Abstract
Background
One of the most challenging issues of chronic Chagas disease is to provide earlier detection of heart involvement. Two-dimensional speckle tracking (2-D ST) echocardiography, a new imaging modality with useful applications in several cardiac diseases, has been validated for subjects with myocardial infarction against cardiac magnetic resonance (CMR). Here we hypothesize that the longitudinal global strain (LGS) has an incremental value to ejection fraction for predicting myocardial fibrosis in subjects with Chagas disease.
Methods
This observational study comprised 58 subjects with Chagas disease, confirmed by two positive serologic tests. All subjects underwent conventional Doppler echocardiogram plus speckle tracking strain, and cardiac magnetic resonance.
Results
The ROC curve analysis revealed that both LGS (area under the curve: 0.78, p = 0.001) and ejection fraction (area under the curve: 0.82, p < 0.001) were significant predictors of myocardial fibrosis. Regarding the percentage of fibrosis, a high correlation was observed with both ejection fraction assessed by echocardiography (r = 0.70, p < 0.001) and LGS (r = 0.64, p < 0.001). However, when adjusted through multiple linear regression, the LGS lost statistical significance as a predictor of myocardial fibrosis (p = 0.111).
Conclusions
LGS has no incremental value to conventional ejection fraction measurement in the prediction of myocardial fibrosis in subjects with Chagas disease.
Keywords
Speckle tracking;Chagas disease;Longitudinal global strain
1. Introduction
In Chagas disease, myocardial fibrosis affects not only the sinoatrial node and Purkinje fibers, but also intracardiac nervous ganglia and the contractile myocardium [1]. Myocardial fibrosis has been directly associated with cardiac alterations such as ventricular dysfunction (highly correlated with left ventricular ejection fraction [2]), and arrhythmias (risk identification for ventricular tachycardia [3]). The estimation of myocardial fibrosis in Chagas disease therefore may help in the early detection of cardiac involvement. The best noninvasive technique to evaluate myocardial fibrosis for ischemic [4]; [5] ; [6] and non-ischemic myocardial diseases is magnetic resonance imaging (MRI), but this method is still quite expensive, not widely available and contraindicated in subjects with pacemakers.
Myocardial fibrosis has already been demonstrated to have a significant inverse correlation with left ventricular ejection fraction, and a direct correlation with end-diastolic and end-systolic volume indices [2], which indicates echocardiography as a possible method to indirectly evaluate myocardial fibrosis. One of the most challenging issues is the identification of fibrosis in subjects with normal ejection fraction. In this context, two-dimensional (2-D) speckle tracking (ST) echocardiography could be a useful approach, since it provides an assessment of myocardial deformation and left ventricular torsion [7]. Several studies have demonstrated that longitudinal myocardial velocity, strain, and strain rate are more sensitive and specific than LVEF for detecting early myocardial dysfunction [8]; [9] ; [10]. Moreover, a previous study has shown that this new technique was also able to demonstrate myocardial involvement, which was not evident in visual analysis from subjects with the indeterminate form of Chagas disease [11].
Currently, the evaluation of Chagas disease subjects is performed by conventional echocardiography and, to a lesser extent, by CMR imaging, which is considered the gold standard method for measuring ejection fraction. Although the 2-D ST for left ventricular quantification has already been validated against cardiac magnetic resonance for subjects with myocardial infarction [12], to date the accuracy of longitudinal global strain against other echocardiographic variables, such as ejection fraction (EF), using cardiac magnetic resonance (CMR) as the reference standard, has not been evaluated in subjects with Chagas disease. Therefore we hypothesize that the longitudinal global strain has an incremental value to ejection fraction when predicting myocardial fibrosis in subjects with Chagas disease. To test our hypothesis, we performed a correlation analysis between longitudinal global strain and ejection fraction, both with myocardial fibrosis, adjusting the variables through multiple linear regression.
