Summary
Background
Severe head injury is known to be a major cause of early mortalities and morbidities. Patients' long-term outcome after acute care, however, has not been widely studied. We aim to review the outcome of severely head-injured patients after discharge from acute care at a designated trauma center in Hong Kong.
Materials and methods
This is a retrospective study of prospectively collected data of patients admitted with severe head injuries between 2004 and 2008. Patients' functional status post-discharge was assessed using the Extended Glasgow Outcome Score (GOSE).
Results
Of a total of 1565 trauma patients, 116 had severe head injuries and 41 of them survived acute hospital care. Upon the last follow-up, 23 (56.1%) of the acute-care survivors had improvements in their GOSE, six (11.8%) experienced deteriorations, and 12 (23.5%) did not exhibit any change. The greatest improvement was observed in patients with GOSE of 5 and 6 upon discharge, but two of the 16 patients with GOSE 2 or 3 also had a good recovery. On logistic regression analysis, old age and prolonged acute hospital stay were found to be independent predictors of poor functional outcome after a mean follow-up duration of 42 months.
Conclusion
Multidisciplinary neurorehabilitation service is an important component of comprehensive trauma care. Despite significant early mortalities, a proportion of severely head-injured patients who survive acute care may achieve good long-term functional recovery.
Keywords
Functional survival;severe head injury;neurorehabilitation;trauma
1. Introduction
Traumatic brain injury (TBI) accounts for a significant proportion of accidental deaths in adults worldwide.1 ; 2 The acute outcome of severe TBI has been studied extensively over the years with reported mortality rates ranging from 20% to 50%.3 ; 4 The majority of TBI-related deaths occur shortly after injury or during acute hospital stay, and patients who survive acute care may also have significantly reduced life expectancies and functional impairment.5 When compared with the general population, head-injured patients continue to have increased risks of mortality after hospital discharge.6 Post-acute-care mortality has been postulated to be secondary to patients' impaired mobility, suicide risks, and altered lifestyle.6 Other risk factors may include old age, male gender, as well as premorbid psychosocial, psychiatric, and seizure disorders.7
The majority of published studies on TBI have focused on patient outcome during acute hospital stay, and relatively less is known about post-acute outcome. Mortality is only one of the many sequelae of severe TBI, and acute-care mortality alone provides an inadequate assessment of patient outcome. In the United States, an estimated 1.59 million patients sustain head injury annually.8 Of these, 20% required inpatient rehabilitation.9 Age, Glasgow Coma Score (GCS), and baseline Functional Independence Measure recorded at the beginning of rehabilitation have been found to be independent predictors for 1-year clinical outcome.10
The disabilities and the psychosocial needs of survivors of TBI have a significant impact on society. A proportion of these patients are unable to live at home or to resume their premorbid employment, which translates into an increased need for care from their families and society, as well as an increased demand on infirmaries, nursing homes, and rehabilitation services. A previous study has demonstrated that an average of 40 years of productivity to the society was lost per victim of all traumatic deaths, with TBI accounting for over 50% of these cases.11 Several previous studies have described predictive factors associated with acute and/or delayed mortality, but reports on functional outcomes are relatively limited.6; 7 ; 12
The aim of this study is to review the post-acute mortality and functional outcomes of patients suffering from severe TBI initially treated at a designated trauma center. Predictive factors associated with post-acute functional outcomes will also be studied.
2. Materials and methods
2.1. Patient selection
This is a retrospective review of the prospective hospital trauma registry of a designated trauma center. All patients with severe head injuries with or without extracranial injuries who were admitted between January 2004 and December 2008 were included. Severe head injury was defined by admission Glasgow Coma Scale (GCS of 8 or lower. Patients who died upon arrival at the center were excluded.
