Cannon 2013) or clinical signs (vomiting, retching and coughing) (Dann et al. 2004; Beynen et al. 2011) were evaluated by cat owners, making the results quite subjective.
In the present study, hair was quantified directly in faeces. Faecal hair excretion was measured daily according to the modified method of Hendriks et al. (1998) and Tournier et al. (2005). Two washing and three sieving steps were needed to separate hair from faeces. Although this method was time consuming, it gave accurate and repeatable measurements (data not shown).
At the beginning of this study, a purge was performed for 3 days in SH and LH cats to eliminate hair accumulated in the digestive tract. Although these cats did not show any clinical signs of hairballs, large quantities of hair were recovered in their faeces within the few days after the purge. As expected, higher faecal hair elimination was observed in LH cats than in SH cats (2.5 times more). Hair accumulated in the digestive tract prior to commencing the study could have altered the results.
Shedding is a natural phenomenon which can have a strong impact on faecal hair excretion. It was therefore assessed throughout the study with a normalized brushing three times a week on all shedding panel cats. The quantity of hair brushed in all cats was not significantly different between 2 phases, indicating that the present study did not occur during a shedding season. The quantity of hair recovered per cat and per brush stroke (63 mg on average) was extremely close to that obtained by Tournier et al. (2005) outside the shedding season. This previous study showed that semi-long-haired cats lost on average 70 mg hair per brush stroke and that this quantity increased up to 140 mg during the shedding season.
The shedding panel lived in the same environment as SH and LH cats. It is therefore unlikely that the variations of faecal hair excretion observed in these cats were the consequence of a change in hair losses during the study. Because this study was performed during the summer (from July to September), the shedding period was probably finished.
Different factors such as gender, flea burdens or breed could have affected grooming behaviour and thus impacted on the faecal hair excretion results. To our knowledge, no data are available on the effect of gender on grooming behaviour in cats. However, balanced panels in terms of male and female cats were used in the present study to minimize this possible effect. Flea exposure can strongly increase grooming rate in cats (Eckstein & Hart 2000b). A flea treatment was thus used monthly in all cats and regular observations of hair and skin were made to detect any infestation.
Several pedigree cat breeds were used in SH and LH panels, which may have had an impact on the results. Indeed hair coat length and density, as well as the relative proportions of guard and undercoat hairs may vary with breed and this may have had an impact on the hair excretion. However, the majority of cats present in each panel (n = 7/panel) were of the same breed (six Chartreux in SH panel and five European domestic LH cats in LH panel), thereby limiting this potential effect.
Because food intake affects the quantity of fibre ingested, it was important to ensure that both panels consumed similarly diet 6, 11 and 15. In this study, cats surprisingly ingested similar quantities of energy, despite ad libitum access to food and differences in food composition. This supports previous studies demonstrating that cats regulate their energy intake when fed foods containing moderate fibre content (9% cellulose; 8.2% crude fibre) (Prola et al. 2006).
Higher energy consumption was only observed in SH cats when fed diet 6 in phase 1. Although not statistically significant, the higher faecal hair excretion observed in SH cats fed diet 6 in phase 1 (1627 ± 156 mg) compared with phase 3 (1084 ± 107 mg) could be explained by this larger consumption (+18%; P < 0.001).
The impact of fibre level on faecal hair excretion varied according to hair coat length. In LH panel, consumption of diets 11 and 15 induced significantly higher faecal hair excretion, compared to diet 6 (P < 0.001), indicating that the combination of psyllium and cellulose facilitate faecal hair elimination in those cats. This supports previous studies based on cat owner questionnaires showing that the supplementation of cellulose in a diet or of psyllium husks in a treat lowered the incidence of clinical signs of hairballs (Dann et al. 2004; Beynen et al. 2011).
The mechanism by which the ingestion of cellulose and psyllium depresses the clinical signs of hairballs and stimulates their excretion in faeces is not known. Psyllium husk is a source of gel-forming soluble fibre which has the property of increasing viscosity of the digestive content. Psyllium husk could thus promote the binding of hair to food particles and thereby increase the transfer of gastric hair into the duodenum and enhanced passage through the intestine. Cellulose is a dietary insoluble fibre source which enhances the intestinal transit rate of digesta (Burrows et al. 1982) and this may promote the excretion of hair in faeces.
The present study cannot determine which of the two fibre sources (psyllium or cellulose) presents the greatest capacity to stimulate hair excretion in cat faeces. The inclusion of a diet containing 6% fibre and 0.5% psyllium or diets with 11% or 15% fibre without psyllium would have been necessary and their lack is a limitation of this study.
Similar low faecal hair excretions were observed when diet 6 was fed in phases 1 and 3 in LH cats, confirming the weak effect of a low-fibre diet on hair transit. No significant difference was found between diet 11 and 15, suggesting that an 11% fibre level would be sufficient to stimulate faecal hair excretion or that the study was too short to observe significantly higher hair excretion in cats fed diet 15.