2. Methods
2.1. Study population
We conducted a cross-sectional study, in which adult subjects with Chagas disease were invited to participate. From January 2011 to December 2013, we included 58 subjects from a convenience sample, in the Chagas disease outpatient clinics at our institution. The inclusion criteria were disease established based on microbiological confirmation by two positive serologic tests (indirect hemagglutination and indirect immunofluorescence), and age between 18 and 70 years. The exclusion criteria were: previous myocardial infarction or history of coronary artery disease; primary valve disease; dialysis treatment of terminal renal failure, chronic liver disease; hematologic, neoplastic or bone diseases; and MRI contraindications.
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital São Rafael. All subjects gave written informed consent before their inclusion in the study.
We obtained a structured medical history, and all subjects underwent physical examination, blood analysis, 12-lead ECG, chest X-ray, 24-h Holter monitoring, conventional Doppler echocardiogram plus speckle tracking strain, and cardiac magnetic resonance.
2.2. Doppler echocardiogram
Standard transthoracic echocardiographic examinations were recorded in all subjects using the Vivid 7 digital ultrasound system (GE Vingmed Ultrasound AS, Horten, Norway). Three cardiac cycles were stored in cine loop format for online analysis. LV and left atrial dimensions were measured according to the American Society of Echocardiography recommendations [13]. The LVEF was measured using the biplane Simpsons method. S0 systolic velocity at the lateral mitral annulus was assessed using pulsed-wave DTI. Diastolic function was evaluated by the analysis of the mitral Doppler inflow, pulsed- wave DTI at the lateral mitral annulus, and mitral propagation velocity using Color M-mode echocardiography. Mitral regurgitation (MR) was obtained using the proximal isovelocity surface area method. Right ventricular function was assessed with the S0 systolic velocity in the lateral wall. Systolic pulmonary arterial pressure was obtained from the tricuspid regurgitation flow. In subjects with adequate Doppler MR signals, dP/dt was determined noninvasively from the rate of change of MR velocity, according to the American Society of Echocardiography guidelines.
2.3. Strain measurements
Two-dimensional gray-scale images were acquired in the standard apical four-chamber, three-chamber, and two-chamber views at a frame rate of 80 frames/s. The dimensions of the computation area were an angle of 80° and at a depth of 13 cm. The left ventricle was divided into 17 segments, and each segment was analyzed individually. GLS and segmental longitudinal strain were obtained as previously described [14]. Average longitudinal strains were calculated automatically by the software. The two-dimensional strain is a non-Doppler-based method for the evaluation of systolic strain using standard 2D acquisitions. After placing three endocardial markers in an end-diastolic frame, the software automatically tracks the contour on subsequent frames. Adequate tracking can be verified in real time and corrected by adjusting the region of interest or by manually correcting the contour to ensure optimal tracking. Two-dimensional longitudinal strain was assessed in apical views. Average longitudinal strains were calculated for the 17 segments.
2.4. Magnetic resonance imaging
Cardiac magnetic resonance exams were performed on all subjects using the General Electric Sigma HDx 1.5-T system (Fairfield, CT, USA). Images were acquired and gated to the ECG during breath-hold, four-chamber, short axis and long axis of the left ventricle, in the exact same location in different sequences. This allows a precise comparison between cardiac function and regional myocardial structure. The parameters used in the dynamic sequences were repetition time (RT) of 3.5 ms, echo time (ET) of 1.5 ms, flip angle 60°, bandwidth of receipt of ± 125 kHz, field of view (FOV) of 35 × 35 cm, 256 × 148 matrix, temporal resolution (TR) 35 ms and 8.0 mm slice thickness, without a space between the slices. Following this acquisition, a dose of gadolinium-based contrast (0.1 mmol/kg) was injected. The myocardial delayed enhancement (MDE) technique was used to investigate myocardial fibrosis, an inversion-recovery prepared gradient-echo acquired 10 to 20 min after contrast application, with the following parameters: Repetition time (RT) 7.1 ms, echo time (ET) 3.1 ms, flip angle 20°, cardiac phase 1, views per segment 16 to 32, matrix 256 × 192, slice thickness 8 mm, gap between slices 2 mm and field of view 32 to 38 cm, inversion time 150 to 300 ms, receiver bandwidth 31.25 kHz, number of excitations 2 and acquisition every heart beat. The mass of fibrosis was estimated by a quantitative visual method.
