Summary
Background
Allied disorders of Hirschsprungs disease (ADHD) have been proposed to be the concept of the functional obstruction of the intestine with the presence of ganglion cells in the terminal rectum. They are classified into two categories based on pathology: (1) abnormal ganglia, including immaturity of ganglia, hypoganglionosis (HG), and intestinal neuronal dysplasia; (2) normal ganglia, including megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS), segmental dilatation (SD), internal anal sphincter achalasia (IASA), and chronic idiopathic intestinal pseudo-obstruction (CIIP). Some of these show poor prognosis, therefore, the establishment of criteria and appropriate treatment strategies is required.
Methods
The questionnaires were sent to the 161 major institutes of pediatric surgery or gastroenterology in Japan, in order to collect the cases of ADHD during 10 years from 2001 and 2010.
Results
In total, 355 cases were collected. They included 28 immaturity of ganglia, 130 HG (121 congenital, 9 acquired), 18 intestinal neuronal dysplasia, 33 MMIHS, 43 SD, three IASA, and 100 CIIP. Of the 95 institutes, 69 (72.6%) had their own criteria for ADHD. Criteria were based on clinical symptoms and signs, and conventional pathological examinations. Prognosis was poor in congenital HG, MMIHS, and CIIP, while the others showed good survival rates.
Conclusion
Almost all Japanese cases of ADHD in the past 10 years were collected. Congenital HG and CIIP showed relatively high incidence, whereas acquired HG and IASA were extremely rare in Japan. The criteria of each disorder were also collected and summarized. Prognosis was poor in congenital HG, MMIHS, and CIIP.
Keywords
allied disorders;chronic idiopathic intestinal pseudo-obstruction;Hirschsprungs disease;hypoganglionosis;megacystis microcolon intestinal hypoperistalsis syndrome;pseudo-obstruction
1. Introduction
Allied disorders of Hirschsprungs disease (ADHD) have been understood as the conditions that clinically resemble Hirschsprungs disease (HD), despite the presence of ganglion cells in the terminal rectum.1 Patients with Hirschsprungs disease generally present in the newborn period with delayed passage of meconium and abdominal distention or as a young child with severe chronic constipation. Patients with ADHD show similar symptoms and signs to HD, but they can be distinguished from HD by the pathological findings. The term pseudo HD was proposed by Ravitch in 1958. 2 They encountered patients referred for treatment of megacolon in whom the difficulty lay elsewhere rather than in the congenital absence of ganglion cells of the myenteric plexuses of a segment of the rectum or of the colon and rectum. Bentley et al3 summarized HD and allied disorders in the Seminar on Pseudo-Hirschsprungs Disease and Related Disorders. The main thing to remember was that the various disease patterns were essentially determined by their underlying pathology, irrespective of what we choose to call them. ADHD was classified into two categories based on histology 3: those with abnormality of ganglion cells and those without abnormality of ganglion cells (Table 1). Puri and Gosemann4 called this group variants of HD, including four disorders: intestinal neuronal dysplasia (IND); isolated hypoganglionosis (HG); internal anal sphincter achalasia (IASA); and megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS) in 2012. 4 They did not treat chronic idiopathic intestinal pseudo-obstruction (CIIP) as one of the variants of HD.
|
|
Okamoto and Toyosaka5 used the term of pseudo-Hirschsprungs disease in the Japanese literature. It was defined as a congenital, nonmechanical obstruction of the intestine with presence of intramural ganglion cells in the terminal rectum. They classified them based on histology into two categories for six disorders: immaturity of ganglia (IG); HG; hypogenesis; IND; CIIP; and MMIHS. 5
According to the literature and Okamoto and Toyosakas5 classification, ADHD was classified into two categories depending on the pathological findings (Table 2): (1) abnormal ganglia, including IG, HG, and IND; (2) normal ganglia, including MMIHS, segmental dilatation (SD), IASA, and CIIP. Some of them show poor prognosis; therefore, establishment of criteria, severity, and treatment strategy are required. In order to examine the incidence and criteria of ADHD, a preliminary nationwide survey was planned in Japan.
