Summary
Esophageal cancer is a common and highly lethal disease. In the Asia-Pacific region, esophageal squamous cell neoplasias are the major forms of the disease. Recent advances in endoscopic therapy enable curative treatment of early esophageal squamous cell neoplasias, however, the technique is complicated and requires a high level of expertise, especially for those with long-segment lesions. Endoscopic radiofrequency ablation is a rapidly evolving treatment modality and has been shown to have good efficacy and safety for the treatment of dysplasia in cases of Barrett’s esophagus. Theoretically, it can also be used to treat squamous dysplasia. We report a case of a 48-year-old man with an 8-cm-long circumferential squamous high-grade dysplasia over the esophagus (from 21 cm to 29 cm below the incisor) that was treated successfully and safely with balloon-based radiofrequency ablation. The procedure took only around 30 minutes to complete. There were no major adverse events during and after the procedure. In addition, we examined the histology of the esophageal coagulum, which showed an extensive cauterization effect with focal dysplasia within the ablated epithelium. Follow-up endoscopy at 1 month, 3 months, and 6 months showed no residual lesion, and biopsies also confirmed complete remission.
Keywords
Endoscopic therapy ; Esophageal cancer ; Esophageal squamous cell neoplasia ; Radiofrequency ablation
Introduction
Esophageal cancer is a highly lethal disease, causing >400,000 deaths/y worldwide [1] . It ranks as the sixth most common cause of cancer death, and >70% of esophageal cancer cases occur in Asia [1] . In the Asia-Pacific region, esophageal squamous cell neoplasia (ESCN) is the major histological type of the disease [2] . Recent advances in image-enhanced endoscopy enable accurate diagnosis of early-stage ESCNs [3] . Endoscopic treatment serves as an excellent option with curative intent, because lesions of squamous high-grade dysplasia or carcinomas are limited to the epithelium or lamina propria with little risk of lymph node metastasis [4] . Recently, endoscopic submucosal dissection (ESD) is gaining attention as a treatment tool [4] . However, in order to acquire the necessary skill-set for good and safe application of ESD techniques, a significant learning curve is required [5] . Due to this limitation, this technique cannot be expanded worldwide and can only be carried out in some high-volume centers. In addition, esophageal stenosis is still a great concern, especially for long-segment lesions or lesions covering more than three-quarters of the circumference of the esophagus [6] . Endoscopic radiofrequency ablation (RFA) is a rapidly evolving treatment modality, and has been shown to have good efficacy and safety for the treatment of dysplasia in cases of Barrett’s esophagus (BE) [7] . Theoretically, it can also be used to treat squamous dysplasia, however, previous reports are very rare [8] ; [9] . We report a case with an 8-cm-long circumferential squamous high-grade dysplasia over the esophagus (from 21 cm to 29 cm below the incisor) that was treated successfully and safely with balloon-based RFA.
Case Report
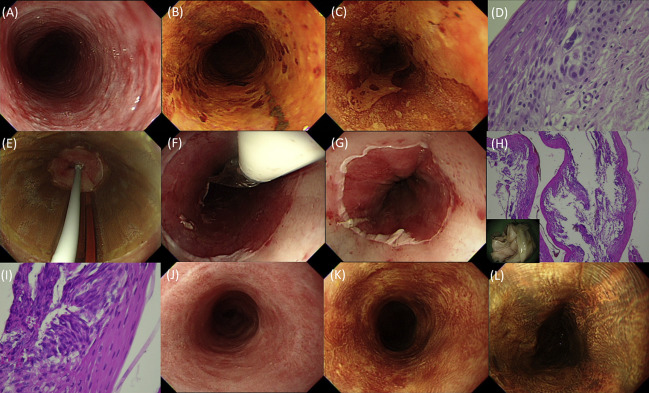
A 48-year-old man with a social history significant for alcohol consumption, smoking, and betel nut chewing for several decades was referred for an esophagogastroduodenoscopic examination due to his acid regurgitation symptoms. Conventional white-light endoscopy showed a faint circumferential flat hyperemic lesion over the upper esophagus. Lugol chromoendoscopy showed an 8-cm-long well-demarcated Lugol-voiding lesion, occupying almost the total circumference of the esophagus from 21 cm to 29 cm below the incisor (Figures 1 A–C). Narrow-band imaging with magnification showed dilated intraepithelial papillary capillary loops, which are consistent with high-grade intraepithelial neoplasia. The histology taken from endoscopic biopsy every 2 cm confirmed a high-grade squamous dysplasia (Figure 1 D). Subsequent endoscopic ultrasound and computer topography scan showed that there was no lymphadenopathy or distant metastasis. After detailed explanations of the use of RFA or ESD and alternative treatment options, the patient agreed to be treated with balloon-type RFA and provided informed consent. The HALO360 System (Covidien GI Solutions, Sunnyvale, CA, USA), which consists of an ablation catheter, an energy generator, and a sizing balloon, was deployed for the eradication of the esophageal lesion. The procedure was performed under general anesthesia. Lugol staining was performed before RFA to determine the location and extent of the Lugol-voiding lesions. Then, a sizing balloon was introduced over a guide-wire to measure the inner diameter of the