2.2. Acute trauma care
Our trauma center has 24-hour neurosurgical coverage and is equipped with staffing and resources compatible with a Level I trauma center in the United States. All patients received initial resuscitation by the hospital trauma team. Severely head-injured patients would receive operative treatment as clinically indicated and postoperative care in the neurointensive care unit. All patients were given prophylactic anticonvulsant medications and received intracranial pressure monitoring. Refractory intracranial hypertension, as defined by intracranial pressure greater than 20 mmHg, was treated with intravenous mannitol infusion and/or reoperations if repeated imaging studies demonstrated the presence of significant intracranial mass lesions. Cerebral perfusion pressure was maintained at 70 mmHg with the use of inotropic support if needed. Tracheostomy was performed if mechanical ventilation was required for more than 5 days.
2.3. Rehabilitation services
Following discharge from the trauma center, decisions on subsequent rehabilitation care was decided by the attending neurosurgeons based on the perceived rehabilitation potential. Patients may be transferred to an affiliated rehabilitation center or a convalescence hospital or were sent home. The rehabilitation center was staffed with a multidisciplinary team of occupational therapists, physiotherapists, speech therapists, and medical social workers. Each patient would receive 4–6 hours of goal-directed and systematic training daily, depending on the individuals progress and clinical state. In contrast, the convalescence was mainly catered for general nursing care. Physiotherapy was given primarily for the prevention of limb spasticity and chest infections. Cognitive and functional training was limited.
2.4. Data and statistical analysis
Patients' demographics as well as mechanisms and severities of head injuries as denoted by the Injury Severity Score (ISS), Revised Trauma Score (RTS), and computerized tomography findings were collected for analysis. Main outcome measures included acute hospital length of stay, acute hospital and post-discharge mortalities, and functional outcomes. The latter was assessed using the Extended Glasgow Outcome Scale (GOSE).13 Statistical analysis was performed using SPSS for Windows v16.0.1 software. Correlative analysis was performed using independent t-test and logistic regression to identify predictive factors for post-acute outcome. Statistical significance was defined as p < 0.05.
3. Results
3.1. Early and delayed mortalities
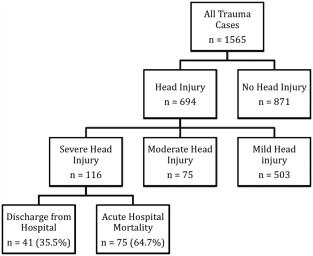
A total of 1565 trauma patients were admitted during the study period, of whom 116 suffered from severe head injuries. Of the latter group, 41 (35.3%) survived acute hospital care, yielding an acute hospital mortality rate of 64.7%. After discharge, five patients subsequently died, yielding a post-acute mortality rate of 12.2% (5/41) and an overall mortality rate of 69.0% (80/116) for patients with severe head injuries (Fig. 1). All five delayed mortalities had GOSE of 2 to 4 upon discharge from the trauma center. The causes of these five deaths included sepsis (2), carcinoma of urinary bladder (1), chronic obstructive airway disease (1), and cerebrovascular accident (1).
|
|
|
Figure 1. Acute and delayed mortalities of patients with head injuries during the study period. |
3.2. Characteristics of acute-care survivors
Of the 41 survivors of acute hospitalization, 27 (65.9%) were men and 14 (34.1%) were women, with a mean age of 47.8 years (range = 19.0–82.0) (Table 1). Over half of the patients (56.1%) suffered from severe head injury as a result of falls. The mean ISS was 18.54, and the mean RTS was 4.786. Around one-third of patients had traumatic intracerebral hemorrhages. The mean length of acute hospital stay was 23.6 days. Almost half of our patients (48.8%) were able to be discharged home, 36.6% were transferred to a convalescence hospital, and the rest (14.6%) were admitted to our rehabilitation center.