2.5. Statistical analysis
Categorical data were presented as numbers (percentages), and continuous data were expressed as mean (SD) or median (interquartile range). Comparisons of continuous variables among groups were performed with the analysis of variance (ANOVA) test or Kruskal–Wallis, depending on normality assessed by the Shapiro–Wilk test. Chi-Square or Fisher tests were applied for proportion comparisons. Correlations between continuous variables were evaluated by Pearson or Spearman coefficients, depending on normality. Receiver-operating characteristic (ROC) curve analysis was used to evaluate if the longitudinal global strain would add accuracy to the ejection fraction in the predicted fibrosis. C-Statistic (area under the curve) was presented as a unified estimate of sensitivity and specificity. To determine the ability to predict myocardial fibrosis, multiple linear regression was performed, including longitudinal global strain, left ventricular ejection fraction, E/E′ ratio, Tei index, propagation velocity by Color M-Mode, and systolic tricuspid annular velocity. The multiple linear regression method used was forced entry, in which all predictors were entered simultaneously. Analyses in subgroups with normal and low ejection fraction were also accomplished. Cases with missing data were not included in the analysis. Analyses were performed using SPSS version 20.0 (IBM), and p < 0.05 (two-tailed) was considered statistically significant.
3. Results
3.1. Clinical and imaging characteristics
The study comprised 58 subjects, 41% were men, and the mean age was 58 ± 9. The prevalence of hypertension, diabetes, hypercholesterolemia and current smoking was 72%, 14%, 47% and 26%, respectively. Regarding the clinical form of presentation, the subjects were distributed as follows: 17 (29%) with the indeterminate form (subjects with no evidence of cardiac involvement or heart failure), 16 (28%) with the cardiac form without ventricular dysfunction and 25 (43%) with the cardiac form with ventricular dysfunction. Thirteen subjects were in NYHA functional classes III–IV (22.4%) and 15 showed episodes of ventricular tachycardia (26.3%). The clinical and demographic characteristics of the subjects are described in Table 1.
| Subjects (n = 58) | |
|---|---|
| Male gender | 24 (41.4) |
| Age (years) | 58 ± 9 |
| Indeterminate form | 17 (29.3) |
| Cardiac form without ventricular dysfunction | 16 (27.6) |
| Cardiac form with ventricular dysfunction | 25 (43.1) |
| NYHA III or IV | 13 (22.4) |
| Hypertension | 42 (72.4) |
| Diabetes | 8 (13.8) |
| Current smoking | 15 (25.9) |
| Hypercholesterolemia | 27 (46.6) |
| RBBB | 28 (49.1)a |
| Cardiomegaly | 27 (46.6) |
| VT | 15 (26.3)a |
| Hemoglobin (g/dl) | 14.0 ± 0.98 |
| Creatinine (mg/dl) | 0.86 ± 0.17 |
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as number of subjects (%). NYHA = New York Heart Association; RBBB = right bundle-branch block; VT = ventricular tachycardia.
a. One missing data for these variables.
The mean left ventricular ejection fraction assessed by Simpson was 54 ± 15%, and the mean left ventricular longitudinal global strain was − 16.8 ± 5.5. Abnormal left ventricular ejection fraction (< 55%) and abnormal longitudinal global strain (> − 18%) were found in 39.7% and 52.7% of the subjects, respectively. Myocardial fibrosis was observed in 37 subjects (63.8%). Echocardiographic and CMR data are described in Table 2.