In SH cats, similar faecal hair excretions were observed when fed diet 6, 11 and 15. This lack of effect could be explained by insufficient ingestion of hair in this population outside the shedding season. Indeed the quantities of hair excreted by SH animals fed diets 11 and 15 were respectively 40 and 60% lower than those found in LH cats fed the same diets. Moreover, when fed diet 15, the highest daily faecal hair excretion observed was equivalent to the lowest faecal hair excretion in LH cats. The exposure to the diets (14 days) might also have been too short to obtain significant results.
Conclusions
The results of this study showed that dietary fibre significantly affects faecal hair excretion in LH cats over only 14 days, and outside shedding periods. Results obtained suggest that a diet containing psyllium husk and 11% or 15% TdF facilitates faecal excretion of hair in LH cats. Clinical studies are however required to determine whether such psyllium and TdF levels result in a significant reduction in hairball related clinical signs in cats.
In SH cats, 11% and 15% fibre levels combined with psyllium have no effect on faecal hair excretion. This could be explained by the low quantity of hair ingested by those animals outside the shedding season, by the relatively short duration of the study or by the small number of cats included in the study.
Therefore, future studies would be valuable to evaluate the effect of these diets during the shedding season in both short and LH cats, over a longer period of time and on a larger number of cats.
Acknowledgement
The authors thank Sophie Clément for assistance in writing this manuscript.
Source of funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
All authors were employees of Royal Canin at the time of writing the manuscript.
Contributions
All authors were involved in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript and in the decision to sub-mit the manuscript for publication.
References
- Agnello K.A. & Kantrowitz L. (2003) Esophageal obstruction. Journal of the American Veterinary Medical Association15, 1115–1116.
- Barrs V.R., Beatty J.A., Tisdall P.L., Hunt G.B. & Gunew M. (1999) Intestinal obstruction by trichobezoars in five cats. Journal of Feline Medicine and Surgery1, 199–207.
- Benjamin L. (1976) Feline behavior. Feline Practice7, 14–17.
- Beynen A.C., Middelkoop J. & Saris D.H.J. (2011) Clinical signs of hairballs in cats fed a diet enriched with cellulose. American Journal of Animal and Veterinary Sciences6, 69–72.
- Burrows C.F., Kronfeld D.S., Banta C.A. & Merritt A.M. (1982) Effects of fibre on digestibility and transit time in dogs. Journal of Nutrition112, 1726–1732.
- Cannon M. (2013) Hair balls in cats, a normal nuisance or a sign that something is wrong. Journal of Feline Medicine and Surgery15, 21–29.
- Dann J.R., Adler M.A., Duffy K.L. & Giffard C.J. (2004) A potential nutritional prophylactic for the reduction of feline hairball symptoms. Journal of Nutrition134, 2124S–2125S.
- De Vos W.C. (1993) Migrating spike complex in the small intestine of the fasting cat. American Journal of Physiology265, G619–G627.
- Eckstein R.A. & Hart B.L. (2000a) The organization and control of grooming in cats. Applied Animal Behaviour Science68, 131–140.
- Eckstein R.A. & Hart B.L. (2000b) Grooming and control of fleas in cats. Applied Animal Behaviour Science68, 141–150.
- Fucci V., Pechman R.D., Hedlund C.S. & Venugopalan C.S. (1995) Large bowel transit times using radiopaque markers in normal cats. Journal of the American Animal Hospital Association31, 473–477.
- German A.J. & German A.C. (2013) Bad hair day. Journal of Feline Medicine and Surgery15, 6–7.
- Hendriks W.H., Tartellin M.F. & Moughan P.J. (1998) Seasonal hair loss in adult domestic cats (Felis catus). Journal of Animal Physiology and Animal Nutrition79, 92–101.
- Hernot D., Biourge V., Martin L., Dumon H. & Nguyen P. (2005) Relationship between total transit time and faecal quality in adult dogs differing in body size. Journal of Animal Physiology and Animal Nutrition89, 189–193.
- Panaman R. (1981) Behavior and ecology of free-ranging female farm cats (Felis catus L). Zeitschrift für Tierpsychologie56, 59–73.
- Peachey S.E., Dawson J.M. & Harper E.J. (2000) Gastrointestinal transit times in young and old cats. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology126, 85–90.
- Prola L., Dobenecker B. & Kienzle E. (2006) Interaction between dietary cellulose content and food intake in cats. Journal of Nutrition136, 1988S–1990S.
- Roche M., Bueno L., Vagne M. & Blourde C. (1982) Patterns of electrical activity in the digestive tract of the conscious cat. British Journal of Nutrition48, 129–135.
- Ryan C.P. & Wolfer J.J. (1978) Recurrent trichophytobezoar in a cat. Veterinary Medicine Small Animal Clinician73, 891–893.
- Tournier C., Dumon H., Nguyen P., Biourge V. (2005) Dietary fiber stimulates fecal hair excretion in cats. Proceeding of the 9th European Society of Veterinary and Comparative Nutrition Congress112, 27–28.
- Willemse T., Mudde M., Josephy M. & Spruijt B.M. (1994) The effect of haloperidol and naloxone on excessive grooming behavior of cats. European Neuropsychopharmacology4, 39–45.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?