Abstract
Background
In patients with heart disease, the presence of a fragmented QRS complex (fQRS) on the surface electrocardiogram (ECG) is associated with an increased risk of mortality. We sought to evaluate the prevalence and location of fQRS before and after left ventricular assist device (LVAD) implantation and any associated risk of mortality.
Methods and results
Twelve-lead surface ECGs before (pre-LVAD, n = 98) and after (early [< 7 days], n = 96, and late [≥ 30 days], n = 85, post-LVAD) LVAD implantation were evaluated for fQRS. Mortality data were gathered via review of medical records. The prevalence of fQRS increased significantly following LVAD implantation on early post-LVAD ECGs (31% to 47%, p < 0.01). Patients with fQRS in the anterior territory (precordial leads V1 to V5) on late post-LVAD ECGs had decreased survival or survival to cardiac transplantation over a 30 month follow-up period compared with patients who did not exhibit anterior fQRS (30% and 59%, respectively, p < 0.01).
Conclusions
The prevalence of fragmented QRS on 12-lead ECG increases significantly in the anterior territory following LVAD implantation and is associated with decreased survival.
Keywords
Fragmented QRS;Left ventricular assist device;Mortality;Survival;ECG
1. Introduction
With advances in technology and design leading to improved patient outcomes, left ventricular assist device (LVAD) support has become a more widely used therapeutic option for patients with advanced heart disease, both as a bridge to cardiac transplantation and destination therapy [1]; [2]; [3] ; [4]. As LVADs become increasingly utilized, the identification of risk factors that help predict clinical outcomes may improve the post-operative course for these patients. To date, several risk factors associated with adverse clinical outcomes — including thrombocytopenia, hypoalbuminemia, low body surface area, elevated INR, and ventricular arrhythmias — have been identified [5]; [6]; [7] ; [8].
In prior studies of patients with heart disease, the presence of a fragmented QRS complex (fQRS) on 12-lead surface electrocardiogram (ECG) has been associated with an increased risk of mortality and arrhythmic events [9]; [10] ; [11]. The prevalence of fQRS has not been evaluated in a population of patients with LVAD. In this retrospective study, we analyzed 12-lead surface ECGs before and after continuous-flow LVAD implantation for the prevalence and distribution of fQRS, and the association between fQRS and post-LVAD survival.
2. Methods
2.1. Patient selection
The medical records of 107 consecutive adult patients receiving a continuous-flow LVAD (HeartMate-II LVAD [Thoratec, Pleasanton, CA, USA] or HeartWare HVAD [HeartWare, Miramar, FL, USA]) at the University of Chicago from 2008 through 2010 were reviewed.
The HeartMate II is a second-generation continuous-flow device designed to be implanted in the muscles of the abdominal wall or preperitoneally. It has one moving part, the rotor, which is powered by an electromagnetic motor and spins at rates between 6000 and 15,000 rpm [1]; [12] ; [13]. The HeartWare HVAD is a third-generation continuous-flow device designed to be implanted in the pericardial space adjacent to the heart. It has one moving part, the impeller, which spins at rates between 2400 and 3200 rpm [14] ; [15].
For all patients, implantation of the LVAD was performed in the standard fashion by using a coring instrument to remove a portion of the left ventricular apex, followed by securing of the LVAD inflow cannula through the ventriculotomy and anastomosis of the LVAD outflow graft to the ascending aorta.
2.2. ECG analysis
Archived resting twelve-lead surface ECGs (GE Marquette, GE Healthcare, Milwaukee, WI; USA, 25 mm/s, 10 mm/mV) performed up to 30-days prior to (pre-LVAD), up to 7-days after (early post-LVAD), and greater than 30-days after (late post-LVAD) LVAD implantation were evaluated for the presence and distribution of fQRS. When multiple ECGs were available, the ECG nearest to LVAD implantation within the above specified intervals was used, unless a preponderance of electromagnetic artifact in the majority of leads (limb or precordial) limited accurate interpretation. In those instances, the next available ECG without artifact in the majority of leads was used. Two investigators independently analyzed ECGs for the presence of fQRS in territories corresponding to major coronary arteries as detailed below. For discrepancies, a third investigator adjudicated the final determination of fQRS presence or absence.
