Summary
Background
Hepatitis C virus (HCV) infection can lead to increased insulin resistance, but the dynamics of insulin resistance in HCV-infected patients receiving pegylated interferon plus ribavirin remain elusive.
Methods
This prospective study enrolled HCV-infected patients who received pegylated interferon plus ribavirin. Patients were classified according to the attainment of sustained virological response (SVR). Insulin resistance was measured using homeostatic model assessment-insulin resistance (HOMA-IR). The change in HOMA-IR at baseline, the end of treatment, and 24 weeks after the end of treatment was compared in patients who achieved SVR and those who did not.
Results
A total of 65 patients participated in this study, of which 46 (71%) achieved SVR. Overall, The HOMA-IR changed significantly during antiviral therapy, with the median values [interquartile range (IQR)] of 3.7 (1.6–10.0) prior to the treatment, 1.5 (0.8–2.9) at the end, and 1.6 (0.9–3.1) at 24 weeks after completion of therapy. However, only patients who achieved SVR had significant off-therapy reduction of HOMA-IR, with median values of 1.3 (IQR, 0.7–2.6) at 24 weeks off therapy and 3.6 (IQR, 1.5–9.9) at baseline (p < 0.0001). In those without SVR, the HOMA-IR measured 24 weeks after treatment completion (median, 2.2; IQR, 1.9–4.7) did not differ from baseline values (median, 3.9; IQR, 2.2–10.0; p = 0.5).
Conclusion
Dual therapy with pegylated interferon plus ribavirin ameliorated IR in HCV-infected patients, but the off-therapy improvement of IR was limited to those who attained SVR.
Keywords
Hepatitis C; Insulin resistance; Sustained virological response
Introduction
Hepatitis C virus (HCV) infection is causally associated with impaired glucose homeostasis. Epidemiological surveys have consistently revealed a higher prevalence of insulin resistance and diabetes mellitus (DM) in HCV-infected patients than in the general population or in those with other chronic liver diseases [1], [2], [3], [4], [5], [6], [7], [8] and [9]. HCV infection may cause insulin resistance, which is the essential component of metabolic syndrome and type 2 DM, through the direct involvement of viral proteins or as an indirect consequence of infection-related cytokine dysregulation [8], [10], [11], [12] and [13].
Because chronic HCV infection is causally associated with impaired glucose homeostasis, it is plausible that viral clearance may ameliorate insulin resistance and prevent DM. Current evidence, however, remains inconclusive in confirming the efficacy of successful HCV eradication in improving glucose intolerance. Several follow-up studies observing treated patients have reported encouraging results by demonstrating a lower incidence of new-onset type 2 DM in HCV-infected patients who achieved sustained virological response (SVR), as compared with those who could not clear the virus [14], [15] and [16]. Nevertheless, these studies could not ascertain whether the apparent reduction of DM incidence was the result of viral eradication or simply reflected that patients who were more likely to develop DM were more difficult to treat. Several studies showed that HCV-infected patients with insulin resistance were more difficult to treat [17] and [18]. In order to elucidate how antiviral treatment as well as viral clearance may impact the glucose homeostasis in patients with HCV infection, it is imperative to measure insulin resistance alongside the therapeutic course.
We conducted this study to explore the change in insulin resistance from the baseline, through the end of, and to 24 weeks after the standard regimen with pegylated interferon plus ribavirin. The association between viral clearance and amelioration of insulin resistance was assessed.
Patients and methods
Study design and patient population
This is a prospective cohort study of HCV-infected patients receiving standard antiviral therapy in a regional teaching hospital in southern Taiwan (E-Da Hospital, Kaohsiung, Taiwan). The study protocol was approved by the Institutional Review Board of E-Da Hospital (EMRP-099-112). Patients were eligible if they were older than 20 years, were seropositive for HCV antibody, had detectable HCV RNA in serum, and had elevated serum alanine aminotransferase. Those who met any of the following exclusion criteria were not enrolled: coinfection with human immunodeficiency virus or hepatitis B virus, alcohol abuse (daily consumption converted to more than 40 mL pure ethanol), presence of other concomitant liver diseases (hemochromatosis, Wilson disease, drug-related hepatitis, or alpha-1 antitrypsin deficiency), pregnant or lactating women, severe comorbidity (malignancy, major psychiatric disorder, or failure of vital organ), contraindication for interferon-based therapy, and lack of informed consent.