| Age | 47.8 y (range, 19.0–82.0) |
| Gender (%) | |
| Men | 27 (65.9%) |
| Women | 14 (34.1%) |
| Mechanisms of injury | |
| Fall | 23 (56.1%) |
| Motor vehicle accidents | 14 (34.1%) |
| Others | 3 (7.3%) |
| Assault | 1 (2.4%) |
| Injury Severity Score (ISS) | 18.54 (range: 1–59) |
| Revised Trauma Score (RTS) | 4.7863 (range: 0.8724–5.9672) |
| Computerized tomography findings | |
| Intracerebral hemorrhages | 13 (31.7%) |
| Subarachnoid hemorrhages | 12 (29.3%) |
| Subdural hemorrhages | 11 (26.8%) |
| Epidural hemorrhages | 5 (12.2%) |
| Length of acute hospital stay days | 23.6 (1–180) |
| Disposal | |
| Rehabilitation center | 6 (14.6%) |
| Convalescence hospital | 15 (36.6%) |
| Home | 20 (48.8%) |
Upon hospital discharge, 24.4%, 19.6%, and 41.4% of acute-care survivors had severe disabilities (GOSE = 3 or 4), moderate disabilities (GOSE 5 or 6), and good recoveries (GOSE = 7 or 8), respectively. Close to 15% were in vegetative states (GOSE = 2) (Table 2). The mean duration of post-acute follow-up was 42 months (range = 12– 60 months).
| GOSE | Description | Discharge (%) | Follow-up (%) |
|---|---|---|---|
| 1 | Dead | 0 (0) | 5 (12.2) |
| 2 | Vegetative | 6 (14.6) | 2 (4.9) |
| 3 | Lower severe disability | 4 (9.8) | 1 (2.4) |
| 4 | Upper severe disability | 6 (14.6) | 4 (9.8) |
| 5 | Lower moderate disability | 4 (9.8) | 1 (2.4) |
| 6 | Upper moderate disability | 4 (9.8) | 3 (7.3) |
| 7 | Lower good recovery | 17 (41.4) | 6 (14.6) |
| 8 | Upper good recovery | 0 (0) | 19 (46.4) |
3.3. Changes in functional outcome after acute care
Overall, 23 (56.1%) of the acute-care survivors had improvements in their GOSE, six (11.8%) experienced deteriorations, and 12 (23.5%) did not exhibit any changes as of the last follow-up (Tables 2 and 3). The number of patients with good recovery (GOSE 7 and 8) increased from 17 (41.6%) to 25 (61%). The number of patients in vegetative state decreased from six (14.6%) to two (4.9%). The greatest improvement was observed in patients with GOSE of 5 and 6 upon hospital discharge. In contrast, among the 16 patients who were vegetative or severely disabled upon hospital discharge, 31.3% subsequently died, while 43.8% remained unimproved. Of note, however, were two of these 16 patients (12.5%) who eventually attained GOSE of 8.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|
| GOSE upon acute hospital discharge | 1 | ||||||||
| 2 | •••• | •• | |||||||
| 3 | • | • | •• | ||||||
| 4 | •• | • | • | •• | |||||
| 5 | • | ••• | |||||||
| 6 | •• | •• | |||||||
| 7 | • | •••• | •••••••••••• | ||||||
| 8 |
“•” represents one patient.
With regard to the impact of post-acute rehabilitation service, 66.7% of patients who were transferred to the rehabilitation center experienced improvement; only 53.3% of those transferred to a convalescence hospital subsequently improved. The two severely disabled patients who subsequently improved to GOSE of 8 were initially discharged home from acute care.
Thirty-two (78%) of all acute-care survivors were able to return home while four (9.8%) remained under institutional care. In terms of ambulatory ability, 25 (61%) could walk unaided and three (7.3%) could walk with aids. Six (14.6%) and two (4.9%) survivors remained wheelchair-bound and bed-bound, respectively.