2.3. Fragmented QRS
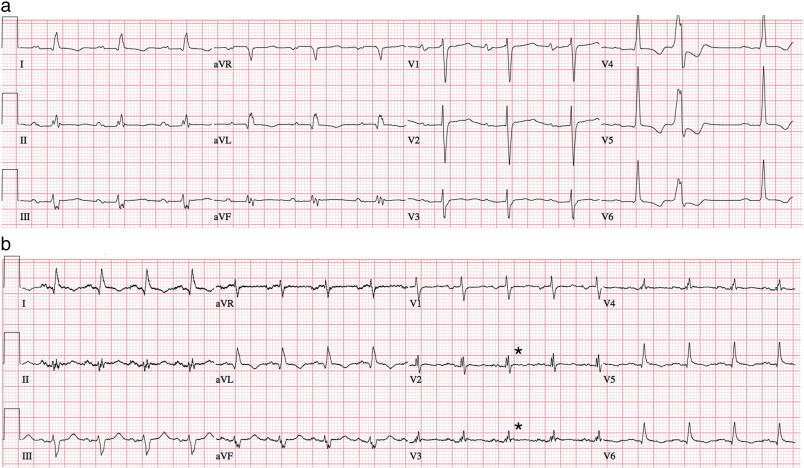
Criteria for identifying fQRS were adapted from Das et al. [10] ; [16]. Fragmented QRS with a narrow complex was defined by a QRS duration less than 120 ms and the presence of an additional R wave (R′) or notching in the S wave in at least two contiguous leads corresponding to a major coronary artery territory [16] (pre- and post-LVAD, Fig. 1a and b). Fragmented QRS with a wide complex was defined by a QRS duration greater than or equal to 120 ms and the presence of more than two R waves, more than two notches in the R wave, or more than two notches in the S wave. In paced QRS complexes, fQRS was defined by the presence of more than two R′ waves or more than two notches in the S wave. Findings must again have been present in at least two contiguous leads [10]. Contiguous leads corresponding to major coronary artery territories were designated as anterior (V1 through V5), lateral (I, aVL, and V6), and inferior (II, III, and aVF).
|
|
|
Fig. 1. a: Pre-LVAD twelve-lead ECG showing fragmented QRS (fQRS) in leads II, III and aVF (inferior territory). b: Post-LVAD twelve-lead ECG in same patient showing new fragmented QRS (fQRS) in leads V2 and V3 (anterior territory). |
2.4. Survival
Mortality data were gathered through review of medical records on all patients with available pre-LVAD and post-LVAD ECGs up to 30 months after LVAD implantation. Causes of death were recorded when available. The primary outcome was a composite of survival or survival to cardiac transplantation.
2.5. Statistical analysis
Comparisons of continuous variables were performed using the Student paired t-test. Comparisons of categorical variables were performed using the McNemar test and Fisher exact test where appropriate. Comparisons of survival outcomes were determined by analyses of Kaplan–Meier plots. Two-tailed p-values of less than 0.05 were considered statistically significant. Data are presented as mean ± standard deviation or median [interquartile range] where appropriate.
3. Results
Of the 107 patients reviewed, seven were excluded due to the presence of a concomitant RVAD. Two patients did not have available pre-LVAD and post-LVAD ECGs, and were also excluded. The remaining 98 patients were included in the analysis. All 98 patients had available pre-LVAD ECGs, 96 patients had available early post-LVAD ECGs, and 85 patients had available late post-LVAD ECGs. Twelve patients did not survive beyond 30 days.
Baseline patient characteristics are summarized in Table 1. Pre-LVAD ECGs were performed at a median of one (1 to 3) day prior to LVAD implantation, early post-LVAD ECGs at one (0 to 1) day after LVAD-implantation, and late post-LVAD ECGs at 83 (38 to 201) days after LVAD implantation.
| Age (years)a | 57 ± 13 |
| Male (%) | 73 |
| Female (%) | 27 |
| Ischemic cardiomyopathy (%) | 49 |
| Non-ischemic (%) | 51 |
| Pre-LVAD LVEF (%)a | 18 ± 6 |
| HeartMate II LVAD (%)b | 83 |
| HeartWare HVAD (%)c | 17 |
| Beta blocker use (%)d | 30 |
| Amiodarone use (%)d | 48 |
| Other antiarrhythmic use (%)de | 16 |
| Narrow QRS complex (%) | 35 |
| Wide QRS complex (%) | 65 |
| Ventricularly paced (%) | 53 |
LVEF = left ventricular ejection fraction.
a. Values presented as mean ± standard deviation.
b. Thoratec, Pleasanton, CA.
c. HeartWare International, Miramar, FL.
d. During hospitalization post-LVAD implant or on discharge.
e. Lidocaine or mexiletine.