Antiviral therapy
All patients were treated with response-guided pegylated interferon alpha-2b (1.5 μg/kg per week; PEG-Intron; Schering-Plough Inc., Kenilworth, NJ, USA) and ribavirin (Rebetol; Schering-Plough Inc., Las Piedras, Puerto Rico) with dosage adjusted on the basis of body weight (1200 mg/d for weight ≥ 100 kg; 1000 mg/d for weight < 75 kg). Patients who achieved rapid virological response received a 24-week therapy; those who failed to attain rapid virological response but could achieve early virological response were treated for 48 weeks. Undetectable HCV RNA 24 weeks after the completion of therapy defined the achievement of SVR. The HCV RNA test was performed at a central laboratory (Taipei Institute of Pathology, Taipei, Taiwan) for HCV RN level using second-generation real-time polymerase chain reaction assay (COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test, version 2.0; Roche Molecular Diagnostics, Branchburg, NJ, USA; detection limit,15 IU/mL) and for HCV RNA genotype by LINEAR ARRAY Hepatitis C Virus Genotyping Test (Roche Molecular Diagnostics, Branchburg, NJ, USA).
Methods of measurement and definition of insulin resistance
Prior to the antiviral therapy, patients were interviewed and physically examined to obtain comprehensive personal information. Body mass index (BMI) was calculated as the patient’s weight in kilograms divided by the square of height in meters. HCV genotyping was performed according to Simmonds' system.
Venous blood for laboratory examinations was collected in the morning after an overnight fast at baseline, at the end of the therapy, and 24 weeks after the cession of therapy. Insulin resistance was measured indirectly with homeostasis model assessment (HOMA-IR) method using the following formula:
|
|
Statistical analysis
The outcome of interest was the change in HOMA-IR during the antiviral therapy. Continuous variables are expressed with median and interquartile range (IQR) and categorical variables with percentage of occurrence. For comparison of continuous variables, the Wilcoxon matched-pairs signed-ranks test was used for values collected at different time points in the same patient, and the rank-sum test for between-group comparison. Fishers exact test was used for analysis of proportions. All statistical analyses were two-tailed and carried out using a commercial software package (Stata, version 9.1; Stata Corp., College Station, TX, USA). A p value < 0.05 was the threshold for statistical significance.
Results
Baseline characteristics of the study population
A total of 65 patients were enrolled in this study (Table 1). This cohort included 41 men (63.1%). The predominant viral genotypes was type 1 (55.4%, n = 36) and followed by type 2 (41.6%, n = 27). The median viral load prior to antiviral therapy was 8.6 × 105 (IQR, 5.9 × 104–3.7 × 106) IU/mL. The pretreatment median value of HOMA-IR was 3.7 (IQR, 1.6–10.0), and the median concentration of C-peptide was 3.3 (IQR, 2.0–6.1) ng/mL. After antiviral treatment, 71% (n = 46) successfully achieved SVR.
| Characteristics | |
|---|---|
| Age, y | 53 (45–59) |
| Male sex | 41 (63.1) |
| Body mass index, kg/m2 | 24.8 (22.7–26.2) |
| Waist circumference, cm | 89.5 (82–96) |
| Hip circumference, cm | 99.3 (94–104.5) |
| Waist/hip ratio | 0.90 (0.86–0.94) |
| HCV genotype | |
| Genotype 1 | 36 (55.4) |
| Genotype 2 | 27 (41.6) |
| Genotype 6 | 1 (1.5) |
| Mixed genotypes | 1 (1.5) |
| HCV RNA, IU/mL | 8.6 × 105 (5.9 × 104–3.7 × 106) |
| AST, IU/L | 93 (69–122) |
| ALT, IU/L | 129 (82–200) |
| Creatinine, mg/dL | 1.1 (0.9–1.2) |
| Platelet, 103/μL | 172 (143–215) |
| Hemoglobin, g/dL | 14.7 (13.8–15.7) |
| Leukocyte, /μL | 6180 (4890–7090) |
| Diabetes mellitus | 12 (18.5) |
| Hypertension | 16 (24.6) |
| Cirrhosis | 5 (7.7) |
| Glucose, mg/dL | 98 (90–120) |
| Insulin, μU/mL | 13.5 (7.3–33.6) |
| HOMA-IR | 3.7 (1.6–10.0) |
| C peptide, ng/mL | 3.3 (2.0–6.1); 4.3 ± 3.1 |
| Cholesterol, mg/dL | 182 (158–212) |
| LDL, mg/dL | 100 (79–125) |
| Triglyceride, mg/dL | 152 (103–201) |
| Triglyceride, mg/dL | 152 (103–201) |
Data are presented as n (%) or n (range).