3.4. Predictive factors of post-acute outcome
To identify predictive factors for functional outcome after acute care, patients were dichotomized into the good recovery and poor recovery groups (Table 4). Good recovery was represented by GOSE of 7 and 8, while GOSE 6 or lower were classified as poor recovery.
| Poor outcome | Good outcome | pa | |
|---|---|---|---|
| Age (y) | 60.5 ± 17.1 | 39.6 ± 15.9 | <0.001 |
| Male (%) | 63 | 68 | 0.725 |
| LOS (d) | 42.3 ± 34.5 | 11.6 ± 10.9 | 0.002 |
| ICH (%) | 50 | 20 | 0.045 |
| SAH (%) | 31 | 28 | 0.829 |
| EDH (%) | 13 | 12 | 0.963 |
| RTS | 4.87 ± 0.96 | 4.73 ± 1.38 | 0.740 |
| ISS | 21 ± 19.16 | 16.96 ± 15.55 | 0.463 |
EDH = epidural hemorrhage; ICH = intracerebral hemorrhage; ISS = Injury Severity Score; LOS = length of stay; SAH = subarachnoid hemorrhage; RTS = Revised Trauma Score.
a. Independent t-test.
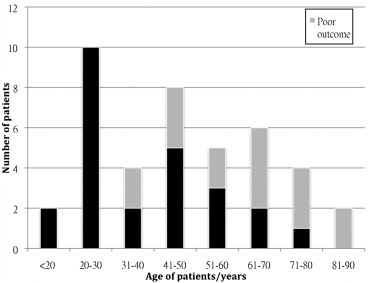
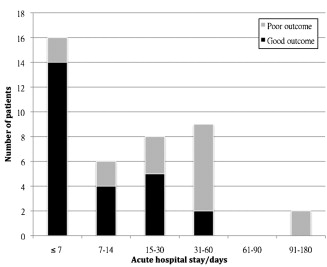
Gender, the presence of subarachnoid/subdural/epidural hemorrhages, RTS, and ISS were not found to be associated with poor functional outcome. The presence of ICH had a weak association with poor GOSE (p = 0.045). Old age (p < 0.001) and prolonged hospital stay (p = 0.002) were significantly associated with poor outcome. Using binary logistic regression, gender, GCS upon admission, and presence of intracranial hemorrhages of all types were not predictive of functional outcome (results not shown). Age (hazard ratio, 1.11; 95% confidence interval, 1.02–1.21; p = 0.012), and the length of acute hospital stay (hazard ratio, 1.16; 95% confidence interval, 1.03–1.31; p = 0.017) were found to be independent predictors of poorer outcome. Fig. 2 illustrates the association between patient age by decades and post-discharge functional outcomes. All patients younger than 30 years achieved good outcomes, while none above the age of 65 did. Fig. 3 illustrates the association between the length of acute hospital stay with functional outcomes. Patients who were transferred out of acute care within 1 week or sooner were particularly likely to achieve good post-discharge functional outcome.
|
|
|
Figure 2. Distribution by age groups for patients with good and poor outcomes upon the last follow-up (n = 41). |
|
|
|
Figure 3. Distribution by length of acute hospital stay days for patients with good and poor outcomes upon the last follow-up (n = 41). |
4. Discussion
4.1. Recovery from severe TBI
Severe TBI is associated with high mortality rates, and we found an overall mortality rate of 69% in this study.1 Our study also found a delayed mortality rate of 12.2%. Similar findings have been reported previously which demonstrated an increase in mortality risk for at least 7 years after the initial trauma. The first-year mortality rate was 9.5%. Thereafter, the annual mortality rate was around 3.2% and the overall mortality rate at the 7th year post-injury was as high as 27%.14 Most of the delayed mortalities were directly related to the initial TBI. When compared with the general population, the risk of death for patients with TBI was 23 times higher in the first 2 months, three times higher in the 3rd to the 12th months, and twice as high thereafter.14 Statistically, younger patients were found to be particularly at risk, probably as a result of the relatively low mortality rates in the younger age groups within the general population.
Causes of post-acute mortality have been reported, with 25% of deaths being due to circulatory conditions, 22% due to respiratory conditions, 14% due to neoplasms, and 5% were seizure-related.15 In the present study, five of our patients died after acute care, and their GOSE upon discharge were poor. It was also interesting to note that none of these deaths was directly neurologically related.