|
|
2. Patients and methods
As a nationwide retrospective cohort study, supported by Ministry of Health and Welfare, Japan, the preliminary questionnaires, requesting the number of cases of ADHD from January 2000 to December 2009 and the criteria of each institute, were sent to the 161 major institutes of pediatric surgery or pediatric gastroenterology representing the core members of the Japanese Society of Pediatric Surgeons, the Japanese Society of Pediatric Nutrition, Gastroenterology, and Hepatology, and the Japanese Study Group of Pediatric Constipation. Therefore almost all institutes that are treating ADHD are considered covered. The number of patients, including the definite and suspected cases, based on the classification of ADHD in Japan (Table 1) and the survival rate and clinical outcome were asked. The criteria of each institute were asked to be answered as free descriptions. The criteria for definitive or suspected were dependent on each institute.
This study was performed according to the Ethical Guidelines for Clinical Research published by the Ministry of Health, Labor, and Welfare of Japan on July 30, 2003. And this study was approved by the Ethical Committee for Clinical Research of Kyushu University Hospital, Fukuoka, Japan (No. 24-163).
3. Results
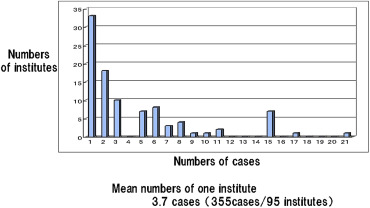
Replies were obtained from 157 of 161 institutes (98%). Out of 157 institutes, 95 (61%) had ADHD. In totally, 355 cases, including 287 definite cases and 68 suspected cases were collected between 2001 and 2010. More than half of the institutes (53 institutes) had three cases or fewer (Figure 1). The mean number of cases per institute was 3.7 cases. There were 165 of 355 cases (47%) treated in university hospitals, 93 (26%) in childrens hospitals, and 97 (27%) in general hospitals. ADHD included 28 IG, 130 HG (121 congenital, 9 acquired), and 18 IND in abnormal ganglia; and 33 MMIHS, 42 SD, three IASA, and 100 CIIP in normal ganglia, and these numbers were compared with those of the previous study in Japan (Table 3).2
|
|
|
Figure 1. Number of cases in each institute. |
| Definitive | Suspected | Total | Okamoto and Toyosaka2 | |
|---|---|---|---|---|
| Abnormal ganglia | ||||
| IG | 22 | 6 | 28 (7.9) | 26 (24.1) |
| HG | 112 | 18 | 30 (36.6) | 44 (40.8) |
| Congenital | 104 | 17 | 121 (34.1) | |
| Acquired | 8 | 1 | 9 (2.5) | |
| IND | 8 | 10 | 18 (5.1) | 5 (4.6) |
| Normal ganglia | ||||
| MMIHS | 27 | 6 | 33 (9.3) | 9 (8.3) |
| SD | 33 | 10 | 43 (12.1) | NE |
| IASA | 1 | 2 | 3 (0.8) | NE |
| CIIP | 84 | 16 | 100 (28.2) | 24 (22.2) |
| Total | 287 | 68 | 355 (100) | 108 (100) |
CIIP = chronic idiopathic intestinal pseudo-obstruction; HG = hypoganglionosis; IASA = internal anal sphincter achalasia; IG = immaturity of ganglia; IND = intestinal neuronal dysplasia; MMIHS = megacystis microcolon intestinal hypoperistalsis syndrome; NE = not examined; SD = segmental dilatation.