esophagus. Accordingly, an 18-mm-diameter balloon catheter with a 3-cm-long bipolar array that can deliver short-burst (1 second) RFA at 40 W/cm2 and 12 J/cm2 was selected and introduced over the guide-wire to the diseased segment. We used a 12 J/cm2 –clean–12 J/cm2 regimen [8] for the procedure (Figures 1 E–G). The treatment area included 1 cm proximal to 1 cm distal of the Lugol-voiding lesion. We started ablation from the oral to the distal side with a 0.5-cm overlap between each application. After electrode activation, the energy generator sensed and reported good efficacy over the treatment area in all applications. After the first pass of ablation, an endoscope equipped with a transparent cap was used to remove adherent coagulum from the ablation zone. Then, the ablation catheter was reintroduced and the area was treated for a second time. The procedure time was only approximately 30 minutes. No immediate adverse events were noted (Figure 1 G). The removed coagulum was sent for a histological examination, and it showed an extensive cauterization effect with focal dysplastic epithelium (Figures 1 H and 1I). After the procedure, the patient was provided with esomeprazole 40 mg/d and sucralfate suspension 5 mL (200 mg/mL) four times daily for 1 month. He started oral intake on Day 2 post-RFA. Chest pain and throat pain developed after the procedure and resolved within 3 days. He was discharged uneventfully on the 3rd day post-RFA. Follow-up endoscopy at 1 month, 3 months, and 6 months showed no residual Lugol-voiding lesion or stricture (Figures 1 J–L). Biopsies taken randomly from the normal-appearing mucosa every 2 cm over the treatment area also confirmed a complete remission.
|
|
|
Figure 1. Endoscopic images prior to, during, and after balloon-based radiofrequency ablation of a long-segment circumferential high-grade dysplasia. (A) A reddish, rough-appearing, and circumferential lesion with conventional white-light imaging. (B, C) Lugol chromoendoscopy showing a well-demarcated long-segment unstained lesion. (D) Endoscopic biopsy showing squamous high-grade dysplasia. (E) Treatment with the first balloon-based RFA. (F) After the first ablation pass, a circumferentially ablated segment was seen. (G) After the removal of coagulum and the second ablation pass, a circumferentially ablated segment with good ablation effect was seen. (H) The histology of retracted coagulum showed an extensive cauterization effect. (I) High-power view of the coagulum revealed focal dysplasia within the ablated epithelium. (J–L) Three months after treatment, a normal-appearing squamous esophagus was seen on conventional white-light imaging and no unstained lesions were seen after Lugol staining. RFA = radiofrequency ablation. |
Discussion
The incidence of esophageal cancer is increasing rapidly in Taiwan [2] . Recent advances in endoscopic techniques have led to an earlier diagnosis of esophageal squamous mucosal cancer or precancerous lesions, which can be cured by ESD [3] ; [4] . However, ESD not only requires considerable expertise, but may also lead to esophageal strictures in larger lesions [6] . Thus, a more convenient and safe method is desired. We report a case with long-segment circumferential squamous high-grade dysplasia that was treated successfully and safely using balloon-based RFA. The RFA procedure does not require a high level of endoscopic expertise, and is a potentially safe and effective procedure to treat early ESCNs. Interestingly, we, for the first time, demonstrated the histology of esophageal coagulum that showed an extensive cauterization effect (Figures 1 H and 1I).
Although ESD is gaining increasing attention, it remains a challenging technique, and is both time consuming and associated with a higher rate of complications (including perforation, massive bleeding, and postprocedural strictures) [4] . The incidence of these complications is strongly related to the size and depth of invasion of the lesion, and the excision extension of the lumen [6] . Safe and satisfactory performance of ESD involves a significant learning curve [5] . This has limited the use of ESD to some high-volume centers, with limited expansion worldwide. However, RFA appears to be less technically demanding, with a smaller learning curve compared to ESD. RFA has the potential to be more widespread due to being technically simpler for endoscopists to perform. RFA is already the first choice of treatment for dysplasia in cases of BE in many countries [7] . Particularly, in our experience, ablation of squamous neoplasia is technically easier than that of BE primarily due to two reasons: (1) squamous epithelium sloughs more readily than BE mucosa; and (2) BE always involves the esophagocardial junction, making endoscopic contact more challenging.