3.1. Fragmented QRS
The prevalence and distribution of fQRS before and after continuous-flow LVAD implantation are summarized in Table 2. There was 95.6% concordance between investigators for presence of fQRS in individual territories on independent review; the remaining 4.4% of fQRS territories were adjudicated by the third investigator. The majority of discrepancies arose in patients with ventricular-paced QRS complexes.
| Pre-LVAD ECG (n = 98) | Early post-LVAD ECG (≤ 7 days) (n = 96) | pa | Delayed post-LVAD ECG (≥ 30 days) (n = 85) | pb | pc | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| fQRS (any territory) | 28 (29) | 46 (48) | < 0.001 | 33 (39) | 0.15 | 0.57 |
| Anterior (V1–V5) | 10 (10) | 31 (32) | < 0.001 | 22 (26) | < 0.01 | 0.73 |
| Inferior (II, III, aVF) | 19 (19) | 23 (24) | 0.45 | 21 (25) | 0.80 | 1.00 |
| Lateral (I, aVL, V6) | 12 (12) | 15 (16) | 0.34 | 9 (11) | 1.00 | 0.51 |
a. Comparison of Pre-LVAD to Post-LVAD.
b. Comparison of Pre-LVAD to Delayed-post LVAD.
c. Comparison of Post-LVAD to Delayed-post LVAD.
On pre-LVAD ECGs, fQRS was present in at least one territory in 29% of patients. On early post-LVAD ECGs, the prevalence of fQRS increased significantly, with 48% of patients having fragmentation in at least one territory (p < 0.01). The anterior territory was the most frequently fragmented on early post-LVAD ECGs, noted in 32% of patients and significantly increased compared to pre-LVAD ECGs (p < 0.001). The prevalence of QRS fragmentation on early post-LVAD ECGs in the inferior and lateral territories did not change significantly compared to pre-LVAD ECGs.
On late post-LVAD ECGs, there remained an increase in the prevalence of fQRS in at least one territory as compared to pre-operative ECGs, though the difference was not statistically significant (p = 0.15). The prevalence of fQRS in the anterior territory on late post-LVAD ECGs remained significantly increased compared to pre-LVAD ECGs (26% versus 10%, p < 0.01).
Following LVAD implantation, a smaller proportion of patients were noted to have fQRS on late post-LVAD ECG compared to early post-LVAD ECGs. While some patients developed, and subsequently lost, fQRS post-LVAD, when comparing early post-LVAD and late post-LVAD ECGs, the prevalence of fQRS in at least one territory, as well as the prevalence of fQRS in the anterior territory, did not change significantly (p = 0.57 and 0.73, respectively).
There were no significant differences in baseline clinical characteristics between patients with fQRS in at least one territory after LVAD and patients without fQRS after LVAD, both on early post-LVAD and late post-LVAD ECGs (Table 3).
| Early post-LVAD ECG (≤ 7 days) (n = 96) | Late post-LVAD ECG (≥ 30 days) (n = 85) | |||
|---|---|---|---|---|
| fQRS + (n = 46) | fQRS − (n = 50) | fQRS + (n = 33) | fQRS − (n = 52) | |
| Age (years) | 56 ± 14 | 57 ± 12 | 55 ± 15 | 56 ± 14 |
| Ischemic cardiomyopathy (%) | 56 | 41 | 56 | 37 |
| Male (%) | 74 | 76 | 79 | 71 |
| LVEF (%) | 16 ± 7 | 18 ± 7 | 17 ± 8 | 17 ± 7 |
| HeartMate II (%) | 83 | 84 | 76 | 89 |
| HeartWare (%) | 17 | 16 | 24 | 11 |
LVEF = left ventricular ejection fraction; HeartMate II, Thoratec, Pleasanton, CA; HeartWare HVAD, HeartWare International, Miramar, FL.
a. Differences in clinical characteristics not statistically significant (p > 0.05).
