Abstract
Bupropion (BUP), a substituted phenyl-ethylamine, has been utilized for the treatment of depression and for smoking cessation, however, one concern is that BUP may increase a risk of psychosis similar to other substituted phenyl-ethylamine amphetamine (AMPH) and methamphetamine (MetAMPH). BUP promotes ambulation in mice and causes behavioral sensitization on the ambulation-promoting effect when repeatedly administered as well as AMPH and MetAMPH. The present study aimed to elucidate brain regions and monoaminergic neurotransmitters that are involved in the ambulation-promoting effect of BUP. c-Fos-like immunoreactivity (c-Fos-IR) mapping in brain in combination with measuring ambulatory activity was conducted to determine brain region(s) that is involved in the ambulatory effect of BUP. Three kinds of statistical analyses for c-Fos-IR in 24 brain regions consistently showed that c-Fos-IR in the Caudate putamen (CPu) is positively correlated with the ambulatory response to BUP. In addition, multiple regression analysis indicated that the ambulatory response is a function of c-Fos-IR not only in the CPu but also in the lateral septum nucleus (LS), median raphe nucleus (MnR), lateral globus pallidus (LGP), medial globus pallidus (MGP), locus coeruleus (LC) and ventral hypothalamic nucleus (VMH). Effects of BUP on monoaminergic neurotransmitters in the CPu were examined using in vivo microdialysis method, as the pharmacological experiments indicated that monoaminergic neurotransmitters, dopamine (DA) in particular, mediate the ambulatory response to BUP. Response of DA in the CPu to BUP was parallel to the ambulatory response, showing that DA in the CPu is involved in the ambulatory response to BUP. The present study also suggests that other brain regions such as the LC, the origin nucleus of norepinephrine (NE) neurons, and another neurotransmitter NE may also play some roles for the ambulatory response to BUP, however, further studies are needed to elucidate the roles.
Chemical compounds studied in this article
Bupropion (PubChem CID: 62884) ; Chlorpromazine HCl (PubChem CID: 6240) ; Fluphenazine 2HCl (PubChem CID: 67356) ; Spiperone (PubChem CID: 5265) ; Haloperidol (PubChem CID: 3559) ; Pimozide (PubChem CID: 16362) ; α-Methyl-p -tyrosine (PubChem CID: 3125) ; Reserpine (PubChem CID: 5770)
Keywords
Mouse ; Ambulatory activity ; Striatum ; Dopamine ; c-Fos mapping ; Microdialysis
1. Introduction
Bupropion (BUP), a substituted phenyl-ethylamine, was found to possess therapeutic benefits as an atypical antidepressant medication [18] , smoking-cessation medication [18] and drug abuse-cessation medication [10] , [14] and [46] . Clinical use of BUP for the treatment of depression and for smoking-cessation was approved by the Food and Drug Administration (FDA) in the United States. Daily dose of 450 mg is recommended for the treatment of depression. The original immediate release formulation of BUP is dosed three times daily, followed by introduction of the sustained-release formulation that is dosed twice daily and the extended-release formulation that is dosed once daily [29] .
Though BUP has been extensively utilized for the treatment of depression and for smoking cessation, one concern is that BUP may increase a risk of psychosis [29] . Several studies report that BUP may cause or worsen psychosis [6] , [21] , [25] , [27] and [62] . BUP inhibits the dopamine (DA) transporter (DAT) and the norepinephrine (NE) transporter (NET), and is an antagonist at the neuronal nicotinic acetylcholine receptors (nAChRs) [48] and [50] . The IC50 of BUP for inhibiting DA uptake is higher than the IC50 values for inhibiting NE uptake and nAChRs function [16] . This pharmacological property also suggests that BUP can have the potential for precipitating psychosis. However, whether or not BUP increase a risk of psychosis and neuronal mechanisms underlying the potential of BUP to increase the risk have not been elucidated sufficiently [29] .
BUP promotes ambulatory activity in mice [54] , [55] and [59] . Repeated administration of BUP to the same mouse augments the ambulation-promoting effect (behavioral sensitization) [58] . These ambulatory effects of BUP are similar to those of amphetamine (AMPH) and methamphetamine (MetAMPH) [22] and [30] , other substituted phenyl-ethylamine, that have the potential to produce psychosis-like mental disorder (amphetamine psychosis) [26] . Mechanism underlying the locomotion-promoting effect of AMPH in mice is elucidated well [7] , [24] , [36] , [51] and [64] , whereas mechanisms underlying the ambulation-promoting effect of BUP have poorly been elucidated.
The present study aimed to elucidate brain region(s) and neurotransmitter(s) that are involved in the ambulation-promoting effect of BUP.
Mapping c-Fos-like immunoreactivity (c-Fos-IR), which indicates neuronal activation, is useful for determining brain regions that are activated by CNS acting drugs and involved in specific behaviors [43] and [66] . BUP increases c-Fos-IR in various rat brain regions [8] , whereas effects of BUP on c-Fos-IR in mouse brain have not been studied. The present study conducted c-Fos-IR mapping in combination with measuring ambulatory activity to determine mouse brain region(s) that is involved in the ambulatory response to BUP.
BUP inhibits dopamine (DA) transporter (DAT) and norepinephrine (NE) transporter (NET) and enhances the extracellular DA and NE levels in rat brain [11] and [33] . On the other hand, effects of BUP on monoaminergic neurotransmitters in mouse brain have not been elucidated well. The present study examined whether or not monoaminergic neurotransmitters mediate the ambulatory response to BUP using drugs that modulate monoaminergic neurotransmission. Drugs used were α-methyl-p -tyrosine (AMPT), reserpine (RES), chlorpromazine (CPZ), fluphenazine (FLU), SCH12679 (SCH), spiperone (SPI), haloperidol (HAL) and pimozide (PIM). AMPT and RES deplete monoaminergic neurotransmitters in brain [17] , [41] and [61] . CPZ, FLU, SCH, SPI, HAL and PIM antagonize DA receptors (DARs) and their affinities for DARs are much higher than those for NE receptors [13] and [47] . Then, the present study examined effects of BUP on monoaminergic neurotransmitters in brain region(s) that was identified by the c-Fos-IR study using in vivo microdialysis in order to elucidate relationships between responses to BUP of monoaminergic neurotransmitters in the brain region(s) and of the ambulatory activity.
2. Materials and methods
2.1. Subjects
Male ICR strain mice (Clea Japan, Tokyo, Japan) aged 7–15 weeks and weighing 35–45 g at the start of experiments were used. Mice were housed in aluminum cages (three mice/cage) with a stainless-steel mesh top and paper bedding. Commercial solid food (Clea Japan) and tap water were provided ad libitum. The cages were placed in a room artificially illuminated by fluorescent lamps on a 12-h light:12-h dark schedule (light period: 07:00–19:00) and a room temperature of 25 ± 1 °C. All experiments were conducted during the light phase.
All experiments were performed in accordance with the guidelines of the Ethics Committee for Experimental Animals of the National Institute for Environmental Studies, Japan.
2.2. Drugs
Bupropion HCl (BUP), chlorpromazine HCl (CPZ), fluphenazine 2HCl (FLU), SCH12679 ((R)-(−)-Phenyl−2,3,4,5-tetrahydro-1H -7,8-dimethoxy-3-benzazepine) maleate (SCH) and spiperone (SPI) (Sigma-Aldrich, Tokyo, Japan) were prepared in 0.9% NaCl (Nacalai Tesque, Kyoto, Japan) solution (saline). Haloperidol (HAL) (Sigma-Aldrich) was prepared in 0.1% acetic acid (Nacalai Tesque) solution. Pimozide (PIM) (Sigma-Aldrich), α-methyl-p -tyrosine (AMPT) (ICN Biomedicals, Solon, OH, USA), and reserpine (RES) (Sigma-Aldrich) were mixed with a small amount of polyoxyethylene sorbitan monooleate (Tween 80) (Nacalai Tesque) and diluted in saline. Doses of BUP, CPZ, FLU, and SCH were expressed as the salt weights.
