Summary
Objectives
Idiopathic granulomatous mastitis (IGM) is a benign rare inflammatory pseudotumor. Bilateral involvement of IGM has been reported in a few cases. To our knowledge, this study is the largest series of bilateral cases to date. The goals of this study were to present clinical features of bilateral IGM and to evaluate the results of treatments.
Materials and methods
We performed a retrospective review of the idiopathic granulomatous mastitis database from 2010 to 2013. Ten female patients who met required histologic and clinical criteria of IGM in both breasts were included in study. Demographic data, clinical findings, medication history, and radiologic findings are presented.
Results
The mean age at onset of the disease was 38.4 ± 8.3 years (range: 29–52 years). Nine patients had no recurrence during a mean follow-up period of 21 months (range: 11–26 months). Additionally, the median time to second breast involvement was 15.6 months.
Conclusion
Bilateral IGMs have a higher rate of more relapse and greater resistance to medical therapies than do unilateral IGMs. Surgical management should be avoided unless all medical treatment options have been exhausted. Nevertheless, expectant management seems a rational option for the treatment of bilateral IGM.
Keywords
breast cancer;cancer;corticosteroids;granulomatous mastitis;idiopathic;tuberculosis
1. Introduction
Idiopathic granulomatous mastitis (IGM), also known as idiopathic granulomatous lobulitis, is an uncommon, benign inflammatory pseudotumor that was first reported by Kessler and Wolloch in 19721. Clinically, IGM presents as a palpable hard lump with erythematous skin changes or lymph node involvement, which may lead to nipple discharge or retraction, sinus formation, breast atrophy, and “apple jelly” scars2; 3; 4; 5; 6; 7 ; 8. IGM is usually unilateral and can be seen in all quadrant regions, except for the subareolar area9 ; 10.
Few cases of bilateral involvement have been reported in the literature11; 12 ; 13. To our knowledge, this study, which includes 10 bilateral IGM cases, is the largest series of bilateral cases to date. The goals of this study were to present the clinical features of bilateral IGM and to evaluate the results of treatment.
2. Materials and methods
Between 2010 and 2013, 10 female patients who met the required histologic criteria of IGM in both breasts were included in this retrospective study. The patients were referred to our mastitis study group from the Outpatient Departments of the General Surgery and Infectious Diseases Clinics. Our study group included three infectious disease physicians, two radiologists, two pathologists, and two breast surgeons. The diagnosis was confirmed by core biopsy of the suspicious breast lesions and biopsy specimens taken from the abscess wall. The pathological criteria for the diagnosis of IGM were the presence of the characteristic histopathological features of a noncaseating granulomatous inflammation centered on breast lobules, in the absence of any evidence of specific underlying causes. The presence of epithelioid histiocytes, lymphocytes, plasma cells, polymorphonuclear leukocytes, and multinucleated Langhans-type giant cells without caseous necrosis were demonstrated in each case. Treatment outcomes were evaluated by physical examination and ultrasonography (US). The clinical data in terms of the presentation, histopathology, management, recurrence, discharge from the clinic, and follow-up at monthly intervals were analyzed by a review of the medical records. The Ethics Committee of Istanbul University Cerrahpasa Medical School, Istanbul, Turkey approved the study. The document number is 83045809/604/02-15545/Date: June 5, 2014.
3. Results
3.1. Demographic data and clinical findings
Table 1 provides the clinical characteristics of the patients. The mean age at onset of the disease was 38.4 ± 8.3 years (range: 29–52 years). The patients had no history of breast carcinoma and no family history of granulomatous mastitis. All but two patients were of reproductive age, and all were parous. The parity of the patients ranged from two to five, with a mean parity of three. None of the patients had a recent history of pregnancy at the time of the presentation; the shortest and longest intervals after childbirth were 1 year and 10 years, respectively. None of the patients were breast-feeding. None of the patients had a history of tuberculosis or systemic granulomatosis, but three had a family history of tuberculosis.