| Subjects (n = 58) | |
|---|---|
| Echocardiogram | |
| LVEF (%) | 54.3 ± 15.2 |
| LVEF < 55% | 23 (39.7) |
| LV ESV (ml) | 78.4 ± 66.4 |
| LV EDV (ml) | 174.5 ± 80.1 |
| LV mass (g) | 169.4 ± 59.8 |
| LV LGS (%) | − 16.8 ± 5.6 |
| LV LGS > − 18% | 29 (52.7)a |
| RV LGS (%) | − 20.4 ± 6.0 |
| E/E′ ratio | 10.3 ± 5.8 |
| Tei index | 0.54 ± 0.14 |
| Systolic tricuspid annular velocity (cm/s) | 11.3 ± 2.8 |
| Propagation velocity by Color M-Mode (cm/s) | 42.1 ± 15.1 |
| CMR | |
| LVEF (%) | 55.8 ± 20.0 |
| LV ESV (ml/m2) | 82.4 ± 76.4 |
| LV EDV (ml/m2) | 161.9 ± 73.1 |
| LV mass (g/m2) | 121.5 ± 49.6 |
| RVEF (%) | 56.6 ± 13.8 |
| RV ESV (ml/m2) | 52.6 ± 33.2 |
| RV EDV (ml/m2) | 112.8 ± 38.9 |
| Fibrosis (%) | 2.2 (0–12.9) |
| DE | 37 (63.8) |
Continuous variables are expressed as mean ± standard deviation or median (interquartile interval) depending on normality assessed by the Shapiro–Wilk test. Categorical variables are expressed as number of subjects (%).CMR = cardiac magnetic resonance; DE = delayed enhancement; EDV = end diastolic volume; ESV = end systolic volume; LGS = longitudinal global strain; LV = left ventricular; LVEF = left ventricular ejection fraction; RV = right ventricular; RVEF = right ventricular ejection fraction.
a. Three missing data for this variable.
3.2. Prediction of myocardial fibrosis
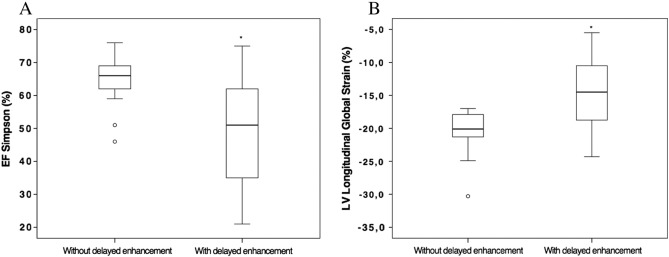
The left ventricular ejection fraction (Simpson method) was elevated in subjects lacking delayed enhancement (64.5 ± 6.7%) when compared to subjects who presented delayed enhancement on CMR (48.5 ± 15.7%, p < 0.001; Fig. 1A). We also found a significant difference in longitudinal global strain between subjects without and with delayed enhancement, − 20.3 ± 3.2% and − 14.7 ± 5.6%, respectively (p < 0.001; Fig. 1B).
|
|
|
Fig. 1. Ejection fraction assessed by Simpson (A) and left longitudinal global strain (B) in subjects with and without delayed enhancement detected by cardiac magnetic resonance. *p < 0.001. |
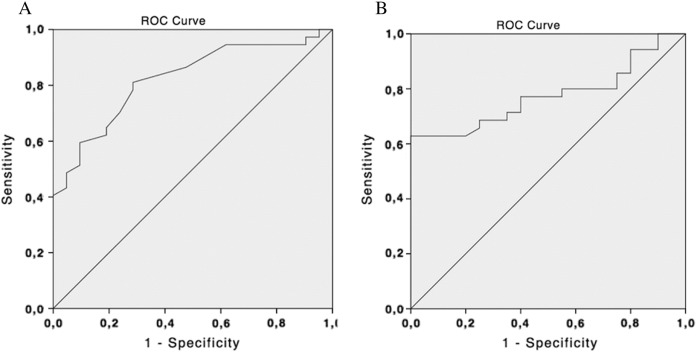
The ROC curve analysis revealed that both longitudinal global strain (area under the curve: 0.78, 95% CI 0.65 to 0.90, p = 0.001) and ejection fraction (area under the curve: 0.82, 95% CI 0.72 to 0.96, p < 0.001) were significant predictors for myocardial fibrosis (Fig. 2). For longitudinal global strain, the cutoff points of − 17.0, − 18.0 and − 19.0 have the following sensitivity and specificity: 63% and 95%, 69% and 70%, and 77% and 60%, respectively. For the left ventricular ejection fraction, the cutoff points of 49%, 55% and 60% have the following sensitivity and specificity: 49% and 95%, 57% and 90%, and 65% and 81%, respectively.