AMPT was intraperitoneally administered to mice, and other drugs were subcutaneously administered. The administration volume was 1 ml/100 g body weight regardless of the type of drug and dosage.
2.3. Measurement of mouse ambulatory activity
Ambulatory activity was measured using an ambulometer (SAM-10; O’Hara and Co., Tokyo, Japan) [55] , [56] , [57] , [58] , [59] , [54] , [52] , [53] and [57] (Appendix A). Each activity cage (20 cm in diameter) is supported by a fulcrum in the center of the bottom; the fulcrum tilts according to movement of the mouse in the activity cage. The tilting movement of the activity cage activates three micro-switches that surround the cage. The number of activations of micro-switches during a set time is recorded, and the result is printed out.
After adapting mice to the activity cages for 30 min, saline, 5 mg/kg or 10 mg/kg of BUP was administered to the mice, followed by measuring the ambulatory activity for 60 min. Combined administration of saline or 50 mg/kg AMPT plus saline or 2, 4, or 8 mg/kg RES was performed on mice at the same time one day before measuring the ambulatory response to 10 mg/kg BUP. Drugs that preferentially antagonize DARs were administered after 30 min of adaptation, 10 min later followed by measuring the ambulatory response to 10 mg/kg BUP.
2.4. Immunocytochemistry for c-Fos
2.4.1. Preparation of brain samples
The following procedures were performed in three separate experiments on mice given saline (n = 8) or BUP (n = 11). We obtained seven brain samples from mice given saline and 10 brain samples from mice given BUP. One brain sample from each of the animal groups was excluded because of unsuccessful transcardial perfusion of fixation.
Individual mice were placed in activity cages, and 30 min later, saline was administered, followed by measurement of ambulatory activity for 60 min. After the measurement, they were returned to their home cages. This procedure was repeated every day for 3 days to reduce stress to the mice (acclimation procedure), because stress induces c-Fos-IR in various brain regions [3] and [23] . On the 4th day, individual mice were placed in activity cages. After 30 min of adaptation, saline or 10 mg/kg BUP was administered, and ambulatory activity was measured for 60 min. Immediately after the end of the ambulatory measurements, the mice were deeply anesthetized with pentobarbital (Nembutal® , Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) and perfused transcardially with saline containing heparin (Wako Pure Chemical Ind. Ltd., Osaka, Japan) followed by Speh’s fixative (4% paraformaldehyde, 0.2% saturated picric acid, and 0.05% glutaraldehyde in 0.1 M phosphate buffer pH 7.4), which is a modification of Zamboni’s fixative [65] . Brains were removed and post-fixed in the same fixative overnight at 4 °C. They were soaked in 0.1 M phosphate buffer (pH 7.4) containing 25% sucrose for cryoprotection until they had completely sunk. Brains were individually frozen using methyl butane cooled by dry ice and stored at −80 °C.
2.4.2. Immunocytochemistry for c-Fos in brain samples
Coronal sections of brains from the olfactory bulb to the midbrain were cut at a thickness of 50 μm using a cryostat. Immunocytochemistry was performed on free-floating sections in four separate batches. Each batch included samples derived from mice administered either saline or BUP to ensure that the extent of staining was balanced between the two groups. After washing with Tris-buffered saline (TBS, pH 7.4) (Nacalai Tesque), sections were incubated for 3 days at 4 °C in primary antibody to c-Fos (F7799; Anti-c-Fos rabbit IgG, Sigma-Aldrich; 1:5000) in antibody diluent (TBS containing 0.25% λ-carrageenan, 1% bovine serum albumin, and 0.3% Triton X-100 (all from Sigma-Aldrich)) with 0.1% sodium azide (Nacalai Tesque). Sections were then washed in TBS and incubated in biotinylated secondary goat anti-rabbit IgG (Vector Labs, Burlingame, CA, USA; 1:500) in antibody diluent for 60 min at room temperature. After washing with TBS, sections were incubated with ABC complex (ABC Elite kit, Vector Labs; 1:750) in antibody diluent for 90 min. Staining was visualized by reaction with H2 O2 and diaminobenzidine (Sigma-Aldrich). The reaction was stopped by washing sections in TBS. Sections were mounted onto subbed slides, allowed to air dry, dehydrated, and coverslipped using Permount (Sigma-Aldrich).
2.4.3. Quantification of c-Fos-IR
Microscopic images of stained sections were captured via a camera (DFC490, Leica, Germany) interfaced with a personal computer. Captured images at 100 × magnification were printed in color for quantification of c-Fos-IR (Appendix B).
Twenty four mouse brain regions were selected by referring to the previous study that examined the effects of BUP on c-Fos expression in various rat brain regions [8] , and c-Fos-IR in the brain regions were quantified. The numbers of c-Fos-IR nuclei in areas 0.11-0.8 mm2 in the 24 brain regions were manually counted. The sizes of the examined area were identical throughout each brain region except the dentate gyrus (DG). Because the morphology of the DG was quite different among each brain, the sizes of the areas in the DG ranged from 0.11 to 0.27 mm2 . The other brain regions examined were the anterior olfactory nucleus (AO), orbital cortex (medial, ventral) (MO, VO), primary motor cortex (M1), medial prefrontal cortex (mPFC), lateral septum nucleus (LS), claustrum (Cl), nucleus accumbens (NAcb), olfactory tubercle (Tu), ventral pallidum (VP), anterior paraventricular thalamic nucleus (PVA), lateral globus pallidus (LGP), caudate putamen (CPu), central amygdaloid nucleus (CeA), medial globus pallidus (MGP), lateral hypothalamic nucleus (LH), ventral hypothalamic nucleus (VMH), subthalamic nucleus (STh), ventral tegmental area (VTA), substantia nigra pars compacta (SNC), substantia nigra pars reticulate (SNR), dorsal raphe nucleus (DR), median raphe nucleus (MnR), and locus coeruleus (LC) (Appendix C). The brain regions were identified according to the mouse brain atlas [42] .
Counting of c-Fos-IR nuclei in the SNR, CPu, and CeA was performed in duplicate, and their correlations were examined in order to confirm the reliability of our c-Fos-IR quantification. Correlation coefficients were 0.9795, 0.8481, and 0.9606 for the CPu, CeA, and SNR, respectively (Appendix D), showing that the quantification of c-Fos-IR was reliable.
2.5. Measurement of brain monoaminergic neurotransmitters and their metabolites using in vivo microdialysis
Mice were anesthetized using 50 mg/kg pentobarbital (Nembutal® ) and fixed in a stereotaxic apparatus equipped with a mouse adapter (David Kopf, CA, USA). A dialysis probe (D-I-6-02, cut-off 50,000 Da, Eicom, Kyoto) was stereotaxically implanted into the brain region determined to be involved in the ambulatory response to BUP by the c-Fos study according to the mouse brain atlas [42] and fixed with dental cement. After surgery, the animals were individually housed in cages and allowed to recover for 2–3 days.
Two to three days after surgery, online measurement of the extracellular monoaminergic neurotransmitters, DA, NE, and 5-HT, and their metabolites, (3,4-dihydroxy-phenylacetic acid (DOPAC), homovanillic acid (HVA), 3-methoxy-4-hydroxy-phenyl-ethylene glycol (MHPG), and 5-hydroxyindolacetic acid (5-HIAA)) was performed using in vivo microdialysis coupled with high-performance liquid chromatography (HPLC). The probe-implanted mouse was placed in a cage for the microdialysis experiment and allowed to move freely in the cage. Food and water were available ad libitum throughout the microdialysis measurement. Ringer’s solution (147 mM Na+ , 4 mM K+ , and 2.3 mM Ca+ , 155.6 mM Cl− ) was perfused at a rate of 2.0 μl/min through the probe using a syringe pump (ESP-64, Eicom). Each dialysate sample was collected for 25 min using an auto injector (EAS-2, Eicom) that automatically injected the dialysate sample into HPLC immediately after the end of each 25-min collection. After at least four dialysate samples were collected to establish baseline levels, saline or 10 mg/kg BUP was administered to the mouse, followed by continuous collection of six dialysate samples. The lag time from the probe tip to the auto injector was considered to determine the time point for the administration. After the end of the microdialysis measurement, eosin solution was perfused through the probe, the mouse was deeply anesthetized using pentobarbital (Nembutal® ), and the brain was removed and fixed using 10% formaldehyde neutral buffer solution (Nacalai Tesque). The placement of the probe was verified histologically (Appendix E).