| Patients characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 52 | 51 | 30 | 33 | 34 | 40 | 43 | 32 | 29 | 40 |
| Parity | 3 | 4 | 4 | 2 | 2 | 4 | 5 | 2 | 3 | 3 |
| Past lactation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Oral contraceptive use | No | No | No | No | No | No | For 45 d 15 y ago | No | No | No |
| Tobacco smoking | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No |
| Trauma history to the breast | No | No | No | No | No | No | No | No | No | No |
| Medical history | DM | Hashimotos thyroiditis | No | No | No | No | No | No | No | No |
| Family history in relation to TB | No | No | Yes | No | Yes | No | Yes | No | No | No |
| First admission to us | No | No | No | No | No | No | No | No | No | No |
| Presentation time | 8 y after childbirth | 6 y after childbirth | 5 y after childbirth | 2.5 y after childbirth | 5 y after childbirth | 1 y after childbirth | Over 2 y after childbirth | 10 y after childbirth | 4 y after childbirth | 6 y after childbirth |
| Total duration of breast-feeding (mo) | 36 | 17 | 60 | 7 | Over 12 mos | 48 | 120 | 12 | 27 | 60 |
| Initial complaint | A hard mass | A hard mass and redness of the skin | A hard mass and redness of the skin | A hard mass and redness of the skin | A hard mass and redness of the skin | A painful hard mass | A hard mass and redness of the skin | A painful hard mass | A hard mass and redness of the skin | A hard mass and redness of the skin |
| Time from onset of symptoms to diagnosis (mo) | 6 | 24 | 14 | 12 | 15 | 14 | 30 | 12 | 4 | 9 |
| First affected breast side | Right | Right | Left | Right | Left | Left | Left | Right | Left | Left |
| Duration for the second breast involvement (mo) | 6 | 12 | 12 | 3 | 15 | 8 | 48 | 24 | 4 | 24 |
| Size and location of mass in right breast | 2 cm, Upper inner quadrant | 2.5 cm, Upper inner quadrant | 3 cm, lower inner quadrant | 4 cm, Upper inner quadrant | 6 mm, Upper outer quadrant | 1 cm, Upper outer quadrant | 4 cm, Upper outer quadrant | 3 cm, Upper inner quadrant | 1 cm, Upper outer quadrant | |
| Size and location of mass in left breast | 2.5 cm, Upper inner quadrant | 2 cm, Upper outer quadrant | 3 cm upper quadrant close to aerola | 4 cm, Upper outer quadrant | 3 cm, Upper inner quadrant | 2 cm, Upper outer quadrant | No data (left lesion 4 y ago) | 3 cm, Upper inner quadrant | 3 cm, Upper outer quadrant | No data(left lesion 2.5 y ago) |
| Core needle biopsy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Tuberculin skin test (mm) | 20 | Anergic | 28 | 9 | 17 | 8 | 20 | 8 | 9 | Not performed |
| Acid fast stain | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Rose Bengal | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Radiologic tool | US | US | US | US | US | US | US | US | US | US |
| Abscess drainage | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Antibiotics | Amoxicillin-clavulanate | Amoxicillin-clavulanate | Ceftidoren pivoxil | Ceftriaxon + ornidazol | Amoxicillin-clavulanate | Amoxicillin-clavulanate | Fusidic acid | Amoxicillin-clavulanate | Amoxicillin-clavulanate | Ciprofloxacin + Metronidazole |
| Steroid therapy | No | Previously | Yes | Yes | Yes, later | Yes | No | No | Yes | No |
| Antituberculous therapy | No | Yes | No | No | Yes, initial | No | Previously | No | No | No |
| Recurrence | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Outcome | Healed | Healed | Healed | Healed | Resistant | Healed | Healed | Healed | Healed | Healed |
| Unsightly scars | Yes, bilateral | Yes, bilateral | Yes, bilateral | Yes, bilateral | Yes, in left breast | Yes, in left breast | Yes, in right breast | Yes, in right breast | Yes, in left breast | Yes, in left breast |
DM = diabetes mellitus; IGM = idiopathic granulomatous mastitis; US = ultrasonography.
a The data are not available due to histopathological sampling of outer clinic.
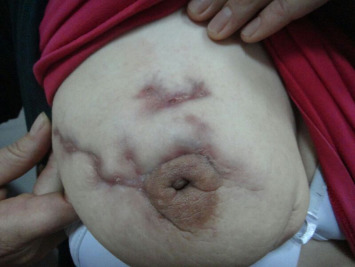
The most common presenting symptom was a hard mass, which was detected in all of the patients (n = 10; 100%). Additionally, seven presented with redness of the skin (70%), and draining sinuses (fistulas) or discharge from the breast skin (n = 7; 70%). Nine patients had no recurrence during a mean follow-up period of 21 months (range: 11–26 months). Only one patient had static disease, at a follow-up duration of 26 months ( Fig. 1).
|
|
|
Figure 1. The picture presents the deformation of the breast due to skin fistulas of IGM. IGM = idiopathic granulomatous mastitis. |
3.2. Radiologic findings
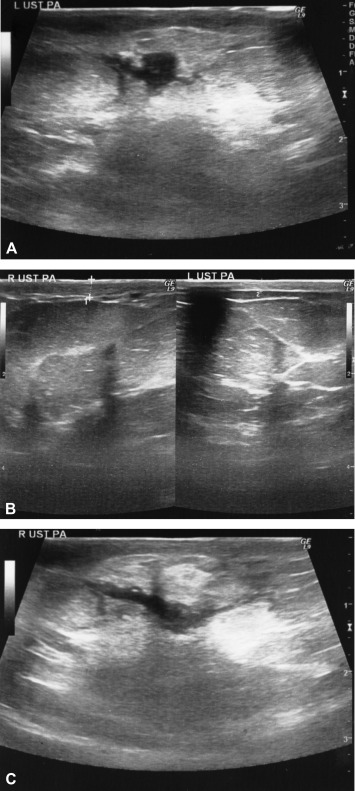
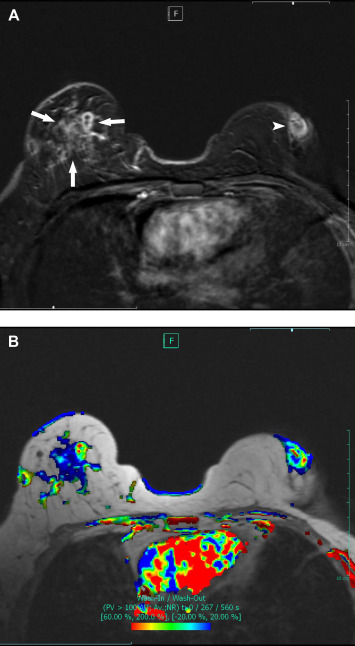
The most useful method for evaluating the radiologic appearance of IGM is sonography. The technique defines valuable data for infectious conditions such as effusions, inflammation of parenchyma and fatty tissues, abscess formations, fistula tracts. These are the key findings for discriminating the suspected lesions from malignancy. Information of the patients' history is essential to assess before evaluation. Sonography examination showed a hypoechoic collection in nine patients (90%), solid lesion in one patient (10%), thickening of the breast skin in eight patients (80%), fistula tracts in four patients (40%), and ductal fillings and postobstructive dilatation in three patients (30%). Mammographic findings were nonspecific in the three patients who underwent mammograms. Magnetic resonance imaging (MRI) evaluation may demonstrate the extension of the disease and may be used to clarify the suspected diagnosis. MRI was performed for three cases; asymmetrical enhancement with nonspecific contrast enhancement kinetics and fluid collections were common.
