Abstract
Pulmonary vein thrombosis (PVT) is often not diagnosed because PVT has subtle symptoms and PVT is believed to be rare. The mechanism for the formation of PVT is unclear. In this case, I describe a small thrombus in a small branch of a pulmonary vein draining into a larger vein, the right lower pulmonary vein (RLPV).
The patient was a 70-year-old male with angina pectoris, and he presented with chest pain. He had no symptoms of cerebral infarction. He previously had been treated with percutaneous coronary intervention and had four stents in the coronary arteries. A 64-slice multidetector computed tomography (64-MDCT) scan was performed to evaluation for in-stent restenosis. A thrombus in the RLPV was shown in axial and sagittal images as a defect in contrast enhancement, representing a small thrombus in a small branch of a pulmonary vein draining into the RLPV.
The 64-MDCT scan depicted them well. The effects of PVT are unknown, and more studies are needed.
Keywords
Pulmonary vein thrombus;64-MDCT;Right lower pulmonary vein
1. Introduction
Stroke is the third-leading cause of death and the leading cause of permanent disability [1]. Stroke prophylaxis is one of the targets of atrial fibrillation (AF) management. The current standard of care for stroke prevention in patients with AF is oral systemic anticoagulation [2]. Presently, we have new options such as rivaroxaban [3], dabigatran [4] and apixaban [5].
Pulmonary vein thrombosis (PVT) is a possible cause of ischemic stroke, but PVT is often not diagnosed and thought to be rare. Pulmonary vein thrombi and left atrial appendage (LAA) thrombi cause ischemic stroke, so diagnosing PVT early and preventing PVT primary and secondary thromboembolic stroke using anticoagulants are important.
A 64-slice multidetector computed tomography (64-MDCT) scan has become an option to assess coronary artery plaques [6]. A 64-MDCT scan can easily assess LAA thrombi and pulmonary vein thrombi. Since 2012, I have published some cases of pulmonary vein thrombi, and in 2014, I reported that 61% (35 patients) of 57 patients with chest pain had pulmonary vein thrombi [7]. Previously, I reported a thrombus in a main branch of the pulmonary vein that almost completely occluded the vessel and extended into the pulmonary vein [8]. However, how pulmonary vein thrombi form remains unclear. I presented a case of pulmonary vein thrombus that started not in a main branch of the pulmonary vein but a small branch.
2. Case presentations
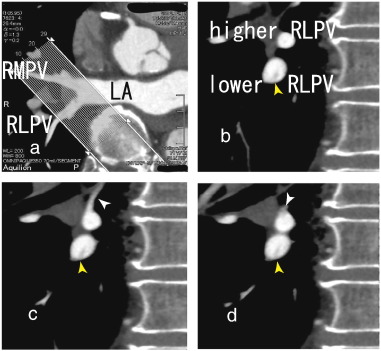
The patient was a 70-year-old man with angina pectoris, and he presented with recent chest pain. He had been treated with percutaneous coronary intervention and had four stents in the coronary arteries. A 64-MDCT scan was performed to evaluate recent chest pain. The scan did not show a coronary artery stenosis and no restenosis in the stents. However, a thrombus in the right lower pulmonary vein (RLPV) was shown in sagittal images (Figs. 1b to 2d) as a defect in contrast enhancements. Axial images of that portion are shown in Fig. 1a. A 64-MDCT scan is useful for identifying pulmonary vein thrombi and preventing stroke because 64-MDCT can depict pulmonary vein thrombi in detail, which helps to estimate the effects of anticoagulants.
|
|
|
Fig. 1. b, 64-MDCT images showed a thrombus in the lower right lower pulmonary vein (lower RLPV) (yellow arrowhead), which seemed to attach to the inferior wall of the lower RLPV. In Fig. 1c, a small vessel approached to the superior wall of the higher RLPV (white arrowhead) and then in Fig. 1d, there appeared a thrombus located on the superior wall as a defect in contrast enhancements (white arrowhead) and extended toward the left atrium (LA) (Fig. 2a to c) that was attached to the superior wall of the lower RLPV (white arrowhead). In Fig. 1d, the higher and lower RLPVs merged into the RLPV. The thrombus attached to the inferior wall of the lower RLPV extended toward the LA. |
|
|
|
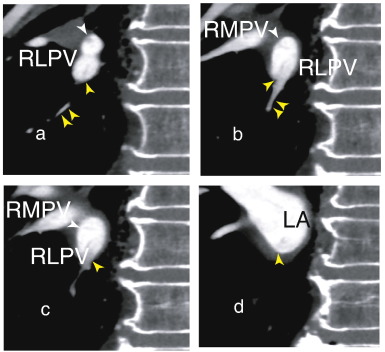
Fig. 2. a and Fig. 2b, a small dark inferior vessel (double yellow arrowhead) has a thrombus that seems to joint a pre-existent thrombus extending from the lower RLPV (Fig. 1b) to the LA (Fig. 2c, yellow arrowhead) and attached to the inferior wall of the RLPV. The upper and lower RLPVs merged into the RLPV (Fig. 2c). In Fig. 2d, the RLPV and the right middle pulmonary vein (RMPV) merged and flowed into the LA. LA; left atrium, RLPV; right lower pulmonary vein, RMPV; right middle pulmonary vein. |
3. Discussion
This paper describes a small thrombus in a small branch of the pulmonary vein extending into a larger pulmonary vein, RLPV. I reported a thrombus in a major branch of the pulmonary vein extending into the pulmonary vein [8]; however, a small branch of pulmonary vein thrombus is rarely reported case because noticing such a thrombus in a small branch is difficult.