Monoaminergic neurotransmitters and their metabolites in dialysate samples were analyzed using HPLC (HTEC-500, Eicom). Each sample was analyzed for 25 min using HPLC during which all analytes of interest appeared in a chromatogram. Before starting the microdialysis experiment for each mouse, HPLC was calibrated using authentic standard substances for monoaminergic neurotransmitters and their metabolites. The column used was SC-50DS (Eicom), and the mobile phase (pH 3.5) consisted of 83% 0.1 M acetic acid-citric acid buffer, 17% methanol (Nacalai Tesque), 190 mg/L octanesulfonic acid (Nacalai Tesque), and 5 mg/L Na2 EDTA (Wako Pure Chemical Ind. Ltd.). The flow rate of the mobile phase was 0.23 ml/min. Monoaminergic neurotransmitters and their metabolites were detected using an electrochemical detector (ECD-300, Eicom) that included a graphite electrode (WE-3G, Eicom). The applied voltage was +700 mV against an Ag/AgCl reference electrode. Data were collected from HPLC via an interface (EPC-300, Eicom) to a personal computer and analyzed using software (PowerChrom, AD instruments Japan, Nagoya).
2.6. Statistical analyses
P < 0.05 was considered statistically significant in all statistical analyses.
2.6.1. Ambulatory activity
To eliminate differences in baseline ambulatory activity, the ambulatory activity of each mouse was normalized using the total activity of the mouse during the 30-min adaptation period before administration of drugs. Normalized ambulatory activity was analyzed using the Kruskal-Wallis test followed by the Wilcoxon test, as data were not distributed normally. In the case of the AMPT/RES administration experiment, not only normalized but also actual values of ambulatory activity were analyzed using the Kruskal-Wallis and Wilcoxon tests to examine the effect of AMPT/RES on the baseline ambulatory activity.
2.6.2. c-Fos-IR
Three kinds of statistical analyses were conducted to determine brain region(s) in which c-Fos-IR was involved in the ambulatory response to BUP.
Differences in numbers of c-Fos-IR nuclei in each brain region between saline-administered and BUP-administered mice were compared using the Wilcoxon test, as data were not distributed normally.
Relationships between ambulatory activity and c-Fos-IR in the twenty four brain regions were analyzed using multiple regression analysis [60] . Square root transformations of the c-Fos-IR data and the ambulatory activity were adopted to normalize the data. The step-wise method was first used to select the brain regions in which c-Fos-IR was correlated with ambulatory activity, followed by multiple regression analysis. The best-fit regression equation was obtained by the least-squares method. Significance of the determined multiple regression equation was evaluated using analysis of variance (ANOVA) and R2 (coefficients of determination), and significance of the partial regression coefficients was tested using the t -test.
Furthermore, the relationship between ambulatory activity and c-Fos-IR in each of the seven brain regions identified by the multiple regression analysis was examined using single regression analysis. The best-fit regression equation was obtained by the least-squares method. Significance of the determined single regression equations was evaluated by ANOVA and R2 .
2.6.3. Monoaminergic neurotransmitters and their metabolites
In normal mice, the measured values for monoaminergic neurotransmitters and their metabolites just before administering saline or BUP for each mouse were defined as baseline values, and the measured values before and after administration were expressed as ratios to the baseline values. In the AMPT- and RES-administered mice, actual amounts of monoaminergic neurotransmitters and their metabolites in dialysates were examined to elucidate the effects of the pretreatment with AMPT and RES on their baseline values and responses of them to BUP.
Measuring 5-HT was difficult because the retention time was close to 25 min in many cases. In addition, as the NE and MHPG measured in the dialysates in the present study were insensitive to pretreatment with AMPT and RES, the measured values for NE and MHPG were believed to be derived from other obstructive sources. Thus, we excluded NE, MHPG, and 5-HT from the examinations. Alterations in DA, DOPAC, HVA, and 5-HIAA in normal mice after administration of saline or BUP were analyzed using two-way repeated ANOVA followed by the Dunnett test that compared BUP data with saline data at each time point. Alterations in DA, DOPAC and HVA after administration of saline or BUP in the mice pretreated with AMPT/RES or saline were analyzed using three-way repeated ANOVA followed by two-way ANOVA, one-way ANOVA, and the Dunnett test.
3. Results
3.1. Effect of BUP on mouse ambulatory activity
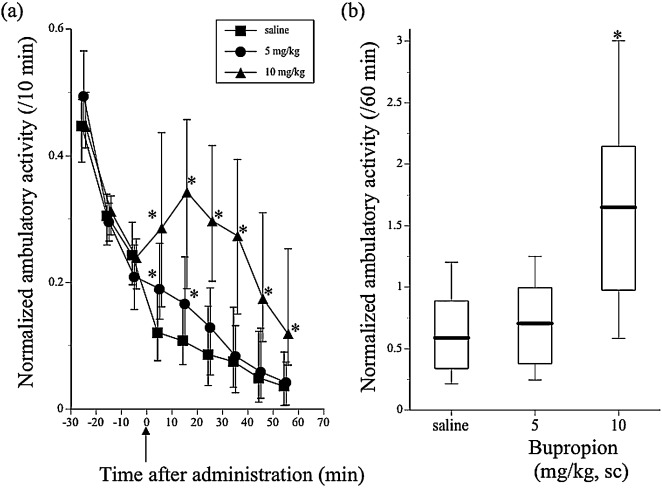
When mice were placed into the activity cages, they exhibited high ambulatory activity, followed by a decrease in activity during the 30-min adaptation period. After the adaptation period, saline or 5 or 10 mg/kg BUP was administered to the mice. The ambulatory activity was significantly promoted by administration of 10 mg/kg BUP (Kruskal-Wallis test; χ22 = 64.9426, P < 0.0001) (Fig. 1 (a), (b)). The ambulatory activity reached a maximum 15 min after administration of 10 mg/kg BUP, followed by a decrease in the ambulatory activity to the level of the saline-administered mice within 60 min (Fig. 1 (a)).
|
|
|
Fig. 1. Effect of bupropion (BUP) on ambulatory activity in ICR mice. The panels show normalized ambulatory activity that was obtained by normalizing actual ambulatory activity to the total ambulatory activity during the 30-min adaptation period before administration in each mouse. (a) Alterations in normalized ambulatory activity before and after subcutaneous administration of saline or 5 or 10 mg/kg BUP. Symbols show median values of normalized ambulatory activity for each 10-min period plotted against the midpoint of the measurement period, and vertical lines denote the first and third quartiles. The arrow indicates the time point for the administration of saline or 5 or 10 mg/kg BUP. (b) Total normalized ambulatory activity for 60 min after administration of saline or 5 or 10 mg/kg BUP. Data are shown using a box plot. *P < 0.05 compared with the saline control. The numbers of animals used were 40–120 (N = 40–120). |
3.2. Effect of BUP on c-Fos-IR in 24 brain regions and the relationships between ambulatory activity and c-Fos-IR in the brain regions
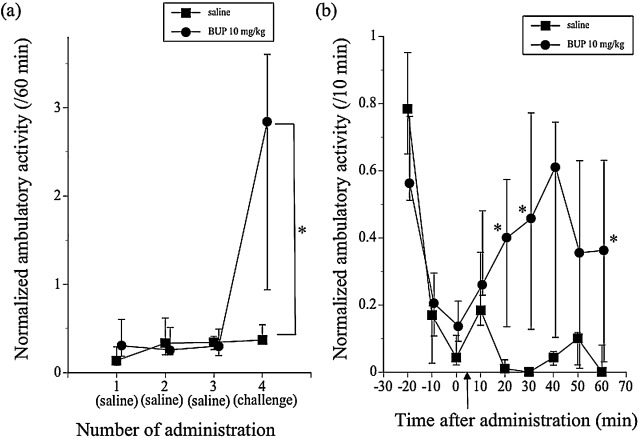
The ambulatory activity on the 4th day (challenge session) was significantly promoted by 10 mg/kg BUP after 3 consecutive days of acclimation (χ21 = 8.5714, P = 0.0034) (Fig. 2 (a), (b)). Immediately after the ambulatory measurement, the brains of the mice were fixed by transcardial perfusion of fixative and used for c-Fos immunocytochemistry.