3.3. Medication history
All patients (100%) had previously taken antibiotics, six patients (60%) had undergone drainage before presenting at our clinic and four patients (40%) (Cases 1, 7, 8, and 10) were treated with a second course of antibiotics (Table 1).
We sent all the biopsy samples to the histopathology department. The characteristic caseating granulomas for TB was observed only in one and this sample was sent for TB culture as well. All were stained with Periodic Acid Schiff (PAS) and Ehrlich Ziehl Neelsen (EZN) to demonstrate the microorganisms within the tissue; all remained negative.
Case 1 had two recurrences and received antibiotics and meloxicam. The patient was offered steroid treatment but refused. Following cessation of medications, the patient had expectant conservative management followed by complete spontaneous recovery. Cases 8 and 10 showed complete disease resolution and no recurrence with expectant management. Case 7 had a history of antituberculous therapy and a complete recovery of IGM in her left breast, and she was referred to us for a lump affecting the right breast. No previous pathological or microbiological data regarding tuberculosis were available. Core needle biopsy was performed, and the specimen was confirmed to be granulomatous mastitis; bacterial culture was negative. This patient was managed conservatively with regular clinical and radiological surveillance. After 2 months of follow-up, the patient showed complete recovery. One month later, she received meloxicam 15 mg once per day for 1 month to treat her pain. Until 2013, none of these four patients recurred without medication. Four (40%) patients (Cases 3, 4, 6, and 9) were treated with oral prednisolone for 6 weeks at an initial dose of 0.5 mg/kg/d in divided doses (4 + 2), which were tapered slowly with clinical improvement. Of these, Case 3 developed recurrence when the prednisolone dose was tapered. As the dose was increased, the patient seemed to respond, but the medication was stopped because she became pregnant. She underwent expectant conservative management, and experienced complete spontaneous recovery. Case 4 developed recurrence 2 months after a complete recovery following cessation of steroid therapy. Nonetheless, the recurrent disease responded well to a second course of oral prednisolone and the dose was tapered slowly. However, after a short time period, she again developed recurrence. Expectant conservative management was preferred, and the patient experienced complete spontaneous recovery after 5 months. Interestingly, this patient became pregnant during this recovery period. Case 9 showed complete recovery after a course of steroid therapy. Case 6 developed recurrence following cessation of steroid therapy, but the patient showed complete recovery after a second course. Case 2 had no clinical response to steroid treatment; however, the patient showed complete remission after undergoing combined antituberculous therapy (rifampicin, ethambutol, and isoniazid) for 6 months. Case 5 had no response to antituberculosis treatment, so she received oral prednisolone. She recurred twice following cessation of steroid therapy because of intolerance, but showed a complete response thereafter. After 2 years, she experienced a second recurrence, which is at present being treated with antibiotic therapy. There was a notable delay between onset of symptoms and diagnosis, a median of 14 months in our study. Additionally, the median time to second breast involvement was 15.6 months (Figure 2, Figure 3 ; Figure 4).
|
|
|
Figure 2. Common sonographic findings of IGM (A–C). (A) The most common finding is hypoechoic collections without well defined contours; (B) skin thickening secondary to inflammation should be compared with contralateral breast; right breast skin was thickened compared with the left breast; and (C) hypoechoic fistula tracts is specific finding and should be followed due to fistula formation. IGM = idiopathic granulomatous mastitis. |
|
|
|
Figure 3. (A,B) MRI images of bilateral IGM. (A) Contrast enhanced T1 weighted reformat substraction (2nd minute) image shows wide asymmetrical nonmass enhancement in right breast and retroareolar disease in left. (B) Corresponding color overlay T1 weighted axial scan provided by the computer-aided diagnosis (CAD) system represents nonspecific contrast enhancement kinetics in both breasts. IGM = idiopathic granulomatous mastitis; MRI = magnetic resonance imaging. |
|
|
|
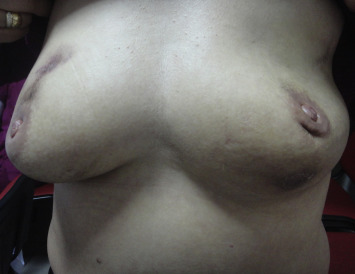
Figure 4. The picture presents the bilateral synchronous involvement in a 34-year-old woman. |
4. Discussion
IGM is a rare, nonmalignant, chronic inflammatory breast condition of unknown etiology that has received little attention in the literature11. Almost 200 cases have been reported during the past three decades, with most cases occurring in females from Mediterranean countries (Turkey and Jordan) and Asia (China, Arabia, and Malaysia)2; 3; 5; 11; 14; 15; 16; 17 ; 18. Although no ethnic predisposition has been documented, underdiagnosis of tuberculosis mastitis might be a major reason for the increased prevalence19 ; 20.