Although LAA occlusion is widely accepted, the evidence base is limited. More studies are required to decide whether LAA occlusion has some merits after we notice the existence of PVT. During mitral valve surgery, the LAA is removed to reduce ischemic stroke risk in patients with valvular heart disease [9] ; [10]. Also removing thrombus from the pulmonary vein may be a good idea.
In this case, complete occlusion by thrombus was observed only in a rather small pulmonary vein, which became hypoxic and under-nourished. This unusual bad circumstance affects the pulmonary vein and the surrounding tissue, which seems to have many effects on the tissues that currently are unknown. Such circumstances may transform normal pulmonary vein cells into pulmonary vein myocardium and cause atrial fibrillation. More studies are needed.
In this manuscript, I have made two important points. Formation of pulmonary vein thrombus may start in a small or branch of the pulmonary vein. Diagnosing a pulmonary vein thrombus may be crucial, and 64-MDCT is reliable to identify a pulmonary vein thrombus.
Conflict of interest
The author reports no relationships that could be construed as a conflict of interest.
References
- [1] G.J. Hankey, C.P. Warlow; Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations; Lancet, 354 (1999), pp. 1457–1463
- [2] V. Fuster, L.E. Ryd'en, D.S. Cannom, H.J. Crijns, A.B. Curtis, K.A. Ellenbogen, et al.; 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American college of cardiology foundation/American heart association task force on practice guidelines; Circulation, 123 (2011), pp. e269–e367
- [3] M.R. Patel, K.W. Mahaffey, J. Garg, G. Pan, D.E. Singer, W. Hacke, et al.; Rivaroxaban versus warfarin in nonvalvular atrial fibrillation; N Engl J Med, 365 (2011), pp. 883–891
- [4] S.J. Connolly, M.D. Ezekowitz, S. Yusuf, J. Eikelboom, J. Oldgren, A. Parekh, et al.; Dabigatran versus warfarin in patients with atrial fibrillation; N Engl J Med, 361 (2009), pp. 1139–1151
- [5] C.B. Granger, J.H. Alexander, J.J. McMurray, R.D. Lopes, E.M. Hylek, M. Hanna, et al.; Apixaban versus warfarin in patients with atrial fibrillation; N Engl J Med, 365 (2011), pp. 981–992
- [6] Takeuchi H. Warfarin improved CT values of the coronary artery artherosclerotic plaque: evaluation by 64-MDCTA BMJ Case Rep Published online: 24 Sep 2012 doi: http://dx.doi.org/10.1136/bcr.02.2012.5941.
- [7] H. Takeuchi; High prevalence of pulmonary vein thrombi in elderly patients with chest pain, which has relationships with aging associated diseases; IJC Heart Vessel, 4 (2014), pp. 129–134 https://doi.org/10.1016/j.ijchv.2014.05.006 [Published online: 6-JUN-2014]
- [8] Takeuchi H. How to find a thrombus in a small pulmonary vein that is not enhanced by contrast agents because of a lack of arterial blood flow BMJ Case Rep Published online: 29 Jul 2013 http://dx.doi.org/10.1136/bcr-2013-010261.
- [9] K. Bando, J. Kobayashi, M. Hirata, T. Satoh, K. Niwaya, O. Tagusari, et al.; Early and late stroke after mitral valve replacement with a mechanical prosthesis: risk factor analysis of a 24-year experience; J Thorac Cardiovasc Surg, 126 (2003), pp. 358–364
- [10] R.O. Bonow, B. Carabello, A.C. de Leon, Edmunds L.H. Jr Jr., B.J. Fedderly, M.D. Freed, et al.; Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease); Circulation, 98 (1998), pp. 1949–1984
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?