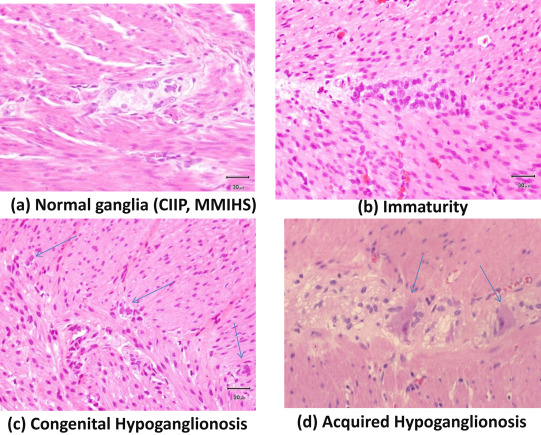
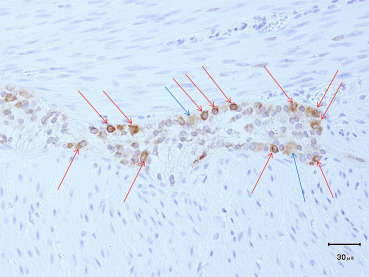
Of the 95 institutes who experienced ADHD, 69 (73%) had their own criteria. The percentages of institutes that had criteria for each disorder were between 28% and 83% (Table 4). More than 80% of institutes had criteria for congenital HG and CIIP, while only ≤ 30% institutes had criteria for acquired HG and IASA. Criteria of each disorder were based on clinical symptoms and signs, examinations including radiography findings, manometric study, and conventional pathological examinations including hematoxylin–eosin (HE; Figure 2) and acetylchoinesterase (AchE). According to answers of the questionnaires, the major criteria listed in each disorder are follows. IG: small ganglion cells, 37/46 (80%); number and distribution of ganglion cells are normal, 19/46 (41%); chronological improvement of clinical symptoms, 8/46 (17%); intestinal obstruction on neonatal onset, 6/46 (13%); normal AchE staining, 3/46 (7%); abdominal distention, 2/46 (4%); and microcolon, 2/46 (4%). Congenital HG: few ganglion cells, 41/55 (75%); few small ganglion cells, 14/55 (25%); intestinal obstruction on neonatal onset, 11/55 (20%); hypoplasia of plexus, 4/55 (7%); normal AchE staining, 4/55 (7%); negative rectospincteric reflex, 4/55 (7%); and delayed meconium pass, 2/55 (4%). Acquired HG: ganglion cells decrease in number after some time, 6/19 (32%); few ganglion cells, 4/19 (21%); normal at birth and symptoms occur after some time, 2/19 (11%); no congenital factors, 2/19 (11%); chronic constipation and persistent bowel dilatation, 2/19 (11%); and normal AchE staining 1 (5%). IND: increased AchE positive fibers in the lamina propria, 17/34 (50%); ectopic ganglion cells, 14/34 (41%); giant ganglia (> 5 ganglion cells per plexus), 13/34 (38%); severe constipation or rectal dysmotility, 9/34 (26%); hyperganglionosis, 6/34 (18%); and dilatation of bowel, 2/34 (6%). MMIHS: megacystis, 39/47 (83%); permanent severe symptoms of intestinal obstruction, 35/47 (74%); microcolon 27/47 (57%); normal histology of intestinal neurons and muscles, 25/47 (53%); neonatal onset, 16/47 (34%); normal AchE staining, 5/47 (11%); and positive rectospincteric reflex 4/47 (9%). SD: persistent segmental dilatation, 36/42 (86%); normal histology of intestinal ganglion cells, 24/42 (57%); no mechanical obstruction distal to dilatation, 13/42 (31%); signs of intestinal obstruction in radiography, 7/42 (17%); complete curability after resection of dilated bowel, 5/42 (12%); abrupt caliber change to the normal intestine, 3/42 (7%); thick or thin muscle layer, 2/42 (5%); and positive rectospincteric reflex, 2/42 (5%). IASA: negative rectospincteric reflex, 9/21 (43%); normal AchE staining, 9/21 (43%); severe constipation since birth, 7/21 (33%); and absence of narrow segment, 4/21 (19%). CIIP: symptoms of intestinal obstruction without mechanical cause, 57/57 (100%); normal histology of intestinal ganglion cells, 46/57 (81%); abnormality of urinary tract, 13/57 (23%); dilatation of intestine in radiography 9/57, (16%); positive rectospincteric reflex, 8/57 (14%); intermittent or recurrent symptoms, 6/57 (11%); and normal AchE staining, 6/57 (11%). For the diagnosis of IG, immunohistochemical studies using Bcl-2 antibody were performed and shown to be effective in a few institutes (Figure 3).