The diagnosis and treatment of IGM remains challenging. IGM can mimic breast abscess or inflammatory breast cancer in terms of physical and radiological findings. It typically presents as a unilateral palpable firm breast mass, with occasional nipple discharge, peau d'orange change, erythema, nipple inversion, breast asymmetry, draining sinuses, and scars, which are manifestations of intrinsic inflammatory or neoplastic breast disease 1; 2; 8; 16 ; 21. Abscess and fistula formation, ulceration, deformity of the breast, and unsightly scars are common complications of IGM. Although this disease is not life-threatening such as other malignancies, it seriously depresses the social life of the patient 3; 16; 17; 21; 22; 23 ; 24.
All of our patients had hard masses as the initial complaint, which were painful in only two patients. Six (60%) patients had redness of the skin. There was a tendency for local recurrence and delayed wound healing. All of our patients had at least one recurrence, and most experienced inadequate or no response to various treatment modalities.
IGM is seen mostly in females of childbearing age with a history of pregnancy and lactation in the previous 5–6 years9; 11 ; 25. Although the youngest and oldest females ever reported were aged 11 years and 83 years, respectively, the disease usually appears in the breast in the third or fourth decade3; 17 ; 19. However, younger patients were reported in recent trials2; 26; 27; 28; 29 ; 30. In our study, the mean age of patients at onset of the disease was 38.4 years.
There was a notable delay between onset of symptoms and diagnosis, a median of 14 months in our study. As IGM is rare, this disease is not routinely considered as a differential diagnosis of a simple breast abscess. Surgical drainage or US-guided aspiration is commonly preferred in practice in place of core-needle biopsy or incisional biopsy from the abscess wall, which may explain the prolonged time to diagnosis. The median time to second breast involvement was 15.6 months. No response to antibiotics was observed in three of the 10 unilateral mastitis cases before the second breast was involved. Two patients showed no response to antibiotics and steroid therapy before bilateral involvement. One patient showed a partial response to antibiotics, but bilateral involvement occurred thereafter. Three patients showed a complete response (1 responded to antibiotics, 1 to antibiotics and steroids, and 1 to antibiotics and abscess drainage), but they later developed bilateral involvement. Only one patient showed complete disease resolution after antituberculosis treatment; unfortunately, the other breast showed involvement 48 months later. We concluded that bilateral IGMs were resistant to medical treatment, and tended to relapse and recur.
All of our patients had a history of pregnancy, were parous, and had breast-fed. No patient experienced symptom-onset during lactation. These results are in accordance with previous reports that pregnancy and breastfeeding conditions are associated with an increased risk of IGM15; 26; 31; 32; 33 ; 34. However, Azlina et al18 and Fletcher et al35 found no association with lactation. In Cases 3 and 4 in our series, the medication was stopped because the patients became pregnant. These patients had expectant conservative management, and experienced complete spontaneous recovery. It is unclear whether being pregnant or receiving expectant management therapy contributed to their recovery. Kaur et al36 reported that prolonged breast-feeding might cause long-term distension of acini and ducts, causing them to rupture. They suggested that this factor might cause a granulomatous response. Baslaim et al2 opposed this idea; they reported that patients, especially nursing mothers who had breast-fed from one breast only, developed the disease in the contralateral breast.
The etiology of IGM is unclear; however, many factors, such as local irritants, oral contraceptives, nicotine addiction, and breast trauma, have been considered2; 3; 9; 15; 16; 30; 37 ; 38. No patients included in the studies by Ozel et al30, Azlina et al18, or Neel et al39 used oral contraceptive hormones. Only one of our patients used oral contraceptives for a short period 15 years ago. The patients in the study by Azlina et al18 were nonsmokers. In addition, the patients had no history of local trauma to the breast in the Ozel et al30 study. In our study, six patients were smokers, but none reported a history of local trauma. An association between periductal mastitis (but not IGM) and nicotine addiction has been established, as seen in more than half of our patients (60%); moreover, smoking may delay recovery and extend IGM disease involvement to the second breast.
Although unilateral IGM is typical in previous reports, bilateral involvement has been included in only a maximum of two patients in these studies4; 12 ; 30. Bilateral involvement has been reported at a lower frequency, and we found no reports of a bilateral IGM series. Some authors reported more common involvement of the right breast16; 19 ; 35, whereas others reported left or equal involvement30 ; 39. In our study, the left breast was the first affected in six of 10 patients.