ALT = alanine aminotransferase; AST = aspartate aminotransferase; HCV = hepatitis C virus; HOMA-IR = homeostatic model assessment-insulin resistance; LDL = low-density lipoprotein.
Change in HOMA-IR during antiviral therapy and the association with SVR
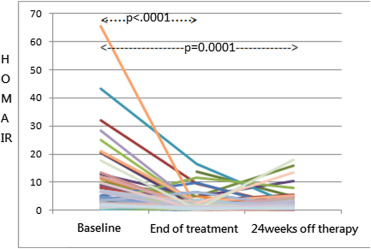
Overall, HOMA-IR changed significantly during the antiviral therapy (Fig. 1), with a median value (IQR) of 3.7 (1.6–10.0) prior to the treatment, 1.5 (0.8–2.9) at the end of course, and 1.6 (0.9–3.1) 24 weeks after the completion of treatment (Fig. 1). From baseline to the end of therapy, HOMA-IR decreased significantly in patients who achieved SVR and in those who did not (Table 2).
|
|
|
Figure 1. Change in HOMA-IR levels during therapy and 24 weeks off therapy in all patients. (HOMA-IR = homeostatic model assessment-insulin resistance).
|
| Baseline | End of therapy | pa | 24 wk off therapy | pa | |
|---|---|---|---|---|---|
| Overall (n = 65) | 3.7 (1.6–10.0) | 1.5 (0.8–2.9) | <0.0001 | 1.6 (0.9–3.1) | 0.0001 |
| SVR (n = 46) | 3.6 (1.5–9.9) | 1.4 (0.6–2.7) | 0.001 | 1.3 (0.7–2.6) | <0.0001 |
| No SVR (n = 19) | 3.9 (2.2–10.0) | 2.1 (0.8–4.8) | 0.002 | 2.2 (1.9–4.7) | 0.46 |
HOMA-IR = homeostatic model assessment-insulin resistance; SVR = sustained virological response.
a. p values calculated by Wilcoxon matched-pairs signed-ranks test, with baseline measurement as the reference for comparison.
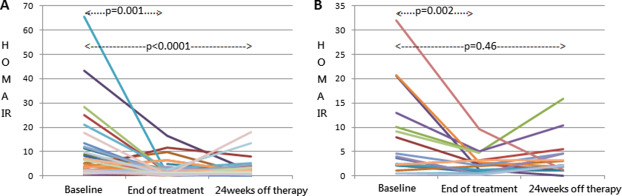
However, only patients who achieved SVR maintained amelioration of insulin resistance after 24 weeks off the antiviral regimen (Fig. 2A), whereas those who failed to achieve viral eradication had HOMR-IR rebound after 24 weeks off treatment (Fig. 2B). The median values of HOMA-IR in patients with SVR were 1.3 (IQR, 0.7–2.6) and 3.6 (IQR, 1.5–9.9) at 24 weeks after completion of therapy and at baseline, respectively (p < 0.0001). In those without SVR, the median HOMA-IR 24 weeks off therapy was 2.2 (IQR, 1.9–4.7), statistically similar with 3.9 (IQR, 2.2–10.0) prior to treatment (p = 0.46).
|
|
|
Figure 2. Change in HOMA-IR levels during therapy and 24 weeks off therapy in (A) patients with sustained virological response (SVR) and (B) those without SVR. (HOMA-IR = homeostatic model assessment-insulin resistance).
|
Change in BMI during antiviral therapy and the association with SVR
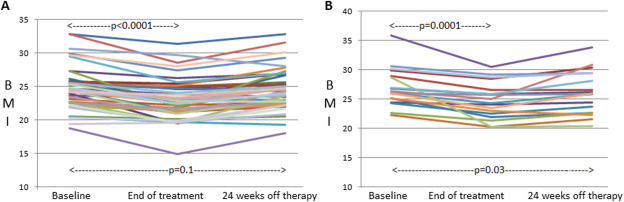
The BMI decreased significantly during the antiviral therapy in both patients who achieved SVR and those who did not, and increased in both groups (Fig. 3 and Table 3). In patients who achieved SVR (Fig. 3A and Table 3), BMI at baseline and that at 24 weeks off therapy did not significantly differ (p = 0.1).