Among our acute-care survivors, there were relatively good functional outcomes with 60.9% of patients attaining GOSE 7 or 8. This was an encouraging finding. A previous study on patients presented with GCS of 3 reported an overall survival rate of 50.8%, with 13.2% of patients attaining good functional outcomes at 6 months post-injury.16 However, since our cohort included patients with admission GCS of 3 to 8, the incidence of good recovery would be expectedly higher.
Previous studies have reported that around 44% of severely head-injured patients were able to achieve satisfactory recovery or were able to care for themselves at 1 year.17 In the present study, 56.1% of patients had improvement in their GOSE. Moreover, over 60% were able to walk unaided and return home. This may add further support to the notion that poor outcomes are not inevitable in severely head-injured patients. In terms of the prognosis for post-acute-care recovery, our patients with GOSE of 5 or 6 upon hospital discharge were found to have the greatest improvement, followed by patients who already had GOSE of 7. It is noteworthy that two individuals from this subgroup of patients were able to achieve good recovery.
4.2. Rehabilitation and functional outcome
One of our discharge institutions was a dedicated rehabilitation hospital. The majority (66.7%) of patients treated there had functional improvement, compared with 53.3% of those transferred to a convalescence hospital. This result is congruent with one of the local studies which reviewed the effects of intensive rehabilitation on the functional outcome of patients with moderate to severe TBI.18 It was noted that although early intensive rehabilitation did not alter the final outcome of patients, those who received it had earlier recovery, which may translate into an expedited resumption of their premorbid productivity. However, since rehabilitation hospitals generally accept patients with better rehabilitation potential, the impact of patient selection bias cannot be excluded. Further investigations into the impact of the level of rehabilitation services in this locality will be of value.
4.3. Predictive factors of functional outcomes
Previous studies have identified age, injury severity, number of comorbidities, mechanisms of injury, and discharge destination as significant predictors of poor outcome.19 ; 20 Our study identified age and prolonged acute hospital stay as significant predicators of poorer functional outcome. Age-related poor outcome has been postulated to be due to the poorer recoverability of the aged brain.19 Another possible explanation is that older patients may have a significant number of comorbidities, leading to a more arduous course of recovery. Prolonged hospital stay may also be related to the higher number of elderly patients included in our study. It was interesting to note that formal injury severity scoring systems, such as RTS or ISS, were not found to be significant predictors.
4.4. Limitations of the study
One of the major weaknesses of this study was that this was a retrospective study with data taken from a large hospital “general” trauma registry. The parameters of the data collected were initially not set out for this study. Post-acute-care assessments were performed by different individual medical personnel who may have different interpretations and experiences. The GOSE upon follow-up were based on retrospective review, which may reduce its reliability. The sample size was also a critical issue. With only 41 severe head injury survivors, the impact of the findings cannot be readily generalized. A structured, protocol-driven, preferably multicentered prospective study would be required. A more thorough assessment on the detailed functional abilities of the patients and their work capacities would also add weight to the study.
5. Conclusion
Severe head injury continued to be a major cause of acute and delayed mortalities and morbidities. The present study illustrated that a proportion of severely head-injured patients who survived acute care may achieve good long-term functional recovery. Advanced age and prolonged acute hospital stay were predictive of poor functional recovery. Multidisciplinary neurorehabilitation service is an important component of comprehensive trauma care. Our findings may serve as a useful reference for the future development of rehabilitation services in this locality and other healthcare systems.