For an accurate diagnosis, IGM must be differentiated from other disorders such as tuberculosis, sarcoidosis, foreign body reaction, ductal ectasia, mycotic infection, Wegeners granuloma, and histoplasmosis40. Diagnostic tests for tuberculosis include the purified protein derivative (PPD) skin test, in vitro interferon-gamma assay and polymerase chain reaction. Ziehl-Neelsen (ZN) stain PPD and routine histology studies are sometimes insufficient to exclude tuberculosis mastitis 16; 41 ; 42. In our study, periodic acid-Schiff (PAS) and ZN acid-fast stains failed to identify specific causative organisms in any of the 10 patients. The PPD skin test was not performed in one patient. Four patients had positive PPD skin test results (> 10 mm indurations). Case 2 was anergic for PPD, and she did not respond to steroid treatment; therefore, she commenced combined antituberculous therapy (rifampicin, ethambutol, and isoniazid) for 6 months and showed complete remission. Further differential diagnoses were negative, including a chest X-ray, the Rose Bengal test, and the antidsDNA test.
US, mammography, and MRI have limited roles in distinguishing granulomatous mastitis from malignancy. Mammographic examination may show a focal asymmetrical density in dense breast parenchyma or a mass with a recognizable margin in fatty breasts. US has been used to describe the disease as inhomogeneous hypoechogenicity with internal hypoechoic tubular lesions4; 10; 19; 25; 43; 44 ; 45. It is possible to discriminate benign neoplastic lesions from cancer using dynamic contrast-enhanced MRI; however, this method has lower specificity for distinguishing granulomatous mastitis from breast cancer. Therefore, MRI may support US and mammography findings in distinguishing benign inflammatory lesions from malignant lesions5; 45; 46 ; 47. MRI aids the surgical approach by delineating the extent of the disease, as edema of the breast skin and tissue prevent precise evaluation by US12. In our study, US revealed loculated fluid collections of various sizes and ill-defined hypoechoic masses in three cases. Two patients underwent breast MRI previously in other centers. In one case, ductal carcinoma in situ was suspected based on MRI; however, US-guided biopsy proved the diagnosis to be IGM. Consequently, there are no IGM-specific radiological findings. US and mammography are used to rule out breast cancer in centers that have experienced numerous cases of IGM. Diagnosis is confirmed on tissue sampling biopsy by revealing noncaseating granuloma with epithelioid histiocytes, giant cells, plasma cells, and eosinophils confined within breast lobules 48.
No clear consensus exists in the literature regarding the optimal treatment approach for IGM, as none seems to be ideal. Treatment options include antibiotics, steroids, methotrexate (MTX), azathioprine, drainage of the wound, wide surgical resection, mastectomy, and expectant management with close follow-up49; 50; 51; 52; 53 ; 54. Aggressive surgery, such as wide resection or mastectomy, is an accepted treatment7; 15 ; 55, whereas some recommend a conservative approach17 ; 39. In addition to being therapeutic, surgical excision provides a definitive diagnosis56. Involvement of oncoplastic surgery, such as mastectomy, followed by autologous breast reconstruction and steroids are alternative treatments for severe and intractable cases33. However, surgical resection can also be associated with complications, including recurrence rates as high as 50%, poor wound healing, sinus tract or abscess formation, and disfigurement9; 15; 16; 17; 22; 33; 40; 57; 58 ; 59. None of our patients with bilateral IGM underwent surgical resection.
Regarding conservative management, steroids as a primary treatment have been shown to be beneficial for shrinking the lesion both pre- and postoperatively in persisting masses60 ; 61. DeHertogh et al60 first reported that high-dose prednisone was more effective in recurrent or refractory cases. Many clinicians recommend an initial treatment of 30–60 mg prednisone orally, which is tapered gradually over several weeks (from 1 week to 22 months)18; 19; 22; 24; 28; 40; 41; 44; 52; 53 ; 62. In our study, four (40%) cases were treated with oral prednisolone for 6 weeks at an initial dose of 0.5 mg/kg/d in divided doses (4 + 2), which were tapered slowly with clinical improvement. Because continued high doses of steroids until complete resolution may be necessary51 ; 60, the patients should be observed closely for side effects, such as glucose intolerance and Cushings syndrome, as well as weight gain, avascular necrosis, depression, and cataracts13; 20 ; 63. One should also take into account relapses following withdrawal of the therapy or tapering off the dose57 ; 60. One of our patients relapsed when the steroid dose was tapered-off. Two patients developed recurrence after a complete recovery following cessation of steroid therapy. Only one of four patients who was given prednisone showed complete recovery after a course of steroid therapy, and no relapse occurred. However, no relationship was detected between the second breast involvement and the withdrawal of the steroid therapy or tapering off the dose in our patients.
As steroids exacerbate infectious disease, all suspicious infectious etiology should be excluded before commencing steroids33 ; 64. If there is a likelihood of a superinfection because of the presence of inflammatory signs, antibiotics as an initial treatment may be offered until a definitive diagnosis is reached, otherwise antibiotics have no therapeutic value for true cases of IGM62. Our policy is to perform surgical drainage and offer antibiotics prior to starting systemic corticosteroids if the disease is complicated by abscess. Even though it was rarely investigated, immunosuppressive therapy including MTX and azathioprine has been considered an alternative option in cases of recurrence, resistance, and side effects of prednisone26; 53; 57 ; 65. We did not prefer this medication for our patients.