|
|
|
Fig. 2. Receiver-operating characteristic (ROC) curves of left ventricular ejection fraction (A) and longitudinal global strain (B) for prediction of myocardial fibrosis. The C-Statistic (area under the curve) with 95% confidence interval (95% CI) for the respective methods were: ejection fraction: 0.82 (95% CI 0.72–0.96, p < 0.001); and longitudinal global strain: 0.78 (95% CI 0.65–0.90, p = 0.001). |
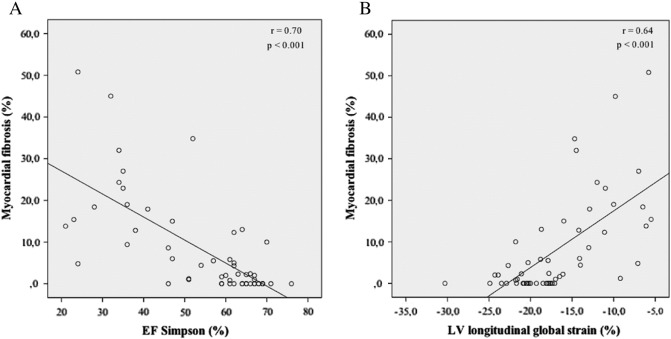
Regarding the percentage of fibrosis, a high correlation was observed with ejection fraction assessed by echocardiography (r = 0.70, beta = − 0.55, 95% CI: − 0.70 to − 0.40, p < 0.001; Fig. 3A) and with longitudinal global strain (r = 0.64, beta = 1.36, 95% CI: 0.91 to 1.82, p < 0.001; Fig. 3B). However, when adjusted to other variables through multiple linear regression, the longitudinal global strain lost statistical significance as a predictor of the percentage of fibrosis (beta = − 0.90, 95% CI: − 2.0 to 0.24, p = 0.111). Left ventricular ejection fraction, Tei index and E/E′ ratio were independent predictors of myocardial fibrosis (p = 0.003, p = 0.023 and p = 0.005, respectively). The results of the multiple linear regression analysis (standardized regression coefficients β, and the probability p) are presented in Table 3.
|
|
|
Fig. 3. Correlation between EF assessed by Simpson (A) and left longitudinal global strain (B) with myocardial fibrosis assessed by CMR (linear regression). EF = ejection fraction. CMR = cardiac magnetic resonance. p < 0.001 for both correlations. |
| Standardized regression coefficients β | Probability p | |
|---|---|---|
| LV ejection fraction | − 0.905 | 0.003 |
| Longitudinal global strain | − 0.904 | 0.111 |
| E/E′ ratio | 3.29 | 0.005 |
| Tei index | 39.49 | 0.023 |
| Propagation velocity by Color M-Mode | 0.110 | 0.369 |
| Systolic tricuspid annular velocity | 0.115 | 0.815 |
3.3. Subgroup analysis
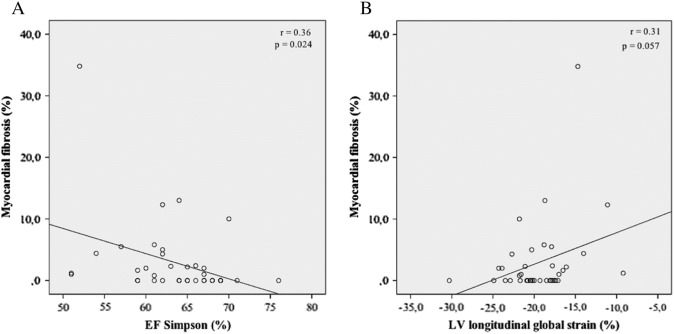
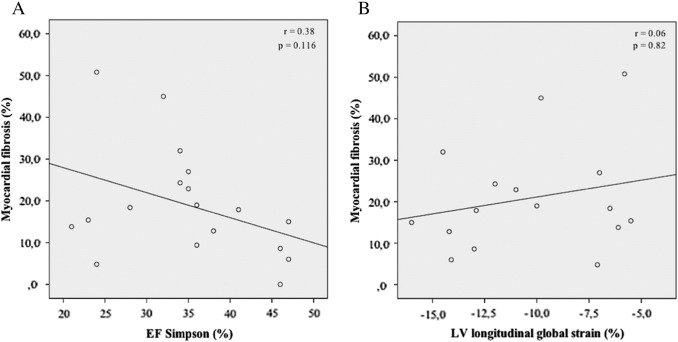
Analyzing subjects with normal left ventricular ejection fraction (n = 35), we found a weak correlation with ejection fraction assessed by echocardiography (r = 0.36, beta = − 0.41, 95% CI: − 0.76 to − 0.06, p = 0.024) and with longitudinal global strain (r = 0.31, beta = 0.51, 95% CI: − 0.02 to 1.04, p = 0.057) with the percentage of myocardial fibrosis (Fig. 4). Considering myocardial fibrosis in subjects with low left ventricular ejection fraction (n = 23), a moderate correlation was observed with ejection fraction (Simpson method; r = 0.38, beta = − 0.60, 95% CI: − 1.37 to 0.17, p = 0.116) and a weak correlation with longitudinal global strain (r = 0.06, beta = − 0.14, 95% CI: − 1.5 to 1.2, p = 0.82; Fig. 5), both without statistical significance.