|
|
|
Fig. 2. (a) Daily changes in total ambulatory activity for 60 min after subcutaneous administration of saline or BUP. All mice were given saline on days 1–3 (acclimation session). On day 4 (challenge session), one group was given saline (N = 7), and another group was given 10 mg/kg BUP (N = 10). Symbols show median values of total normalized ambulatory activity for 60 min after administration, and vertical lines denote the first and third quartiles. *P < 0.05 BUP vs. saline on day 4 (challenge session). (b) Time course of ambulatory activity before and after administration of saline or 10 mg/kg BUP on day 4. The arrow indicates time point for the administration. Immediately after the end of the ambulatory measurement, the mice were transcardially perfused with fixative, and their brains were later used for c-Fos immunocytochemistry. Symbols show median values of normalized ambulatory activity for each 10-min period. Symbols were plotted against the midpoint of the measurement period. Vertical lines denote the first and third quartiles. *P < 0.05 BUP vs. saline. |
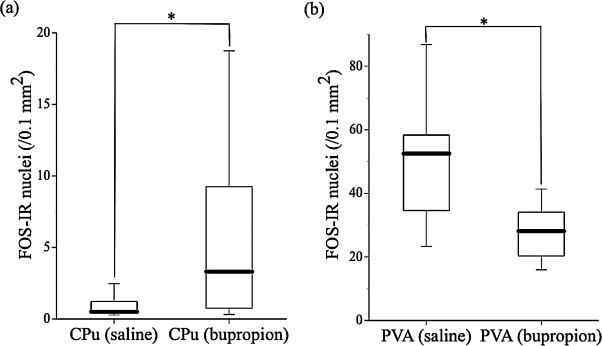
c-Fos-IR nuclei were quantified in 24 brain regions of the mice given saline or BUP. The number of c-Fos-IR nuclei in the CPu of mice that received BUP was significantly larger (Fig. 3 (a)), and the number of c-Fos-IR nuclei in the PVA was significantly smaller (Fig. 3 (b)) than those of mice that received saline (CPu; χ21 = 4.047, P = 0.0442, PVA; χ21 = 5.4857, P = 0.0192). No significant differences were observed in the numbers of c-Fos-IR nuclei in other brain regions between mice given BUP and saline (data not shown).
|
|
|
Fig. 3. c-Fos-IR in the CPu (a) and PVA (b) of mice that were given saline or10 mg/kg BUP. Data are shown using a box plot. *P < 0.05. |
The relationships between the ambulatory activity and the numbers of c-Fos-IR nuclei in the 24 brain regions were examined. Seven brain regions were selected by the step-wise method, and multiple regression analysis indicated the following equation:
|
|
where SQRT is square root transformation of the measured value, and a, b, c……. are partial regression coefficients for the variables. Determined values for the partial regression coefficients and the results of statistical tests for them are shown in Table 1 . The multiple regression equation was significant (ANOVA: F(7, 8) = 7.3737, P = 0.0057), and R2 was 0.865808.
| Explanatory variables | Partial regression coefficients | Determined value | t value | Probability |
|---|---|---|---|---|
| SQRT(c-Fos-IR in the CPu) | a | 0.6740728 | 4.6 | 0.0018 |
| SQRT(c-Fos-IR in the LS) | b | 0.1291112 | 0.82 | 0.4363 |
| SQRT(c-Fos-IR in the MnR) | c | 0.2988483 | 2.35 | 0.0464 |
| SQRT(c-Fos-IR in the LGP) | d | −0.326261 | −1.53 | 0.1637 |
| SQRT(c-Fos-IR in the MGP) | e | 0.2327495 | 2.02 | 0.0775 |
| SQRT(c-Fos-IR in the LC) | f | −0.371602 | −2.43 | 0.0414 |
| SQRT(c-Fos-IR in the VMH) | g | −0.182014 | −2.62 | 0.0307 |
| Intercept | 0.333934 | 0.73 | 0.486 |
Abbreviations: SQRT; square transformation of the measured value, c-Fos-IR; c-Fos-like immunoreactivity, CPu; caudate putamen, LS; lateral septum nucleus, MnR; median raphe nucleus, LGP; lateral globus pallidus, MGP; medial globus pallidus, LC; locus coeruleus, VMH; ventral hypothalamic nucleus.
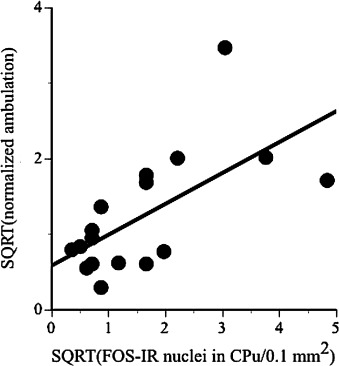
The relationships between the ambulatory activity and the number of c-Fos-IR nuclei in each of the seven brain regions were examined using single regression analysis. The determined regression equations for each of the seven brain regions and their statistics are shown in Table 2 . The single regression equation for the CPu was significant (Fig. 4 ).
| Explanatory variable | Regression coefficient | Intercept | F value | Probability | R2 |
|---|---|---|---|---|---|
| SQRT(c-Fos-IR in the CPu) | 0.409049 | 0.5862859 | F(1.15)=10.6043 | 0.0053 | 0.414162 |
| SQRT(c-Fos-IR in the LS) | 0.0092656 | 1.2033837 | F(1.15)=0.0037 | 0.9525 | 0.000245 |
| SQRT(c-Fos-IR in the MnR) | 0.3405928 | 0.357357 | F(1.15)=4.1809 | 0.0588 | 0.217971 |
| SQRT(c-Fos-IR in the LGP) | 0.2623237 | 0.9954338 | F(1.15)=1.5488 | 0.2324 | 0.093589 |
| SQRT(c-Fos-IR in the MGP) | 0.2928349 | 0.9146221 | F(1.15)=4.0894 | 0.0614 | 0.214223 |
| SQRT(c-Fos-IR in the LC) | 0.0956728 | 1.007142 | F(1.14)=0.2736 | 0.6091 | 0.01917 |
| SQRT(c-Fos-IR in the VMH) | −0.036343 | 1.3765973 | F(1.15)=0.2451 | 0.6277 | 0.016079 |
Abbreviations: SQRT; square transformation of the measured value, c-Fos-IR; c-Fos-like immunoreactivity, CPu; caudate putamen, LS; lateral septum nucleus, MnR; median raphe nucleus, LGP; lateral globus pallidus, MGP; medial globus pallidus, LC; locus coeruleus, VMH; ventral hypothalamic nucleus.