|
|
|
Figure 3. Change in body mass index (BMI) during therapy and 24 weeks off therapy in (A) patients with sustained virological response (SVR) and (B) those without SVR.
|
| Baseline | End of therapy | pa | 24 wk off therapy | pa | |
|---|---|---|---|---|---|
| Overall (n = 65) | 25.1 (23.5–26.3) | 23.2 (21.3–25.1) | <0.0001 | 24.6 (22.7–26.7) | 0.01 |
| SVR (n = 46) | 24.4 (22.7–25.8) | 22.9 (21.2–24.8) | <0.0001 | 24.3 (22.6–25.6) | 0.1 |
| No SVR (n = 19) | 26.0 (24.6–28.9) | 24.3 (22.5–26.6) | 0.0001 | 25.9 (22.7–29.4) | 0.03 |
BMI = body mass index; SVR = sustained virological response.
a. p values calculated by Wilcoxon matched-pairs signed-ranks test, with baseline measurement as the reference for comparison.
Discussion
This prospective study unraveled how insulin resistance would change during the antiviral treatment for HCV infection. We demonstrated a significant reduction of insulin resistance in infected patients after they finished the therapy with pegylated interferon plus ribavirin. However, off-therapy amelioration of insulin resistance was observed only in patients who achieved SVR, indicating that viral clearance is essential for the efficacy to be sustained. Collectively, these findings corroborate the causative role of HCV in impairing glucose homeostasis and implicate the clinical effectiveness of viral eradication in extrahepatic outcomes.
Consistent with our results, several studies from Western countries have recently reported the efficacy of anti-HCV therapy in improving insulin resistance [19], [20] and [21]. All of these studies also indicated the importance of successful clearance. For instance, investigators of the HALT-C (Hepatitis C Antiviral Long-term Treatment against Cirrhosis) trial showed that HOMA-IR changed in a linear fashion according to virological response during the antiviral treatment, after adjusting for the baseline insulin resistance and other potential confounders including BMI, age, sex, and liver fibrosis [20]. Similar results were reported from the Virahep-C study, which exclusively enrolled genotype 1-infected patients with insulin resistance (HOMA-IR >2) at baseline [19]. Given that relevant data from the East remains relatively sparse [22], [23], [24] and [25], this study, which was conducted in an ethnic Chinese population, adds to the growing body of literature in elucidating the influence of HCV on mediating glucose and energy balance.
It is intriguing to note that insulin resistance decreased at the end of the antiviral therapy in most patients regardless of their virological response. The direct pharmacological activity of interferon is unlikely to be the reason because available data indicate that interferon actually induces insulin resistance, instead of ameliorating it [26], [27] and [28]. The most plausible explanation for this finding probably relates to the effects on appetite and body weight during the interferon-based regimen. Conjeevaram and colleagues [19] reported in a large cohort that BMI significantly decreased during interferon therapy irrespective of virological response. They further pointed out that weight loss was usually transient and maintained until completion of the therapy. Accordingly, improvement of insulin resistance that results from weight loss should also be short-lived. Sustained amelioration of insulin resistance in patients who achieved SVR, therefore, cannot be accounted for by the change in body weight. This is consistent with our study. It also appears rational that remission of insulin resistance may derive from improvement of liver fibrosis after viral clearance, insomuch as that the stage of fibrosis correlates with the severity of glucose abnormality [6]. Nonetheless, regression of liver fibrosis usually takes a long time and unlikely to occur 6 months off therapy. All in all, these lines of evidence indicate that HCV per se is the inciting etiology for aberrant glucose homeostasis in infected individuals.
Our findings implicate that antiviral treatment for HCV infection may decrease clinical complications that pathogenically result from impaired glucose control. Previous studies have shown that HCV not only increases the risk of DM [1], [2] and [3], but also raises the hazard of relevant clinical complications such as renal failure and stroke [29] and [30]. Through amelioration of insulin resistance and subsequent betterment of glycemic control, viral eradication could thereby improve renal and cardiovascular outcomes in diabetic patients. Therefore, this study lends support to a recent nationwide cohort study that utilized a national claim database to uncover the association of antiviral treatment with improved renal and cardiovascular outcomes in diabetic patients with HCV infection [31].