Complicated and resistant cases might benefit from steroids after excision19. Gurleyik et al58 recommended consecutive surgical excision of the remaining tissue after steroids and claimed that this treatment would provide better cosmesis and lower recurrence. The surgeon as well as the patient should have patience during the course of the disease because it is characterized by slow resolution and tends to gradually resolve spontaneously2. Observation without any therapy, known as expectant conservative management, is an effective treatment approach63; however, close follow-up is essential. If untreated, spontaneous regression in up to half of cases has been reported8. Thus Lai et al3 reported complete disease resolution and no recurrence in 50% of their patients with expectant management. Correlatively, six (60%) of our patients underwent expectant conservative management and showed spontaneous complete recovery, as mentioned above. In the absence of inflammatory signs in mild disease, expectant management is preferred. If the condition worsens clinically and radiologically, steroids are administered for 6–8 weeks at an initial dose of 0.5 mg/kg/d in divided doses (4 + 2), which are tapered slowly with clinical improvement. As there is a strong tendency for persistence or recurrence, surgery should be considered in untreatable cases.
For follow-up, all of the patients were invited to our mastitis study group for examination once or twice per week until the symptoms resolved. The disease course was followed by routine US at 1- and 3-month intervals. After significant wound healing, 6-month follow-up US is preferred. Finally, when the disease had resolved completely, annual examination and screening were advised.
Although the diagnosis and management of IGM remain challenging, interdisciplinary approaches to this disorder are essential. Unnecessary intervention and delays in the diagnosis and treatment can be avoided by precise radiological and pathological evaluation. In conclusion, bilateral IGMs have a higher rate of more relapse and greater resistance to medical therapies than do unilateral IGMs. Surgical management should be avoided unless all medical treatment options have been exhausted. Nevertheless, expectant management seems a rational option for the treatment of bilateral IGM. Nicotine addiction may worsen the course of the disease and lead to involvement of the second breast.
To our knowledge, this is the first review of 10 bilateral IGM cases in the literature. However, this study had limitations, including its retrospective nature and descriptive review of a limited number of cases. Although this study does not provide strong evidence of the common features of IGM, it does suggest the clinical features of bilateral IGMs.
Acknowledgments
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/dY0pZX.
References
- 1 E. Kessler, Y. Wolloch; Granulomatous mastitis: a lesion clinically simulating carcinoma; Am J Clin Pathol, 58 (1972), pp. 642–646
- 2 M.M. Baslaim, H.A. Khayat, S.A. Al-Amoudi; Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation; World J Surg, 31 (2007), pp. 1677–1681
- 3 E.C. Lai, W.C. Chan, T.K. Ma, A.P. Tang, C.S. Poon, H.T. Leong; The role of conservative treatment in idiopathic granulomatous mastitis; Breast J, 11 (2005), pp. 454–456
- 4 T. Sakurai, S. Oura, H. Tanino, et al.; A case of granulomatous mastitis mimicking breast carcinoma; Breast Cancer, 9 (2002), pp. 265–268
- 5 B. Cakir, N. Tuncbilek, H.M. Karakas, E. Unlu, F. Ozyilmaz; Granulomatous mastitis mimicking breast carcinoma; Breast J, 8 (2002), pp. 251–252
- 6 S. Kuba, J. Yamaguchi, H. Ohtani, I. Shimokawa, S. Maeda, T. Kanematsu; Vacuum-assisted biopsy and steroid therapy for granulomatous lobular mastitis: report of three cases; Surg Today, 39 (2009), pp. 695–699
- 7 L.J. Hovanessian Larsen, B. Peyvandi, N. Klipfel, E. Grant, G. Iyengar; Granulomatous lobular mastitis: imaging, diagnosis, and treatment; AJR Am J Roentgenol, 193 (2009), pp. 574–581
- 8 F.A. Pereira, A.V. Mudgil, E.S. Macias, K. Karsif; Idiopathic granulomatous lobular mastitis; Int J Dermatol, 51 (2012), pp. 142–151
- 9 S. Imoto, T. Kitaya, T. Kodama, T. Hasebe, K. Mukai; Idiopathic granulomatous mastitis: case report and review of the literature; Jpn J Clin Oncol, 27 (1997), pp. 274–277
- 10 E. Yilmaz, B. Lebe, C. Usal, P. Balci; Mammographic and sonographic findings in the diagnosis of idiopathic granulomatous mastitis; Eur Radiol, 11 (2001), pp. 2236–2240