|
|
|
Fig. 4. A result of single regression analysis between ambulatory activity and c-Fos-IR in the CPu. The best-fit regression line was determined using the least-squares method. Statistics for the regression equation are shown in Table 2 . |
3.3. Effect of pretreatment with AMPT and RES on ambulatory effect of BUP
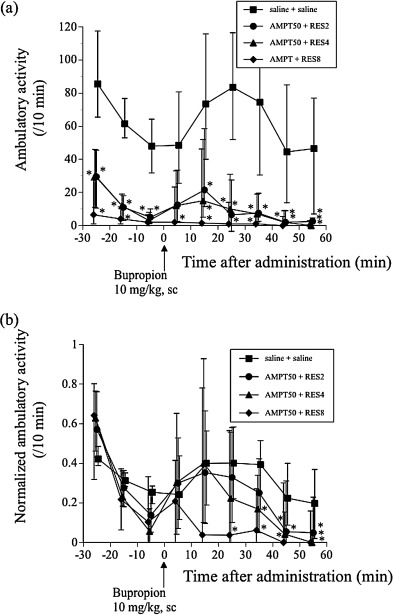
Pretreatment with 50 mg/kg AMPT and 2–8 mg/kg RES extensively reduced the baseline ambulatory activity (Fig. 5 (a)). In addition, the pretreatment also extensively reduced the effect of 10 mg/kg BUP on the actual ambulatory activity (Fig. 5 (a)). Response of the normalized ambulatory activity to BUP also significantly decreased depending on time and the dose of RES (at 25 min, χ23 = 8.4874, P = 0.0369: at 35 min, χ23 = 15.0212, P = 0.0018: at 45 min, χ23 = 13.3536, P = 0.0039: at 55 min, χ23 = 20.3453, P = 0.0001) (Fig. 5 (b)). Of note, pretreatment with 50 mg/kg AMPT and 8 mg/kg RES abolished the ambulatory response to BUP.
|
|
|
Fig. 5. Effect of pretreatment with saline or α-methyl-p -tyrosine (AMPT) + reserpine (RES) on response of ambulatory activity to 10 mg/kg BUP. (a) Changes in actual ambulatory activity. (b) Changes in normalized ambulatory activity. Symbols show the median values of ambulatory activity for each 10-min period that were plotted against the midpoint of the measurement period, and vertical lines denote the first and third quartiles. *P < 0.05 compared with saline control. N = 32–36. |
3.4. Effects of drugs that preferentially antagonize DARs on response of ambulatory activity to BUP
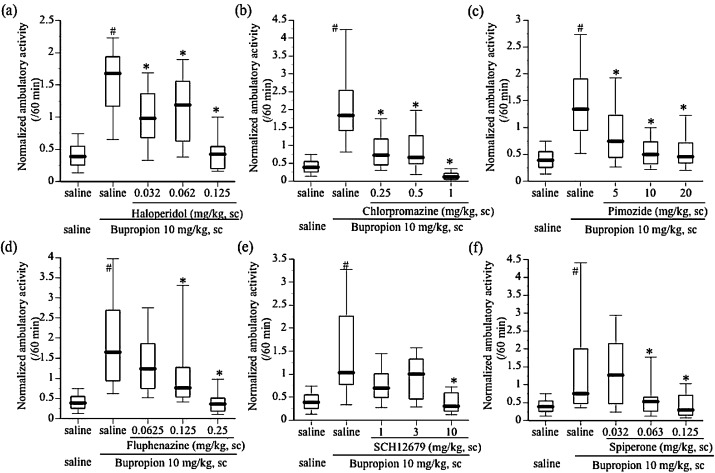
Fig. 6 illustrates the dose effects of (a) HAL, (b) CPZ, (c) PIM, (d) FLU, (e) SCH, and (f) SPI on the ambulatory effect of 10 mg/kg BUP. All drugs consistently attenuated the ambulation-promoting effect of BUP. (HAL: χ24 = 71.982, P < 0.0001; CPZ: χ24 = 78.4963, P < 0.0001; PIM: χ24 = 71.7352, P < 0.0001; FLU: χ24 = 118.4002, P < 0.0001; SCH: χ24 = 27.9333, P < 0.0001; SPI: χ24 = 43.8773, P < 0.0001).
|
|
|
Fig. 6. Effects of 0.032–0.125 mg/kg haloperidol (HAL) (a), 0.25–1 mg/kg chlorpromazine (CPZ) (b), 5–20 mg/kg pimozide (PIM) (c), 0.0625–0.25 mg/kg fluphenazine (FLU) (d), 1–10 mg/kg SCH12679 (SCH) (e), and 0.032–0.125 mg/kg spiperone (SPI) (f) on an effect of 10 mg/kg BUP on ambulatory activity. Data are shown using a box plot on normalized total ambulatory activity for 60 min after administration. (a) N = 20, (b) N = 20, (c) N = 30–40, (d) N = 30-50, (e) N = 10, (f) N = 18. #P < 0.05 compared with saline + saline. *P < 0.05 compared with saline + BUP. |
3.5. Effects of BUP on extracellular DA, DOPAC, HVA, and 5-HIAA in the CPu
The c-Fos-IR study indicated that the CPu was involved in the ambulatory response to BUP, and the pharmacological studies suggested that monoaminergic neurotransmitters, DA in particular, mediated the ambulatory response to BUP. Thus, we examined effects of BUP on extracellular monoaminergic neurotransmitters in the CPu using in vivo microdialysis. A dialysis probe was stereotaxically implanted into the CPu (AP: +0.1 mm, ML: +2.0 mm, DV: −2.8 mm) of mice according to the mouse brain atlas [42] (Appendix E).
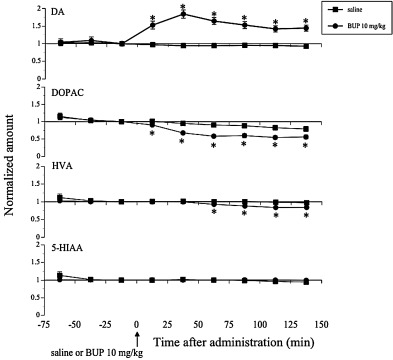
Fig. 7 illustrates alterations of DA, its metabolites DOPAC and HVA, and 5-HIAA, a metabolite of serotonin (5-HT), in dialysates of the CPu before and after administration of saline or 10 mg/kg BUP. The level of DA in the dialysates significantly increased and reached a maximum at 37.5 min after administration of BUP compared with that after saline administration (repeated-measures ANOVA; dose: F(1, 33) = 119.1605, P < 0.0001; time: F(8, 26) = 18.6742, P < 0.0001; interaction: F(8, 26) = 23.1516, P < 0.0001). Significantly higher levels of DA persisted until 137.5 min after BUP administration. Levels of DOPAC and HVA after BUP administration significantly decreased compared with those after saline administration (DOPAC: dose: F(1, 34) = 18.726, P = 0.0001; time: F(8, 27) = 11.0811, P < 0.0001; interaction: F(8, 27) = 9.6829, P < 0.0001; HVA; dose: F(1, 34) = 5.5205, P = 0.0247, time: F(8, 27) = 5.4972, P = 0.0004, interaction: F(8, 27) = 5.4972, P = 0.0004). On the other hand, the level of 5-HIAA did not alter after BUP administration (dose; F(1, 34) = 0.0222, P = 0.8825, time; F(8, 27) = 1.7864, P = 0.124, interaction; F(8, 27) = 1.2068, P = 0.3321).
|
|
|
Fig. 7. Alterations in dopamine (DA), its metabolites DOPAC and HVA, and 5-HIAA, a metabolite of serotonin (5-HT), in microdialysis samples obtained from the CPu of mice before and after administration of saline or 10 mg/kg BUP. The actual measured values for each compound just before administering saline or BUP in each mouse were defined as the baseline values, and all measured values were expressed as ratios to the baseline values. Symbols denote mean values that were plotted against the midpoint of the measurement period, and vertical lines indicate standard error of the mean (SEM). *P < 0.05 compared with saline. N = 17–20. |
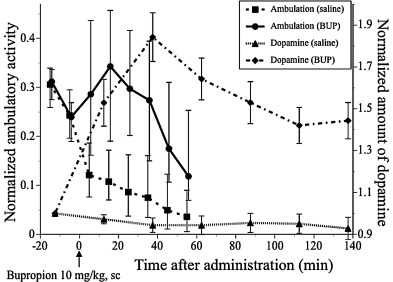
The time course of alteration in ambulatory activity (Fig. 1 ) was compared with that in the extracellular DA in the CPu (Fig. 7 ) after administration of BUP or saline (Fig. 8 ). The ambulatory activity appeared to increase, accompanied by an increase in the extracellular level of DA in the CPu, and the ambulatory activity appeared to decrease to the control level when the extracellular level of DA in the CPu decreased, although the level of DA in BUP-administered mice was significantly higher than that of control mice up to 137.5 min after administration.