Previous studies related to RFA for treating early ESCNs, especially long-segment ESCNs, are rare [8] ; [9] . When making decisions regarding the choice of endoscopic treatment, one must consider additional merits of each technique. Complete ESD has the advantage of providing the whole resected specimens for histologic evaluation. RFA is a tissue-destructive treatment modality and does not allow pathology to be examined for evaluating curability after ablation. This is the major concern of RFA and thus it should be used to treat esophageal squamous cancer conservatively. It is still unclear what the actual depth of ablation is using RFA for squamous neoplasia. Only one previous study reported the ablation depth in humans over the normal squamous epithelium [3 of 5 patients achieved histological ablation depth to the muscularis mucosa (m3) layer with 2 ablations of 12 J/cm2 regimen] [10] . A recent study from the UK registry demonstrated that 30% of patients with early squamous cell neoplasias progressed to invasive cancers after only one ablation at each treatment session [9] . Therefore, the treatment using RFA with a 12-J/cm2 –clean–12J/cm2 regimen, like in our case, may be effective and indicated for m1 (intraepithelial neoplasia) or m2 (lamina propria invasion) ESCNs. In our opinion, RFA is justified only for truly flat lesions after detailed endoscopic inspections to exclude signs of submucosal invasions. Furthermore, we should keep in mind that the risks of incomplete ablation and recurrences of ESCNs after treatment dictate the need for strict patient selection and continuing endoscopic surveillance. Despite the fact that there was no residual dysplasia or local recurrence in this case during the follow-up period, biopsy specimens from the treatment area even with normal Lugol staining might be insufficient to exclude the presence of “buried” squamous neoplasia. A longer-term follow up is needed to clarify this issue.
In summary, we demonstrated that RFA has the potential to become an easier and effective technique for treating large early ESCNs. It does not require a high level of endoscopic expertise and is also associated with fewer adverse events.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] J. Ferlay, H.R. Shin, F. Bray, D. Forman, C. Mathers, D.M. Parkin; Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008; Int J Cancer, 127 (2010), pp. 2893–2917
- [2] Taiwan Cancer Registry (1972–2010). Department of Health, Executive Yuan. Cancer Registry Annual Report. Available at: http://crs.cph.ntu.edu.tw .
- [3] C.T. Lee, C.Y. Chang, Y.C. Lee, C.M. Tai, W.L. Wang, P.H. Tseng, et al.; Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers; Endoscopy, 42 (2010), pp. 613–619
- [4] T. Oyama, A. Tomori, K. Hotta, S. Morita, K. Kominato, M. Tanaka, et al.; Endoscopic submucosal dissection of early esophageal cancer; Clin Gastroenterol Hepatol, 3 (2005), pp. S67–S70
- [5] F.G. van Vilsteren, R.E. Pouw, L.A. Herrero, F.P. Peters, R. Bisschops, M. Houben, et al.; Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program; Endoscopy, 44 (2012), pp. 4–12
- [6] S. Ono, M. Fujishiro, K. Niimi, O. Goto, S. Kodashima, N. Yamamichi, et al.; Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms; Endoscopy, 41 (2009), pp. 661–665
- [7] N.J. Shaheen, P. Sharma, B.F. Overholt, H.C. Wolfsen, R.E. Sampliner, K.K. Wang, et al.; Radiofrequency ablation in Barretts esophagus with dysplasia; N Engl J Med, 360 (2009), pp. 2277–2288
- [8] J.J. Bergman, Y.M. Zhang, S. He, B. Weusten, L. Xue, D.E. Fleischer, et al.; Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus; Gastrointest Endosc, 74 (2011), pp. 1181–1190
- [9] R.J. Haidry, M.A. Butt, J. Dunn, M. Banks, A. Gupta, H. Smart, et al.; Radiofrequency ablation for early oesophageal squamous neoplasia: outcomes form United Kingdom registry; World J Gastroenterol, 19 (2013), pp. 6011–6019
- [10] B.J. Dunkin, J. Martinez, P.A. Bejarano, C.D. Smith, K. Chang, A.S. Livingstone, et al.; Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device; Surg Endosc, 20 (2006), pp. 125–130
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?