- 11 N. Gautier, L. Lalonde, D. Tran-Thanh, et al.; Chronic granulomatous mastitis: imaging, pathology and management; Eur J Radiol, 82 (2013), pp. e165–175
- 12 C.A. Pistolese, R. Di Trapano, V. Girardi, E. Costanzo, I. Di Poce, G. Simonetti; An unusual case of bilateral granulomatous mastitis; Case Rep Radiol, 2013 (2013), p. 694697
- 13 S. Mohammed, A. Statz, J.S. Lacross, et al.; Granulomatous mastitis: a 10 year experience from a large inner city county hospital; J Surg Res, 184 (2013), pp. 299–303
- 14 C.H. Lin, C.W. Hsu, T.Y. Tsao, J. Chou; Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia; Diagn Pathol, 7 (2012), pp. 1–6
- 15 O. Asoglu, V. Ozmen, H. Karanlik, et al.; Feasibility of surgical management in patients with granulomatous mastitis; Breast J, 11 (2005), pp. 108–114
- 16 Y. Erhan, A. Veral, E. Kara, et al.; A clinicopthologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis; Breast, 9 (2000), pp. 52–56
- 17 K.E. Bani-Hani, R.J. Yaghan, I.I. Matalka, N.J. Shatnawi; Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies; Breast J, 10 (2004), pp. 318–322
- 18 A.F. Azlina, Z. Ariza, T. Arni, A.N. Hisham; Chronic granulomatous mastitis: diagnostic and therapeutic considerations; World J Surg, 27 (2003), pp. 515–518
- 19 K. Ocal, A. Dag, O. Turkmenoglu, et al.; Granulomatous mastitis: clinical, pathological features, and management; Breast J, 16 (2010), pp. 176–182
- 20 A. Akcan, H. Akyildiz, M.A. Deneme, H. Akgun, Y. Aritas; Granulomatous lobular mastitis: a complex diagnostic and therapeutic problem; World J Surg, 30 (2006), pp. 1403–1409
- 21 T.M. Milward, M.H. Gough; Granulomatous lesions in the breast presenting as carcinoma; Surg Gynecol Obstet, 130 (1970), pp. 478–482
- 22 M. Lacambra, T.A. Thai, C.C. Lam, et al.; Granulomatous mastitis: the histological differentials; J Clin Pathol, 64 (2011), pp. 405–411
- 23 R. Ahmed, F. Sultan; Granulomatous mastitis: a review of 14 cases; J Ayub Med Coll Abbottabad, 18 (2006), pp. 52–54
- 24 B. Al-Khaffaf, F. Knox, N.J. Bundred; Idiopathic granulomatous mastitis: a 25-year experience; J Am Coll Surg, 206 (2008), pp. 269–273
- 25 H.A. Al-Khawari, H.A. Al-Manfouhi, J.P. Madda, A. Kovacs, M. Sheikh, O. Roberts; Radiologic features of granulomatous mastitis; Breast J, 17 (2011), pp. 645–650
- 26 S. Akbulut, Z. Arikanoglu, A. Senol, et al.; Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis?; Arch Gynecol Obstet, 284 (2011), pp. 1189–1195
- 27 P. Jayia, E. Oberg, H. Tuffaha, D.R. Leff, R. Al-Mufti, D. Hadjiminas; Should we manage all cases of granulomatous mastitis conservatively? A 14 year experience; Breast J, 19 (2013), pp. 215–216
- 28 K.Y. Kok, P.U. Telisinghe; Granulomatous mastitis: presentation, treatment and outcome in 43 patients; Surgeon, 8 (2010), pp. 197–201
- 29 M. Dursun, S. Yilmaz, A. Yahyayev, et al.; Multimodality imaging features of idiopathic granulomatous mastitis: outcome of 12 years of experience; Radiol Med, 117 (2012), pp. 529–538
- 30 L. Ozel, A. Unal, E. Unal, et al.; Granulomatous mastitis: is it an autoimmune disease? Diagnostic and therapeutic dilemmas; Surg Today, 42 (2012), pp. 729–733
- 31 J.D. Davies, P.A. Burton; Postpartum lobular granulomatous mastitis; J Clin Pathol, 36 (1983), p. 363
- 32 K.L. Brown, P.H. Tang; Postlactational tumoral granulomatous mastitis: a localized immune phenomenon; Am J Surg, 138 (1979), pp. 326–329
- 33 R. Taghizadeh, O.P. Shelley, B.K. Chew, E.M. Weiler-Mithoff; Idiopathic granulomatous mastitis: surgery, treatment, and reconstruction; Breast J, 13 (2007), pp. 509–513
- 34 R. Heer, J. Shrimankar, C.D. Griffith; Granulomatous mastitis can mimic breast cancer on clinical, radiological, or cytological examination: a cautionary tale; Breast, 12 (2003), pp. 283–286
- 35 A. Fletcher, I.M. Magrath, R.H. Riddell, I.C. Talbot; Granulomatous mastitis: a report of seven cases; J Clin Pathol, 35 (1982), pp. 941–945
- 36 A.C. Kaur, H. Dal, B. Muezzinoglu, N. Paksoy; Idiopathic granulomatous mastitis. Report of a case diagnosed with fine needle aspiration cytology; Acta Cytol, 43 (1999), pp. 481–484
- 37 G. Cserni, K. Szajki; Granulomatous lobular mastitis following drug-induced galactorrhea and blunt trauma; Breast J, 5 (1999), pp. 398–403
- 38 A. Olfatbakhsh, T. Beheshtian, G.E. Djavid; Granulomatous mastitis, erythema nodosum, and oligoarthritis in a pregnant woman; Breast J, 14 (2008), pp. 588–590