|
|
|
Fig. 8. Comparisons of alterations in ambulatory activity with alterations in DA levels in dialysates of the CPu before and after administration of saline or 10 mg/kg BUP. Data are those shown in Fig. 1 and Fig. 7 . |
3.6. Effect of pretreatment with AMPT and RES on response of extracellular DA in the CPu to BUP
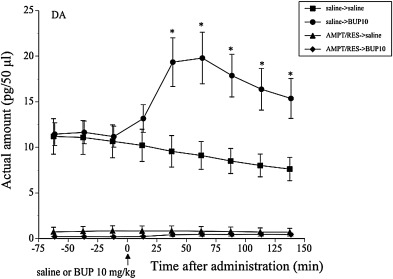
Pretreatment with 50 mg/kg AMPT and 8 mg/kg RES, that remarkably decreased the baseline ambulatory activity (Fig. 5 (a)), extensively reduced the actual amounts of DA in the dialysates of the CPu (three-way ANOVA; pretreatment; F(1, 25) = 79.9599, P < 0.0001; saline or BUP; F(1, 25) = 2.727, P = 0.065; time; F(8, 18) = 2.9876, P = 0.0256) (Fig. 9 ). The actual amount of DA significantly increased after administration of 10 mg/kg BUP in mice pretreated with saline (two-way ANOVA; saline or BUP; F(1, 12) = 5.3938, P = 0.0386, time; F(8, 5) = 1.8636, P = 0.2554, interaction; F(8, 5) = 1.99, P = 0.2326), whereas the actual amounts of DA did not show any significant alterations after BUP administration in mice pretreated with AMPT and RES (two-way ANOVA; saline or BUP; F(1, 12) = 0.9457, P = 0.35; time; F(8, 5) = 1.324, P = 0.3945, interaction; F(8, 5) = 0.4796, P = 0.8302). Thus, pretreatment with 50 mg/kg AMPT and 8 mg/kg RES, that abolished the ambulatory response to BUP (Fig. 5 ), abolished the response of extracellular DA in the CPu to BUP.
|
|
|
Fig. 9. Alterations in actual measured values of DA in dialysates obtained from the CPu before and after subcutaneous administration of saline or 10 mg/kg BUP. The mice were pretreated with saline or AMPT + RES. Symbols denote the mean values that were plotted against the midpoint of the measurement period, and vertical lines indicate the standard error of the mean (SEM). *P < 0.05 compared with the saline pretreated control. N = 6–8. |
4. Discussion
This is the first study that examined c-Fos-IR expression in brain of mice to which BUP was administered. In the present study, significant increase in c-Fos-IR relative to saline was observed only in the CPu of mice given 10 mg/kg BUP in the activity cage, though significant increase in c-Fos-IR relative to saline is observed in 42 of 64 brain regions including the CPu and PVA in rats given 20 mg/kg BUP in their home cages [8] . These different results could be due to several factors including differences in experimental conditions, animal species, and dose administered. It is known that AMPH induces c-Fos-IR in brain in different manners depending on the experimental environment [5] and [39] .
Three kinds of analyses for c-Fos-IR (comparison between saline treated group and BUP administered group, multiple regression analysis, single regression analysis) consistently indicated that c-Fos-IR in CPu positively correlated with the ambulatory activity, showing that neuronal activation in the CPu is involved in the ambulatory response to BUP. This result could be due to the characteristic of the tilting cage method that is more sensitive to horizontal movement such as locomotion of mouse in the activity cage. AMPH increases locomotion and c-Fos-IR in the CPu in mice, and the degree of c-Fos-IR in the CPu is positively correlated with the degree of locomotion promoted by AMPH [66] . It is probable that 10 mg/kg BUP principally increases horizontal movement of mouse in the activity cage through activating the CPu.
Co-administration of AMPT and RES abolished the ambulatory response to BUP. Furthermore, HAL, CPZ, PIM, FLU, SCH, and SPI reduced the ambulatory response to BUP. These pharmacological studies indicate that monoaminergic neurotransmitters, DA in particular, mediate the ambulatory response to BUP. Then, the present study examined effects of BUP on monoaminergic neurotransmitters in the CPu, as the c-Fos-IR study showed the CPu is involved in the ambulatory response to BUP. BUP significantly increased extracellular DA level in the CPu at the dose that promoted the ambulatory activity. The ambulatory activity appeared to increase, accompanied by an increase in the extracellular level of DA in the CPu, and the ambulatory activity appeared to decrease to the control level when the extracellular level of DA in the CPu decreased. The pretreatment with AMPT and RES, that abolished the ambulatory response to BUP, abolished the response of DA in the CPu to BUP. These findings show that DA in the CPu is involved in the ambulation-promoting effect of BUP. DAT is expressed in the mouse CPu [49] , suggesting that BUP increases extracellular level of DA primarily through inhibiting DAT in the mouse CPu as in the case of the rat CPu [33] , [37] and [38] . Increase of extracellular level of DA must increase c-Fos-IR in the CPu in mice given BUP as DA induces c-Fos-IR through DARs [64] , [36] and [15] .
The number of c-Fos-IR was significantly smaller in the PVA, however, c-Fos-IR in the PVA was not correlated with the ambulatory response to BUP. The results suggest that BUP affects the PVA functions [4] and [40] but the PVA is not related to the ambulatory response to BUP. Multiple regression analysis indicated that the ambulatory activity was a function of c-Fos-IR not only in the CPu but also in the LS, MnR, LGP, MGP, LC, and VMH. These brain regions interact one another. For example, the LGP and MGP receive neuronal input from the CPu that is known as an indirect pathway from the CPu to the SNR. The indirect pathway mediates the locomotor effect of AMPH in mice [24] . The CPu receives neuronal input from the LC and MnR in mice [19] and [35] . Interactions among these brain regions may also be involved in the ambulatory response to BUP.
Multiple regression analysis suggests that neuronal activity in the LC, the origin nucleus of NE neurons, is involved in the ambulatory response to BUP. The mouse CPu receives NE input from the LC and expresses NET [19] and [45] . BUP inhibits NET [16] . NE induces c-Fos through NE receptors [9] that are expressed in the CPu of mice [20] . NE may also play some roles for the effects of BUP on the neuronal activity in the CPu and the ambulatory activity, however, the current microdialysis study unfortunately failed to elucidate reliable responses to BUP of NE and its metabolite MHPG in the CPu. The results are probably due to low levels of NE and MHPG in the mouse CPu and the low IC50 of BUP for inhibiting NET [16] . BUP did not affect extracellular level of 5-HIAA, a metabolite of serotonin (5-HT), in the CPu in mice similar to in rats [11] , [33] and [37] . As increase of extracellular DA was accompanied by decrease of extracellular HVA and DOPAC in mice given BUP, BUP may not affect serotonergic state in the CPu in mice.
Striatal DA system is involved in the locomotion-promoting effects of AMPH and MetAMPH in mice [7] , [24] , [36] , [51] and [64] , and sensitization to the locomotor effects of AMPH and MetAMPH has been well studied as animal models of AMP and/or MetAMPH psychosis [28] and [44] . On the other hand, the “endogenous sensitization” hypothesis of schizophrenia has been proposed. The hypothesis postulates that a sensitized DA system is intrinsic to the disease and is responsible for the genesis of psychotic symptoms [1] , [12] , [32] and [34] . The hypothesis is supported by the reports that demonstrate enhanced striatal DA release induced by an acute AMPH challenge in first-episode schizophrenia patients relative to healthy controls [1] , [12] and [32] and overexpression of mesolimbic D2 receptors in the patients [2] , [31] and [63] . As BUP produces sensitization to the ambulation-promoting effect in mice [58] and the present study revealed that the DA in the CPu is involved in the ambulation-promoting effect of BUP, the concern about the potential of BUP to increase a risk of psychosis [29] seems reasonable.