There are several limitations in our study. First, the sample size did not suffice for a subgroup analysis. As a result, we could not further analyze how viral genotype or viral load might influence the change in insulin resistance. From the existing literature, HCV-related insulin resistance is most likely present across different genotypes [4], although some studies suggest it is more frequent in genotypes 1 and 4 [6] and [32]. Further studies are warranted to elucidate whether change in insulin resistance during antiviral treatment will differ according to viral genotype. Second, therapeutic duration was not standardized in our study cohort because the regimen was guided by virological response. This limitation apparently has led to variable dosage of antiviral drugs among participants. However, it did not affect insulin resistance measured 24 weeks off treatment because direct pharmacological effects of these drugs should have already waned completely by then.
In conclusion, antiviral therapy improves insulin resistance during HCV treatment. The durability persists only in patients who achieve SVR. These findings suggest that HCV plays an etiological role in the pathogenesis of impaired glucose homeostasis. Moreover, the reversibility of insulin resistance through viral eradication implicates tremendous clinical benefits by readily available regimens.
Conflicts of interest
Yao-Chun Hsu has received lecture fees from Merck Sharp & Dohme (Taipei, Taiwan) and Roche (Taipei, Taiwan). The authors declare no conflicts of interest.
Acknowledgments
We are grateful to Ms Wei-Lin Huang for her assistance in the patient care and data collection. This study was funded by the Taiwan Ministry of Science and Technology (101-2314-B-650-003), the Taipei Institute of Pathology (10008A), and the E-Da Hospital (EDAHP102006). The funding sources had no role in any part of the research including study design, data collection, data analysis, result interpretation, manuscript writing, and submission for publication.
References
- [1] J.F. Huang, C.Y. Dai, S.J. Hwang, C.K. Ho, P.J. Hsiao, M.Y. Hsieh, et al.; Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication; Am J Gastroenterol, 102 (2007), pp. 1237–1243
- [2] J.F. Huang, M.L. Yu, C.Y. Dai, M.Y. Hsieh, S.J. Hwang, P.J. Hsiao, et al.; Reappraisal of the characteristics of glucose abnormalities in patients with chronic hepatitis C infection; Am J Gastroenterol, 103 (2008), pp. 1933–1940
- [3] D.L. White, V. Ratziu, H.B. El-Serag; Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis; J Hepatol, 49 (2008), pp. 831–844
- [4] A. Mangia, M. Ripoli; Insulin resistance, steatosis and hepatitis C virus; Hepatol Int, 7 (2013), pp. 782–789
- [5] K.L. Milner, D. van der Poorten, M. Trenell, A.B. Jenkins, A. Xu, G. Smythe, et al.; Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance; Gastroenterology, 138 (2010), pp. 932–941 e933
- [6] R. Moucari, T. Asselah, D. Cazals-Hatem, H. Voitot, N. Boyer, M.P. Ripault, et al.; Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis; Gastroenterology, 134 (2008), pp. 416–423
- [7] M. Romero-Gómez; Insulin resistance and hepatitis C; World J Gastroenterol, 12 (2006), p. 7075
- [8] E. Vanni, M.L. Abate, E. Gentilcore, I. Hickman, R. Gambino, M. Cassader, et al.; Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C; Hepatology, 50 (2009), pp. 697–706
- [9] C.Y. Dai, M.L. Yeh, C.F. Huang, C.H. Hou, M.Y. Hsieh, J.F. Huang, et al.; Chronic hepatitis C infection is associated with insulin resistance and lipid profiles; J Gastroenterol Hepatol (2013) http://dx.doi.org/10.1111/jgh.12313
- [10] T. Kawaguchi, Y. Nagao, K. Tanaka, T. Ide, M. Harada, R. Kumashiro, et al.; Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study; Int J Mol Med, 16 (2005), pp. 109–114
- [11] T. Kawaguchi, T. Yoshida, M. Harada, T. Hisamoto, Y. Nagao, T. Ide, et al.; Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3; Am J Pathol, 165 (2004), pp. 1499–1508
- [12] M.Y. Sheikh, J. Choi, I. Qadri, J.E. Friedman, A.J. Sanyal; Hepatitis C virus infection: molecular pathways to metabolic syndrome; Hepatology, 47 (2008), pp. 2127–2133