|
|
|
Fig. 4. Correlation between EF assessed by Simpson (A) and left longitudinal global strain (B) with myocardial fibrosis assessed by CMR (linear regression), in subjects with normal left ventricular EF. EF = ejection fraction. CMR = cardiac magnetic resonance. |
|
|
|
Fig. 5. Correlation between EF assessed by Simpson (A) and left longitudinal global strain (B) with myocardial fibrosis assessed by CMR (linear regression), in subjects with low left ventricular EF. EF = ejection fraction. CMR = cardiac magnetic resonance. |
4. Discussion
The present study evaluated myocardial deformation and LV torsion assessed by 2-D ST echocardiography in a cohort of subjects in different forms of chronic Chagas disease, to test the hypothesis that this new parameter would add accuracy to the ejection fraction in the prediction of myocardial fibrosis. In current medical practice, ventricular contractility is evaluated based on LVEF using the Simpson biplane model by conventional echocardiography. However, this parameter lacks sensitivity to identify early contractility impairment, since longitudinal component and endocardial layers are the first affected. Moreover, ejection fraction measurement is highly dependent not only on operator experience, but also on loading, which may lead to abnormal results under unusual loading situations [15].
In this context, 2-D ST has emerged as a promising imaging modality to calculate regional tissue deformation, less subject to artifact, less dependent on endocardial visualization and independent of geometric assumptions. This method quantifies myocardial deformation through frame-by-frame tracking of a small image block of myocardial acoustic markers. Already applied to several clinical conditions, this technique has been shown to be an effective method for assessing LV function, regardless of operator experience [7]; [14] ; [16]. Nahum and co-workers previously reported that longitudinal global strain by ST is superior to LVEF and other longitudinal markers in identifying subjects with poor outcome in a population with heart failure [17].
Previous studies have validated 2-D ST for LV quantification against magnetic resonance imaging, demonstrating the reliability of the method in a clinical trial [18]. Although myocardial deformation by 2-D ST had been characterized in a cohort of subjects with different forms of Chagas disease [11], the data did not include validation against left ventricular ejection fraction. Garcia-Álvarez and coworkers showed that the average value of LGS for subjects with chronic Chagas cardiomyopathy was − 16.5%, whereas in our study the average was − 11.0%, possibly featuring a population of greater severity.
Our study showed a high percentage of delayed enhancement in the three different forms of the disease: 41% for the indeterminate form, 47% for the cardiac form without ventricular dysfunction and 92% for subjects with ventricular dysfunction. A previous study demonstrated rates of 7.4%, 15.8% and 52.4% for these three groups of subjects, respectively [19]. Despite this difference, the overall prevalence of delayed enhancement in our population of subjects with chronic Chagas cardiomyopathy, estimated at 92%, is consistent with literature data, since global prevalence has demonstrated delayed enhancement ranging from 68.6% to 88.9% in this profile of subjects [2].