References
- 1 F. Tagliaferri, C. Compagnone, M. Korsic, F. Servadei, J. Kraus; A systematic review of brain injury epidemiology in Europe; Acta Neurochirurg Wien, 148 (2006), pp. 255–268
- 2 D.M. Sosin, J.E. Sniezek, J. Waxweiler; Trends in death associated with traumatic brain injury, 1979 through 1992; JAMA, 273 (1995), pp. 1778–1780
- 3 G.R. Boto, P.A. Go ́mez, J. De La Cruz, R.D. Lobato; Severe head injury and the risk of early death; J Neurol Neurosurg Psychiatry, 77 (2006), pp. 1054–1059
- 4 D. Mellick, K.A. Gerhart, G.G. Whiteneck; Understanding outcomes based on the post-acute hospitalization pathways followed by persons with traumatic brain injury; Brain Inj, 17 (2003), pp. 55–71
- 5 G. Ratcliff, A. Colantonio, M. Escobar, S. Chase, L. Vernich; Long term survival following traumatic brain injury; Disabil Rehabil, 27 (2005), pp. 305–314
- 6 L. Chan, J. Doctor, N. Temkin, et al.; Discharge disposition from acute care after traumatic brain injury: the effect of insurance type; Arch Phys Med Rehabil, 82 (2001), pp. 1151–1154
- 7 W.S. Poon, X.L. Zhu, S.C. Ng, G.K. Wong; Predicting one year clinical outcome in traumatic brain injury (TBI) at the beginning of rehabilitation; Acta Neurochir Suppl, 93 (2005), pp. 207–208
- 8 S. Thornhill, G.M. Teasdale, G.D. Murray, J. McEwen, C.W. Roy, N.I. Penny; Disability in young people and adults one year after head injury: prospective cohort study; BMJ, 320 (2000), pp. 1631–1635
- 9 B. Pentland, L.S. Hutton, P.A. Jones; Late mortality after head injury; J Neurol Neurosurg Psychiatry, 76 (2004), pp. 395–400
- 10 S. Corkin, E.V. Sullivan, F.A. Carr; Prognostic factors for life expectancy after penetrating head injury; Arch Neurol, 41 (1984), pp. 975–977
- 11 S.R. Shackford, R.C. Mackersie, T.L. Holbrook, et al.; The epidemiology of traumatic death. A population-based analysis; Arch Surg, 128 (1993), pp. 571–575
- 12 M.A. Schreiber, N. Aoki, B.G. Scott, J.R. Beck; Determinants of mortality in patients with severe blunt head injury; Arch Surg, 137 (2002), pp. 285–290
- 13 J.T.L. Wilson, L.E.L. Pettigrew, G.M. Teasdale; Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use; J Neurotrauma, 15 (1998), pp. 573–585
- 14 T.M. McMillan, G.M. Teasdale; Death rate is increased for at least 7 years after head injury: a prospective study; Brain, 130 (2007), pp. 2520–2527
- 15 C.L. Harrison-Felix, G.G. Whiteneck, A. Jha, M.J. DeVivo, F.M. Hammond, D.M. Hart; Mortality over four decades after traumatic brain injury rehabilitation: a retrospective cohort study; Arch Phys Med Rehabil, 90 (2009), pp. 1506–1513
- 16 R.B. Chamoun, C.S. Robertson, S.P. Gopinath; Outcome in patients with blunt head trauma and a Glasgow Coma Scale score of 3 at presentation; J Neurosurg, 111 (2009), pp. 683–687
- 17 A. Bricolo, S. Turazzi, G. Feriotti; Prolonged posttraumatic unconsciousness. Therapeutic assets and liabilities; J Neurosurg, 52 (1980), pp. 625–634
- 18 X.L. Zhu, W.S. Poon, C.C. Chan, S.S. Chan; Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial; Brain Inj, 21 (2007), pp. 681–690
- 19 D.G. Vollmer, J.C. Torner, H.M. Eisenberg, M.A. Foulkes, A. Marmarou, L.F. Marshall; Age and outcome following traumatic coma: why older patients fare worse; J Neurosurg, 75 (1991), pp. S37–S49
- 20 A. Colantonio, M. Escobar, M. Chipman, et al.; Predictors of post acute mortality following traumatic brain injury in a seriously injured population; J Trauma, 64 (2008), pp. 876–882
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?