- [13] Y. Shintani, H. Fujie, H. Miyoshi, T. Tsutsumi, K. Tsukamoto, S. Kimura, et al.; Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance; Gastroenterology, 126 (2004), pp. 840–848
- [14] Y. Arase, F. Suzuki, Y. Suzuki, N. Akuta, M. Kobayashi, Y. Kawamura, et al.; Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C; Hepatology, 49 (2009), pp. 739–744
- [15] M. Romero-Gómez, C.M. Fernández-Rodríguez, R.J. Andrade, M. Diago, S. Alonso, R. Planas, et al.; Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C; J Hepatol, 48 (2008), pp. 721–727
- [16] R. Simó, A. Lecube, J. Genescà, J.I. Esteban, C. Hernández; Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection; Diabetes Care, 29 (2006), pp. 2462–2466
- [17] C.-Y. Dai, J.-F. Huang, M.-Y. Hsieh, N.-J. Hou, Z.-Y. Lin, S.-C. Chen, et al.; Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients; J Hepatol, 50 (2009), pp. 712–718
- [18] M. Khattab, M. Eslam, M.A. Sharwae, M. Shatat, A. Ali, L. Hamdy; Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients; Am J Gastroenterol, 105 (2010), pp. 1970–1977
- [19] H.S. Conjeevaram, A.S. Wahed, N. Afdhal, C.D. Howell, J.E. Everhart, J.H. Hoofnagle; Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C; Gastroenterology, 140 (2011), pp. 469–477
- [20] A. Delgado-Borrego, S.H. Jordan, B. Negre, D. Healey, W. Lin, Y. Kamegaya, et al.; Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial; Clin Gastroenterol Hepatol, 8 (2010), pp. 458–462
- [21] K.L. Milner, A. Jenkins, M. Trenell, J. Tid-Ang, D. Samocha-Bonet, M. Weltman, et al.; Eradicating hepatitis C virus ameliorates insulin resistance without change in adipose depots; J Viral Hepatitis, 21 (2014), pp. 325–332
- [22] H.J. Jung, Y.S. Kim, S.G. Kim, Y.N. Lee, S.W. Jeong, J.Y. Jang, et al.; The impact of pegylated interferon and ribavirin combination treatment on lipid metabolism and insulin resistance in chronic hepatitis C patients; Clin Mol Hepatol, 20 (2014), pp. 38–46
- [23] T. Kawaguchi, T. Ide, E. Taniguchi, E. Hirano, M. Itou, S. Sumie, et al.; Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2; Am J Gastroenterol, 102 (2007), pp. 570–576
- [24] Y. Kawaguchi, T. Mizuta, N. Oza, H. Takahashi, K. Ario, T. Yoshimura, et al.; Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C; Liver Int, 29 (2009), pp. 871–877
- [25] H.J. Kim, J.H. Park, D.I. Park, Y.K. Cho, C.I. Sohn, W.K. Jeon, et al.; Clearance of HCV by combination therapy of pegylated interferon alpha-2a and ribavirin improves insulin resistance; Gut Liver, 3 (2009), pp. 108–115
- [26] E. Imano, T. Kanda, Y. Ishigami, M. Kubota, M. Ikeda, M. Matsuhisa, et al.; Interferon induces insulin resistance in patients with chronic active hepatitis C; J Hepatol, 28 (1998), pp. 189–193
- [27] V.A. Koivisto, R. Pelkonen, K. Cantell; Effect of interferon on glucose tolerance and insulin sensitivity; Diabetes, 38 (1989), pp. 641–647
- [28] T. Wada, M. Hoshino, Y. Kimura, M. Ojima, T. Nakano, D. Koya, et al.; Both type I and II IFN induce insulin resistance by inducing different isoforms of SOCS expression in 3T3-L1 adipocytes; Am J Physiol Endocrinol Metab, 300 (2011), pp. E1112–E1123
- [29] F.-H. Su, C.-T. Su, S.-N. Chang, P.-C. Chen, F.-C. Sung, C.-C. Lin, et al.; Association of hepatitis C virus infection with risk of ESRD: a population-based study; Am J Kidney Dis, 60 (2012), pp. 553–560
- [30] M.-H. Lee, H.-I. Yang, C.-H. Wang, C.-L. Jen, S.-H. Yeh, C.-J. Liu, et al.; Hepatitis C virus infection and increased risk of cerebrovascular disease; Stroke, 41 (2010), pp. 2894–2900
- [31] Y.C. Hsu, J.T. Lin, H.J. Ho, Y.H. Kao, Y.T. Huang, N.W. Hsiao, et al.; Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients; Hepatology, 59 (2014), pp. 1293–1302
- [32] T. Sersté, M. Nkuize, R. Moucari, M. Van Gossum, M. Reynders, R. Scheen, et al.; Metabolic disorders associated with chronic hepatitis C: impact of genotype and ethnicity; Liver Int, 30 (2010), pp. 1131–1136
Document information
Published on 01/01/2017
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?