We also demonstrated a high correlation between EF assessed by Simpson and longitudinal global strain with myocardial fibrosis assessed by CMR, both with statistical significance. However, one of the issues that we wanted to address in our study was if the use of 2-D ST would have an incremental role to ejection fraction on the prediction of myocardial fibrosis, which was shown not to be true, since the longitudinal global strain became non-significant when adjusted to the ejection fraction and to other important echocardiographic variables. Unlike the longitudinal global strain, the Tei index and the E/E′ ratio were statistically significant as independent predictors of myocardial fibrosis. Since myocardial fibrosis increases the left ventricular diastolic pressure, leading to alterations in the left ventricular relaxation, these parameters may be indicative of early cardiac involvement.
One limitation of our study was that a more complex investigation to rule out coronary artery disease was not performed. However, subjects with a history of acute myocardial infarction or chronic coronary artery disease were excluded. In addition, all subjects underwent a treadmill stress test, an exam with a high negative predictive value for subjects without symptoms of ischemic heart disease, and no ischemia was demonstrated.
It is already known that the evaluation of LV function is crucial in subjects with Chagas disease, as ventricular dysfunction is an independent predictor of mortality in this group of subjects [20]. We studied the role of the longitudinal global strain as a possible method to add accuracy to ejection fraction in the prediction of myocardial fibrosis, but we did not demonstrate superiority of this new technique, leading to the conclusion that the longitudinal global strain has no incremental value to conventional ejection fraction measurement in subjects with Chagas disease. Since every new technique comes up with a great enthusiasm in its use, even before strong scientific evidence confirms its usefulness, it is crucial to develop studies testing a new method against other well-established ones, in order to have a critical view of the diagnostic exams available in clinical practice.
Funding
This work was financially supported by FAPESB: Fundação de Amparo à Pesquisa do Estado da Bahia (00003841).
Conflicts of interest
None.
Acknowledgments
The authors thank Dr. Kyan James Allahdadi for a careful review of the manuscript.
References
- [1] Z.A. Andrade, S.G. Andrade, G.B. Oliveira, D.R. Alonso; Histopathology of the conducting tissue of the heart in Chagas' myocarditis; Am Heart J, 95 (3) (1978), pp. 316–324
- [2] C.E. Rochitte, P.F. Oliveira, J.M. Andrade, B.M. Ianni, J.R. Parga, L.F. Avila, et al.; Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas' disease: a marker of disease severity; J Am Coll Cardiol, 46 (8) (2005), pp. 1553–1558 https://doi.org/10.1016/j.jacc.2005.06.067 [published Online First: Epub Date]
- [3] R.P. Mello, G. Szarf, P.R. Schvartzman, E.M. Nakano, M.M. Espinosa, D. Szejnfeld, et al.; Delayed enhancement cardiac magnetic resonance imaging can identify the risk for ventricular tachycardia in chronic Chagas' heart disease; Arq Bras Cardiol, 98 (5) (2012), pp. 421–430
- [4] A. Wagner, H. Mahrholdt, T.A. Holly, M.D. Elliott, M. Regenfus, M. Parker, et al.; Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study; Lancet, 361 (9355) (2003), pp. 374–379 https://doi.org/10.1016/S0140-6736(03)12389-6 [published Online First: Epub Date]
- [5] C.E. Rochitte, J.A. Lima, D.A. Bluemke, S.B. Reeder, E.R. McVeigh, T. Furuta, et al.; Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction; Circulation, 98 (10) (1998), pp. 1006–1014
- [6] J.A. McCrohon, J.C. Moon, S.K. Prasad, W.J. McKenna, C.H. Lorenz, A.J. Coats, et al.; Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance; Circulation, 108 (1) (2003), pp. 54–59 https://doi.org/10.1161/01.CIR.0000078641.19365.4C [published Online First: Epub Date]