3.2. Survival
Mortality data were available for all 98 patients included in the analysis; 42 (43%) survived with the LVAD or survived to cardiac transplantation. The clinical outcomes and causes of death over 30 months are summarized in Table 4. The most common cause of death included sepsis (13%) and intracranial hemorrhage or stroke (10%); it was not available or unknown in 18%.
| Patients included in survival analysis | Patients with available late post-LVAD ECG (≥ 30 days) | pa | ||
|---|---|---|---|---|
| (n = 98) | Fragmented V1–V5 (n = 23) | Non-fragmented V1–V5 (n = 62) | ||
| Alive/transplanted | 43% | 26% | 55% | 0.03b |
| Expired | 57% | 74% | 45% | |
| Sepsis | 13% | 13% | 11% | NS |
| ICH/stroke | 10% | 17% | 5% | 0.08 |
| Hemorrhage | 3% | 4% | 2% | NS |
| LVAD complication | 6% | 13% | 3% | 0.12 |
| VA | 1% | 0% | 0% | NS |
| RHF | 1% | 0% | 2% | NS |
| Otherc | 4% | 4% | 5% | NS |
| Unknown | 18% | 22% | 18% | NS |
ICH = intracranial hemorrhage; RHF = right heart failure; VA = ventricular arrhythmia.
a. 2-Tailed p-value by Fisher exact test for differences between fragmented and non-fragmented V1–V5.
b. Based on combined survival and survival to transplant outcome in Kaplan–Meier estimate.
c. Includes cancer, ARDS, amiodarone toxicity, elective withdrawal of care.
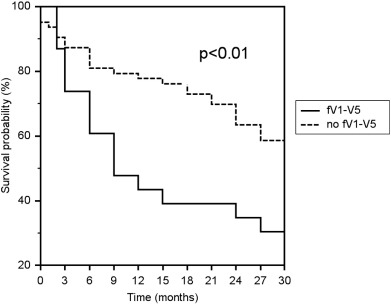
Patients with an fQRS in the anterior territory (V1–V5) on late post-LVAD ECGs had a significantly decreased rate of survival or survival to cardiac transplantation compared to patients without fQRS in the anterior territory over 30 months (30% and 59%, respectively, p < 0.01, Fig. 2). The causes of death were not significantly different between these two groups (Table 4). Patients with an fQRS in the anterior territory on pre-LVAD and early post-LVAD ECG did not have a significantly different rate of survival compared to their counterparts without fQRS in the anterior territory.
|
|
|
Fig. 2. Kaplan–Meier survival estimate for patients with and without QRS fragmentation in the anterior territory (fV1–V5) over 30 months. Patients with fV1–V5 had a significantly decreased rate of survival or survival to cardiac transplantation (p < 0.01). |
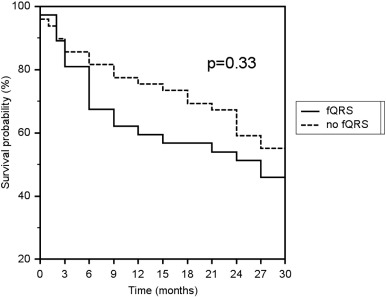
For patients with fQRS in at least one territory on late post-operative ECG, survival was not significantly different compared to the cohort of patients with no fQRS (p = 0.33, Fig. 3). Patients with fQRS in at least one territory on pre-LVAD and early post-LVAD ECGs did not have a significantly different rate of survival compared to their counterparts without fQRS.
|
|
|
Fig. 3. Kaplan–Meier survival estimate for patients with and without QRS fragmentation in any territory (fQRS) over 30 months. Patients with fQRS in any territory on late post-operative ECG did not have a significantly different rate of survival or survival to cardiac transplantation compared to patients without fQRS (p = 0.33). |
Durations of QRS on pre-, early post-, and late post-LVAD ECG were not significantly associated with survival (Table 5). In patients with QRS durations greater than or equal to 120 ms, QRS complex morphologies and ventricular pacing were not associated with survival.
| Pre-LVAD | Early post-LVAD | Late post-LVAD | |
|---|---|---|---|
| QRS < 120 ms | 10.5 [4–30] | 21 [4–30] | 25 [6–30] |
| QRS ≥ 120 ms | 30 [3.5–30] | 30 [3.5–30] | 30 [15–30] |
| p | 0.093 | 0.192 | 0.183 |
| QRS morphologies | |||
| LBBB | 30 [24–30] | 24 [21–27] | 24 [13–27] |
| RBBB | 27 [21–28.5] | 22.5 [17–28] | 30 [22.5–30] |
| IVCD | 30 [22.5–30] | 30 [28.5–30] | 30 [22.5–30] |
| V-paced | 27 [2.5–30] | 28.5 [2.5–30] | 30 [12–30] |
| p | 0.850 | 0.796 | 0.982 |
Values presented as median [interquartile range]. LBBB = left bundle branch block, RBBB = right bundle branch block, IVCD = non-specific intraventricular conduction delay, V-paced = ventricularly paced.