In conclusion, the present study elucidated that DA in the CPu is involved in the ambulation-promoting effect of BUP in mice. The results of the present study suggest that other brain regions such as the LS, MnR, LGP, MGP, LC and VMH and another neurotransmitter NE also play some roles for the ambulatory response to BUP, however, elucidating the roles must await further research.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was partly supported by the Smoking Research Foundation , Tokyo, and the Study of the Health Effects of DPAA organized by the Ministry of the Environment, Japan.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [1] A. Abi-Dargham, R. Gil, J. Krystal, R.M. Baldwin, J.P. Seibyl, M. Bowers, C.H. van Dyck, D.S. Charney, R.B. Innis, M. Laruelle; Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort; Am. J. Psychiatry, 155 (1998), pp. 761–767
- [2] A. Abi-Dargham, J. Rodenhiser, D. Printz, Y. Zea-Ponce, R. Gil, L.S. Kegeles, R. Weiss, T.B. Cooper, J.J. Mann, R.L. Van Heertum, J.M. Gorman, M. Laruelle; Increased baseline occupancy of D2 receptors by dopamine in schizophrenia; Proc. Natl. Acad. Sci. U.S.A., 97 (2000), pp. 8104–8109
- [3] A. Armario; The contribution of immediate early genes to the understanding of brain processing of stressors; R. Pinaud, L. Tremere (Eds.), Immediate Early Genes in Sensory Processing, Cognitive Performance and Neurological Disorders, Springer Science, New York (2006), pp. 199–221
- [4] A. Armario; The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors?; CNS Neurol. Disord. Drug Targets, 5 (2006), pp. 485–501
- [5] A. Badiani, M.M. Oates, H.E.W. Day, S.J. Watson, H. Akil, T.E. Robinson; Amphetamine induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty; J. Neurosci., 18 (1998), pp. 10579–10593
- [6] J. Bailey, S. Waters; Acute psychosis after bupropion treatment in a healthy 28-year-old woman; J. Am. Board Fam. Med., 21 (2008), pp. 244–245
- [7] C. Bay-Richter, M.J. O’Callaghan, N. Mathur, C.M.P. O’Tuathaigh, D.M. Heery, K.C.F. Fone, J.L. Waddington, P.M. Moran; D-amphetamine and antipsychotic drug effects on latent inhibition in mice lacking dopamine D-2 receptors; Neuropsychopharmacol, 38 (2013), pp. 1512–1520
- [8] C.H.M. Beck; Acute treatment with antidepressant drugs selectively increases the expression of c-Fos in the rat-brain; J. Psychiatry Neurosci., 20 (1995), pp. 25–32
- [9] G.Y. Bing, E.A. Stone, Y. Zhang, D. Filder; Immunohistochemical studies of noradrenergic-induced expression of c-Fos in the rat CNS; Brain Res., 592 (1992), pp. 57–62
- [10] T. Brackins, N.C. Brahm, J.C. Kissack; Treatments for methamphetamine abuse: a literature review for the clinician; J. Pharm. Pract., 24 (2011), pp. 541–550
- [11] P. Bredeloux, I. Dubuc, J. Costentin; Comparisons between bupropion and dexamphetamine in a range of in vivo tests exploring dopaminergic transmission; Br. J. Pharmacol., 150 (2007), pp. 711–719
- [12] A. Breier, T.P. Su, R. Saunders, R.E. Carson, B.S. Kolachana, A. de Bartolomeis, D.R. Weinberger, N. Weisenfeld, A.K. Malhotra, W.C. Eckelman, D. Pickar; Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method; Proc. Natl. Acad. Sci. U.S.A., 94 (1997), pp. 2569–2574
- [13] L. Brunton, B. Chabner, B. Knollman; 12th ed.; Goodman and Gilman’s The Pharmacological Basis of Therapeutics, McGraw-Hill Professional, New York (2010)
- [14] X. Castells, M. Casas, X. Vidal, R. Bosch, C. Roncero, J.A. Ramos-Quiroga, D. Capella; Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials; Addict, 102 (2007), pp. 1871–1887
- [15] V. Colelli, M.T. Fiorenza, D. Conversi, C. Orsini, S. Cabib; Strain-specific proportion of the two isoforms of the dopamine D2 receptor in the mouse striatum: associated neural and behavioral phenotypes; Genes Brain Behav., 9 (2010), pp. 703–711
- [16] M.I. Damaj, F.I. Carroll, J.B. Eaton, H.A. Navarro, B.E. Blough, S. Mirza, R.J. Lukas, B.R. Martin; Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors; Mol. Pharmacol., 66 (2004), pp. 675–682
- [17] D.E. Dluzen, S. Bhatt, J.L. McDermott; Differences in reserpine-induced striatal dopamine output and content between female and male mice: implications for sex differences in vesicular monoamine transporter 2 function; Neurosci, 154 (2008), pp. 1488–1496
- [18] L.P. Dwoskin, A.S. Rauhut, K.A. King-Pospisil, M.T. Bardo; Review of the pharmacological and clinical profile of bupropion, an antidepressant and tobacco use cessation agent; CNS Drug Rev., 12 (2006), pp. 178–207
- [19] F. Fornai, M.T. Torracca, L. Bassi, D.A. D’Errigo, V. Scalori, G.U. Corsini; Norepinephrine loss selectively enhances chronic nigrostriatal dopamine depletion in mice and rats; Brain Res., 735 (1996), pp. 349–353
- [20] R. Gilsbach, A. Faron-Gorecka, Z. Rogoz, M. Bruss, M.G. Caron, M. Dziedzicka-Wasylewska, H. Bonisch; Norepinephrine transporter knockout-induced up-regulation of brain alpha(2A/C)-adrenergic receptors; J. Neurochem., 96 (2006), pp. 1111–1120
- [21] R.N. Golden, S.P. James, M.A. Sherer, M.V. Rudorfer, D.A. Sack, W.Z. Potter; Psychoses associated with bupropion treatment; Am J Psychiatry, 142 (1985), pp. 1459–1462
- [22] T. Hayashi, K. Ohashi, S. Tadokoro; Conditioned drug effects to d-amphetamine and morphine-induced motor acceleration in mice: experimental approach for placebo effect; Jpn. J. Pharmacol., 30 (1980), pp. 93–100
- [23] J.P. Herman, H. Figueiredo, N.K. Mueller, Y. Ulrich-Lai, M.M. Ostrander, D.C. Choi, W.E. Cullinan; Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness; Front. Neuroendocrinol., 24 (2003), pp. 151–180
- [24] T. Hikida, K. Kimura, N. Wada, K. Funabiki, S. Nakanishi; Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior; Neuron, 66 (2010), pp. 896–907
- [25] W.T. Howard, J.K. Warnock; Bupropion-induced psychosis; Am J Psychiatry, 156 (1999), pp. 2017–2018
- [26] J.H. Hsieh, D.J. Stein, F.M. Howells; The neurobiology of methamphetamine induced psychosis; Front Human Neurosci, 8 (2014) http://dx.doi.org/10.3389/fnhum.2014.00537 (article No. 537)
- [27] H. Javelot, A. Baratta, L. Weiner, T. Javelot, C. Nonnenmacher, J.F. Westphal, M. Messaoudi; Two acute psychotic episodes after administration of bupropion: a case of involuntary rechallenge; Pharm World & Sci, 31 (2009), pp. 238–240
- [28] P.W. Kalivas, B.A. Sorg, M.S. Hooks; The pharmacology and neural circuitry of sensitization to psychostimulants; Behav. Pharmacol., 4 (1993), pp. 315–334
- [29] S. Kumar, S. Kodela, J.G. Detweiler, K.Y. Kim, M.B. Detweiler; Bupropion-induced psychosis: folklore or a fact? A systematic review of the literature; Gen Hospit Psychiat, 33 (2011), pp. 612–617
- [30] H. Kuribara; Dopamine D-1 receptor antagonist SCH-23390 retards methamphetamine sensitization in both combined administration and early posttreatment schedules in mice; Pharmacol. Biochem. Behav., 52 (1995), pp. 759–763