- 39 A. Neel, M. Hello, A. Cottereau, et al.; Long-term outcome in idiopathic granulomatous mastitis: a Western multicentre study; QJM, 106 (2013), pp. 433–441
- 40 S.M. Mirsaeidi, M.R. Masjedi, S.D. Mansouri, A.A. Velayati; Tuberculosis of the breast: report of four clinical cases and literature review; East Mediterr Health J, 13 (2007), pp. 670–676
- 41 R. Diallo, T. Frevel, C. Poremba, U. Cirkel, D. Metze, B. Dockhorn-Dworniczak; Lupus vulgaris as the etiology of tuberculous mastitis; Pathologe, 18 (1997), pp. 67–70
- 42 D. Ponce de Leon, E. Acevedo-Vasquez, S. Alvizuri, et al.; Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population; J Rheumatol, 35 (2008), pp. 776–781
- 43 B.K. Han, Y.H. Choe, J.M. Park, et al.; Granulomatous mastitis: mammographic and sonographic appearances; AJR Am J Roentgenol, 173 (1999), pp. 317–320
- 44 K. Boarki, M. Labib; Imaging findings in Idiopathic lobular granulomattous mastitis, case report and review of literature; Gulf J Oncolog (2010), pp. 46–52
- 45 M. Kocaoglu, I. Somuncu, F. Ors, et al.; Imaging findings in idiopathic granulomatous mastitis. A review with emphasis on magnetic resonance imaging; J Comput Assist Tomogr, 28 (2004), pp. 635–641
- 46 M. Ozturk, E. Mavili, G. Kahriman, A.C. Akcan, F. Ozturk; Granulomatous mastitis: radiological findings; Acta Radiol, 48 (2007), pp. 150–155
- 47 N. Tuncbilek, H.M. Karakas, O.O. Okten; Imaging of granulomatous mastitis: assessment of three cases; Breast, 13 (2004), pp. 510–514
- 48 G.M. Tse, C.S. Poon, K. Ramachandram, et al.; Granulomatous mastitis: a clinicopathological review of 26 cases; Pathology, 36 (2004), pp. 254–257
- 49 A.B. Ergin, M. Cristofanilli, H. Daw, G. Tahan, Y. Gong; Recurrent granulomatous mastitis mimicking inflammatory breast cancer; BMJ Case Rep, 2011 (2011)
- 50 F.M. Yau, S.A. Macadam, U. Kuusk, M. Nimmo, N. Van Laeken; The surgical management of granulomatous mastitis; Ann Plast Surg, 64 (2010), pp. 9–16
- 51 N. Sato, H. Yamashita, N. Kozaki, et al.; Granulomatous mastitis diagnosed and followed up by fine-needle aspiration cytology, and successfully treated by corticosteroid therapy: report of a case; Surg Today, 26 (1996), pp. 730–733
- 52 G. Schmajuk, M.C. Genovese; First report of idiopathic granulomatous mastitis treated with methotrexate monotherapy; J Rheumatol, 36 (2009), pp. 1559–1560
- 53 N. Raj, R.D. Macmillan, I.O. Ellis, C.M. Deighton; Rheumatologists and breasts: immunosuppressive therapy for granulomatous mastitis; Rheumatology (Oxford), 43 (2004), pp. 1055–1056
- 54 F. Maffini, F. Baldini, F. Bassi, A. Luini, G. Viale; Systemic therapy as a first choice treatment for idiopathic granulomatous mastitis; J Cutan Pathol, 36 (2009), pp. 689–691
- 55 M. Hladik, T. Schoeller, F. Ensat, G. Wechselberger; Idiopathic granulomatous mastitis: successful treatment by mastectomy and immediate breast reconstruction; J Plast Reconstr Aesthet Surg, 64 (2011), pp. 1604–1607
- 56 T.D. Koelmeyer, D.E. MacCormick; Granulomatous mastitis; Aust N Z J Surg, 46 (1976), pp. 173–176
- 57 J. Kim, K.E. Tymms, J.M. Buckingham; Methotrexate in the management of granulomatous mastitis; ANZ J Surg, 73 (2003), pp. 247–249
- 58 G. Gurleyik, A. Aktekin, F. Aker, H. Karagulle, A. Saglamc; Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma; J Breast Cancer, 15 (2012), pp. 119–123
- 59 H.E. Guven, I. Pak, S. Oral; Granulomatous mastitis: surgical outcomes; J Coll Physicians Surg Pak, 16 (2006), pp. 431–433
- 60 D.A. DeHertogh, A.H. Rossof, A.A. Harris, S.G. Economou; Prednisone management of granulomatous mastitis; N Engl J Med, 303 (1980), pp. 799–800
- 61 J.R. Miliauskas, A.S. Pieterse, R.S. Williams; Granulomatous lobular mastitis; Aust N Z J Surg, 65 (1995), pp. 139–141
- 62 J.P. Wilson, N. Massoll, J. Marshall, et al.; Idiopathic granulomatous mastitis: in search of a therapeutic paradigm; Am Surg, 73 (2007), pp. 798–802
- 63 W. Donn, P. Rebbeck, C. Wilson, C.B. Gilks; Idiopathic granulomatous mastitis. A report of three cases and review of the literature; Arch Pathol Lab Med, 118 (1994), pp. 822–825
- 64 A.A. Pathirana, A. Fernando, M.V. de Silva; Three patients with granulomatous mastitis showing good response to oral prednisolone; Ceylon Med J, 52 (2007), pp. 14–15
- 65 S. Akbulut, D. Yilmaz, S. Bakir; Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases; Breast J, 17 (2011), pp. 661–668
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?