- [7] H. Blessberger, T. Binder; Two dimensional speckle tracking echocardiography: clinical applications; Heart, 96 (24) (2010), pp. 2032–2040 https://doi.org/10.1136/hrt.2010.199885 [published Online First: Epub Date]
- [8] J. Koyama, P.A. Ray-Sequin, R.H. Falk; Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis; Circulation, 107 (19) (2003), pp. 2446–2452 https://doi.org/10.1161/01.CIR.0000068313.67758.4F [published Online First: Epub Date]
- [9] V. Richand, S. Lafitte, P. Reant, K. Serri, M. Lafitte, S. Brette, et al.; An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy; Am J Cardiol, 100 (1) (2007), pp. 128–132 https://doi.org/10.1016/j.amjcard.2007.02.063 [published Online First: Epub Date]
- [10] S. Lafitte, M. Perlant, P. Reant, K. Serri, H. Douard, A. DeMaria, et al.; Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis; Eur J Echocardiogr, 10 (3) (2009), pp. 414–419 https://doi.org/10.1093/ejechocard/jen299 [published Online First: Epub Date]
- [11] A. García-Álvarez, M. Sitges, A. Regueiro, S. Poyatos, M. Jesus Pinazo, E. Posada, et al.; Myocardial deformation analysis in Chagas heart disease with the use of speckle tracking echocardiography; J Card Fail, 17 (12) (2011), pp. 1028–1034 https://doi.org/10.1016/j.cardfail.2011.08.007 [published Online First: Epub Date]
- [12] J. Brown, C. Jenkins, T.H. Marwick; Use of myocardial strain to assess global left ventricular function: a comparison with cardiac magnetic resonance and 3-dimensional echocardiography; Am Heart J, 157 (1) (2009), pp. 102.e1–102.e5 https://doi.org/10.1016/j.ahj.2008.08.032 [published Online First: Epub Date]
- [13] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; J Am Soc Echocardiogr, 18 (12) (2005), pp. 1440–1463 https://doi.org/10.1016/j.echo.2005.10.005 [published Online First: Epub Date]
- [14] M. Leitman, P. Lysyansky, S. Sidenko, V. Shir, E. Peleg, M. Binenbaum, et al.; Two-dimensional strain—a novel software for real-time quantitative echocardiographic assessment of myocardial function; J Am Soc Echocardiogr, 17 (10) (2004), pp. 1021–1029 https://doi.org/10.1016/j.echo.2004.06.019 [published Online First: Epub Date]
- [15] T.H. Marwick; Should we be evaluating the ventricle or the myocardium? Advances in tissue characterization; J Am Soc Echocardiogr, 17 (2) (2004), pp. 168–172 https://doi.org/10.1016/j.echo.2003.10.021 [published Online First: Epub Date]
- [16] H. Belghitia, S. Brette, S. Lafitte, P. Reant, F. Picard, K. Serri, et al.; Automated function imaging: a new operator-independent strain method for assessing left ventricular function; Arch Cardiovasc Dis, 101 (3) (2008), pp. 163–169
- [17] J. Nahum, A. Bensaid, C. Dussault, L. Macron, D. Clémence, B. Bouhemad, et al.; Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients; Circ Cardiovasc Imaging, 3 (3) (2010), pp. 249–256 https://doi.org/10.1161/CIRCIMAGING.109.910893 [published Online First: Epub Date]
- [18] B.H. Amundsen, T. Helle-Valle, T. Edvardsen, H. Torp, J. Crosby, E. Lyseggen, et al.; Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging; J Am Coll Cardiol, 47 (4) (2006), pp. 789–793 https://doi.org/10.1016/j.jacc.2005.10.040 [published Online First: Epub Date]
- [19] A. Regueiro, A. García-Álvarez, M. Sitges, J.T. Ortiz-Pérez, M.T. De Caralt, M.J. Pinazo, et al.; Myocardial involvement in Chagas disease: insights from cardiac magnetic resonance; Int J Cardiol, 165 (1) (2013), pp. 107–112 https://doi.org/10.1016/j.ijcard.2011.07.089 [published Online First: Epub Date]
- [20] A. Rassi, W.C. Little, S.S. Xavier, S.G. Rassi, A.G. Rassi, G.G. Rassi, et al.; Development and validation of a risk score for predicting death in Chagas' heart disease; N Engl J Med, 355 (8) (2006), pp. 799–808 https://doi.org/10.1056/NEJMoa053241355/8/799 [pii] [published Online First: Epub Date]
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?