- [31] M. Laruelle; Imaging dopamine transmission in schizophrenia: a review and meta-analysis; Q. J. Nucl. Med., 42 (1998), pp. 211–221
- [32] M. Laruelle; The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies; Brain Res. Brain Res. Rev., 31 (2000), pp. 371–384
- [33] S.X.M. Li, K.W. Perry, D.T. Wong; Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats; Neuropharmacol, 42 (2002), pp. 181–190
- [34] J.A. Lieberman, B.B. Sheitman, B.J. Kinon; Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity; Neuropsychopharmacol, 17 (1997), pp. 205–229
- [35] S. Martin, M. van den Buuse; Phencyclidine-induced locomotor hyperactivity is enhanced in mice after stereotaxic brain serotonin depletion; Behav. Brain Res., 191 (2008), pp. 289–293
- [36] R. Moratalla, M. Xu, S. Tonegawa, A.M. Graybiel; Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor; Proc. Natl. Acad. Sci. U.S.A., 93 (1996), pp. 14928–14933
- [37] G.G. Nomikos, G. Damsma, D. Wenkstern, H.C. Fibiger; Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo micro-dialysis; Neuropsychopharmacol, 2 (1989), pp. 273–279
- [38] G.G. Nomikos, G. Damsma, D. Wenkstern, H.C. Fibiger; In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis; Synapse, 6 (1990), pp. 106–112
- [39] M.M. Ostrander, A. Badiani, H.E.W. Day, C.S. Norton, S.J. Watson, H. Akil, T.E. Robinson; Environmental context and drug history modulate amphetamine-induced c-fos mRNA expression in the basal ganglia central extended amygdala, and associated limbic forebrain ; Neurosci, 120 (2003), pp. 551–571
- [40] M. Palkovits; Interconnections between the neuroendocrine hypothalamus and the central autonomic system; Front. Neuroendocrinol., 20 (1999), pp. 270–295
- [41] J. Park, P. Takmakov, R.M. Wightman; In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry; J. Neurochem., 119 (2011), pp. 932–944
- [42] G. Paxinos, K.B.J. Franklin; The Mouse Brain in Stereotaxic Coordinates; Elsevier, Academic Press, San Diego, CA, USA (2004)
- [43] J.S. Rhodes, A.E. Ryabinin, J.C. Crabbe; Patterns of brain activation associated with contextual conditioning to methamphetamine in mice; Behav. Neurosci., 119 (2005), pp. 759–771
- [44] T.E. Robinson, J.B. Becker; Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis; Brain Res., 396 (1986), pp. 157–198
- [45] K.S. Rommelfanger, D. Weinshenker, G.W. Miller; Reduced MPTP toxicity in noradrenaline transporter knockout mice; J. Neurochem., 91 (2004), pp. 1116–1124
- [46] M.E. Rose, J.E. Grant; Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions; Annal Clinic Psychiat, 20 (2008), pp. 145–155
- [47] B.L. Roth, J. Driscol; PDSP Ki Database. Psychoactive Drug Screening Program (PDSP) ; University of North Carolina at Chapel Hill and the United States National Institute of Mental Health (2013)
- [48] J.E. Slemmer, B.R. Martin, M.I. Damaj; Bupropion is a nicotinic antagonist; J. Pharmacol. Exp. Ther., 295 (2000), pp. 321–327
- [49] T. South, X.F. Huang; High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice; Neurochem. Res., 33 (2008), pp. 598–605
- [50] S.M. Stahl, J.F. Pradko, B.R. Haight, J.G. Modell, C.B. Rockett, S. Learned-Coughlin; A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor; Prim Care Companion J Clin Psychiatry, 6 (2004), pp. 159–166
- [51] T. Steinkellner, L. Mus, B. Eisenrauch, A. Constantinescu, D. Leo, L. Konrad, M. Rickhag, G. Sorensen, E.V. Efimova, E. Kong, M. Willeit, T.D. Sotnikova, O. Kudlacek, U. Gether, M. Freissmuth, D.D. Pollak, R.R. Gainetdinov, H.H. Sitte; In vivo amphetamine action is contingent on alpha CaMKII; Neuropsychopharmacol, 39 (2014), pp. 2681–2693
- [52] T. Umezu, J. Yonemoto, Y. Soma, T. Suzuki; Tris(2-chloroethyl)phosphate increases ambulatory activity in mice: pharmacological analyses of its neurochemical mechanism; Toxicol. Appl. Pharmacol., 148 (1998), pp. 109–116
- [53] T. Umezu, A. Sakata, H. Ito; Ambulation-promoting effect of peppermint oil and identification of its active constituents; Pharmacol. Biochem. Behav., 69 (2001), pp. 383–390
- [54] T. Umezu, M. Morita; Evidence for the involvement of dopamine in ambulation promoted by menthol in mice; J. Pharmacol. Sci., 91 (2003), pp. 125–135
- [55] T. Umezu; Evidence for dopamine involvement in ambulation promoted by menthone in mice; Pharmacol. Biochem. Behav., 91 (2009), pp. 315–320
- [56] T. Umezu; Evidence for dopamine involvement in ambulation promoted by pulegone in mice; Pharmacol. Biochem. Behav., 94 (2010), pp. 497–502
- [57] T. Umezu, K. Nakamiya, K. Kita, T. Ochi, Y. Shibata, M. Morita; Diphenylarsinic acid produces behavioral effects in mice relevant to symptoms observed in citizens who ingested polluted well water; Neurotoxicol. Teratol., 34 (2012), pp. 143–151
- [58] T. Umezu; Unusual effects of nicotine as a psychostimulant on ambulatory activity in mice; ISRN Pharmacol., 2012 (2012) http://dx.doi.org/10.5402/2012/170981 Article ID 170981
- [59] T. Umezu; Evaluation of Central Nervous System acting effects of plant-derived essential oils using ambulatory activity in mice; Pharmacol Pharm, 4 (2013), pp. 160–170 http://dx.doi.org/10.4236/pp.2013.42023(http://www.scirp.org/Journal/PaperInformation.aspx?PaperID=29704)
- [60] T. Umezu, Y. Shibata; Different behavioral effect dose-response profiles in mice exposed to two-carbon chlorinated hydrocarbons: influence of structural and physical properties; Toxicol. Appl. Pharmacol., 279 (2014), pp. 103–112
- [61] B.J. Venton, A.T. Seipel, P.E.M. Phillips, W.C. Wetsel, D. Gitler, P. Greengard, G.J. Augustine, R.M. Wightman; Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool; J. Neurosci., 26 (2006), pp. 3206–3209
- [62] T.S. Wang, I.S. Shiah, C.B. Yeh, C.C. Chang; Acute psychosis following sustained release bupropion overdose; Prog Neuro-Psychopharmacol Biol Pscychiat, 29 (2005), pp. 149–151
- [63] D.F. Wong, H.N. Wagner Jr., L.E. Tune, R.F. Dannals, G.D. Pearlson, J.M. Links, C.A. Tamminga, E.P. Broussolle, H.T. Ravert, A.A. Wilson, J.K. Toung, J. Malat, J.A. Williams, L.A. O'Tuama, S.H. Snyder, M.J. Kuhar, A. Gjedde; Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics; Science, 234 (1986), pp. 1558–1563
- [64] M. Xu, R. Moratalla, L.H. Gold, N. Hiroi, G.F. Koob, A.M. Graybiel, S. Tonegawa; Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphine and in dopamine-mediated behavioral-responses; Cell, 79 (1994), pp. 729–742
- [65] L. Zamboni, C. De Martino; Buffered picric acid-formaldehyde: a new, rapid fixative for electron microscopy; J. Cell Biol., 35 (1967), p. 148A
- [66] J.A. Zombeck, A.D. Lewicki, K. Patel, T. Gupta, J.S. Rhodes; Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6; Neuroscience, 165 (2010), pp. 1087–1099
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?