4. Discussion
Previous studies have suggested that fractionated intracardiac electrograms are the result of delayed conduction through infarcted, damaged, or ischemic myocardium [17]; [18] ; [19]. Similarly, the fragmented QRS (fQRS) on 12-lead surface ECG has been shown to be a marker of prior myocardial infarction, correlating to regional perfusion abnormalities on myocardial perfusion imaging [16]. In patients with coronary artery disease, fQRS is predictive of increased mortality [9], and in patients with ischemic and nonischemic cardiomyopathy, fQRS is associated with sudden cardiac death and ICD-treated ventricular arrhythmias [11] ; [20].
Patients receiving continuous-flow LVAD in this analysis were commonly found to have fQRS on pre-LVAD ECG, reflective of baseline myocardial disease. However, a significant increase in the prevalence of fQRS was noted following LVAD implantation, both on early and late post-LVAD ECGs. The fQRS was commonly seen in the anterior territory (V1–V5) on post-LVAD ECGs, accounting for the majority of all newly fragmented QRS territories following LVAD implantation. Fragmentation of the QRS in the anterior distribution may be related to disruption of myocardial depolarization and possible collateral damage induced by the presence of the LVAD inflow cannula, which is generally inserted into the left ventricular apex.
In previous studies of fQRS, it has been postulated that the increased cardiac event rate and mortality associated with the presence of fQRS reflects an association between the fQRS and significant myocardial disease [9] ; [20]. In this cohort of LVAD-supported advanced heart failure patients, fQRS was a frequent finding on pre-LVAD ECGs, but this alone was not associated with decreased survival or survival to cardiac transplantation at 30 months. However, the sustained increase in fQRS evident on late post-LVAD ECGs was associated with decreased survival, suggesting that even in a milieu of significant baseline myocardial disease, new ventricular conduction abnormalities following LVAD implantation may be associated with adverse outcomes. Other alterations in ventricular conduction, including bundle branch blocks and nonspecific intraventricular conduction delays, were not associated with decreased survival in this cohort.
Decreased survival was not seen in patients with fQRS in the anterior territory on early post-LVAD ECGs. Fewer patients had anterior territory fQRS in the late post-LVAD period when compared to the early post-LVAD period, suggesting that changes in ventricular depolarization manifesting as fQRS following LVAD implantation may be transient in some patients, and without associated mortality risk. Long-standing or permanent alterations of conduction properties induced by LVAD implantation may impart an increased risk of adverse outcomes, which in turn may result in a decreased likelihood of survival.
It was not clear from this retrospective analysis how alterations in ventricular conduction, as reflected by the presence of the fQRS post-LVAD, might directly contribute to mortality in LVAD-supported patients. The causes of death in patients with and without fQRS in the anterior territory were varied and did not differ significantly between the two groups. In previous studies of patients with heart disease, the fQRS has been associated with an increased risk of sudden cardiac death due to ventricular arrhythmias (VA). While none of the patients with available late post-operative ECGs in this analysis died directly from ventricular arrhythmias (VA), VA following LVAD-implantation has been previously identified as a significant risk factor for increased rates of re-hospitalization and mortality [7] ; [8].
In light of these findings, further study of the fQRS in a prospective population of LVAD-supported patients is merited. The pathogenesis of fQRS in this population is not well understood, and investigations of fQRS development following LVAD implantation may illuminate additional details regarding the nature of conduction system and myocardial muscle alterations following mechanical support. As a potential prognostic indicator of patient outcomes, post-LVAD fQRS may be of clinical utility in the management of LVAD-supported patients.
5. Conclusions
The prevalence of the fragmented QRS complex (fQRS) on 12-lead surface ECG increases significantly following continuous-flow LVAD placement, predominantly in the anterior territory. When fQRS persists on ECGs performed 30-days or more after LVAD implantation, there is an associated decrease in rate of survival or survival to cardiac transplantation over 30 months.
Funding
None.
Conflicts of interest
None declared.
Acknowledgments
None.
References
- [1] L.W. Miller, F.D. Pagani, S.D. Russell, R. John, A.J. Boyle, K.D. Aaronson, et al.; Use of a continuous-flow device in patients awaiting heart transplantation; N Engl J Med, 357 (2007), pp. 885–896
- [2] M.S. Slaughter, J.G. Rogers, C.A. Milano, S.D. Russell, J.V. Conte, D. Feldman, et al.; Advanced heart failure treated with continuous-flow left ventricular assist device; N Engl J Med, 361 (2009), pp. 2241–2251
- [3] R.C. Starling, Y. Naka, A.J. Boyle, G. Gonzalez-Stawinski, R. John, U. Jorde, et al.; Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support); J Am Coll Cardiol, 57 (2011), pp. 1890–1898
- [4] R. John, F. Kamdar, K. Liao, M. Colvin-Adams, A. Boyle, L. Joyce; Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy; Ann Thorac Surg, 86 (2008), pp. 1227–1234 [discussion 34–5]
- [5] K. Lietz, J.W. Long, A.G. Kfoury, M.S. Slaughter, M.A. Silver, C.A. Milano, et al.; Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection; Circulation, 116 (2007), pp. 497–505
- [6] J. Cowger, K. Sundareswaran, J.G. Rogers, S.J. Park, F.D. Pagani, G. Bhat, et al.; Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score; J Am Coll Cardiol, 61 (2013), pp. 313–321
- [7] A. Brenyo, M. Rao, S. Koneru, W. Hallinan, S. Shah, H.T. Massey, et al.; Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients; J Cardiovasc Electrophysiol, 23 (2012), pp. 515–520
- [8] H. Raasch, B.C. Jensen, P.P. Chang, J.P. Mounsey, A.K. Gehi, E.H. Chung, et al.; Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation; Am Heart J, 164 (2012), pp. 373–378
- [9] M.K. Das, C. Saha, H. El Masry, J. Peng, G. Dandamudi, J. Mahenthiran, et al.; Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease; Heart Rhythm, 4 (2007), pp. 1385–1392
- [10] M.K. Das, H. Suradi, W. Maskoun, M.A. Michael, C. Shen, J. Peng, et al.; Fragmented wide QRS on a 12-lead ECG; Clinical perspective Circ Arrhythm Electrophysiol, 1 (2008), pp. 258–268
- [11] M.K. Das, W. Maskoun, C. Shen, M.A. Michael, H. Suradi, M. Desai, et al.; Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy; Heart Rhythm, 7 (2010), pp. 74–80
- [12] B.P. Griffith, R.L. Kormos, H.S. Borovetz, K. Litwak, J.F. Antaki, V.L. Poirier, et al.; HeartMate II left ventricular assist system: from concept to first clinical use; Ann Thorac Surg, 71 (2001), pp. S116–S120
- [13] O.H. Frazier, R.M. Delgado III, B. Kar, V. Patel, I.D. Gregoric, T.J. Myers; First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report; Tex Heart Inst J, 31 (2004), pp. 157–159
- [14] A.M. Dell'Aquila, S.R.B. Schneider, D. Schlarb, B. Redwan, J.R. Sindermann, B. Ellger, et al.; Initial clinical experience with the HeartWare left ventricular assist system: a single-center report; Ann Thorac Surg, 95 (2013), pp. 170–177
- [15] L.E. Rodriguez, E.E. Suarez, M. Loebe, B.A. Bruckner; Ventricular assist devices (VAD) therapy: new technology, new hope?; Methodist Debakey Cardiovasc J, 9 (2013), pp. 32–37
- [16] M.K. Das, B. Khan, S. Jacob, A. Kumar, J. Mahenthiran; Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease; Circulation, 113 (2006), pp. 2495–2501
- [17] P.I. Gardner, P.C. Ursell, J.J. Fenoglio, A.L. Wit; Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts; Circulation, 72 (1985), pp. 596–611
- [18] M.D. Lesh, J.F. Spear, M.B. Simson; A computer model of the electrogram: what causes fractionation?; J Electrocardiol, 21 (1988), pp. S69–S73 [Supplement]
- [19] N.C. Flowers, L.G. Horan, J.R. Thomas, W.J. Tolleson; The anatomic basis for high-frequency components in the electrocardiogram; Circulation, 39 (1969), pp. 531–539
- [20] A. Brenyo, G. Pietrasik, A. Barsheshet, D.T. Huang, B. Polonsky, S. McNitt, et al.; QRS fragmentation and the risk of sudden cardiac death in MADIT II; J Cardiovasc Electrophysiol, 23 (2012), pp. 1343–1348
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?