| Abnormal ganglia | |
| IG | 46/69 (67%) |
| HG | |
| Congenital HG | 55/69 (80%) |
| Acquired HG | 19/69 (28%) |
| IND | 34/69 (49%) |
| Normal ganglia | |
| MMIHS | 47/69 (68%) |
| SD | 42/69 (61%) |
| IASA | 21/69 (30%) |
| CIIP | 57/69 (83%) |
CIIP = chronic idiopathic intestinal pseudo-obstruction; HG = hypoganglionosis; IASA = internal anal sphincter achalasia; IG = immaturity of ganglia; IND = intestinal neuronal dysplasia; MMIHS = megacystis microcolon intestinal hypoperistalsis syndrome; SD = segmental dilatation.
|
|
|
Figure 2. Typical pathology of allied disorders of Hirschsprungs disease (ADHD) in hematoxylin–eosin staining. (A) Normal ganglia (megacystis microcolon intestinal hypoperistalsis syndrome, MMIHS; chronic idiopathic intestinal pseudo-obstruction, CIIP; segmental dilatation). (B) Immaturity of ganglia. (C) Congenital hypoganglionosis. Arrows indicate small ganglion cells. (D) Acquired hypoganglionosis. Arrows indicate degenerated ganglion cells. |
|
|
|
Figure 3. Bcl-2 immunostaining for immaturity of ganglia (2-day-old boy). Mature ganglion cells (blue arrows) show weekly positive, while immature ganglion cells (red arrows) show strongly positive in Bcl-2 immunostaining. Glial cells are not stained. Therefore, this staining is considered to be able to distinguish mature ganglion cells, immature ganglion cells, and glial cells. |
The survival rates of each entity for which the follow-up data were available are shown in Table 5. Three entities, congenital HG, MMIHS, and CIIP, showed poor survival rate, compared with those of the other five entities. These three entities required long-term nutritional support, including parenteral and enteral nutrition (Table 6). In particular, outcome is extremely poor in MMIHS.
| Abnormal ganglia | Survival rate |
| IG | 28/28 (100%) |
| HG | |
| Congenital HG | 70/90 (78%) |
| Acquired HG | 8/8 (100%) |
| IND | 11/11 (100%) |
| Normal ganglia | |
| MMIHS | 10/19 (53%) |
| SD | 27/27 (100%) |
| IASA | 3/3 (100%) |
| CIIP | 50/56 (89%) |
CIIP = chronic idiopathic intestinal pseudo-obstruction; HG = hypoganglionosis; IASA = internal anal sphincter achalasia; IG = immaturity of ganglia; IND = intestinal neuronal dysplasia; MMIHS = megacystis microcolon intestinal hypoperistalsis syndrome; SD = segmental dilatation.
| Survival rate | Normal diet in survivors | Normal diet in all cases | |
|---|---|---|---|
| Congenital HG | 70/90 (78%) | 42/69 (60%) | 42/89 (48%) |
| CIIP | 50/56 (89%) | 13/50 (26%) | 13/56 (23%) |
| MMIHS | 10/19 (53%) | 1/10 (10%) | 1/19 (5%) |
CIIP = chronic idiopathic intestinal pseudo-obstruction; HG = hypoganglionosis; MMIHS = megacystis microcolon intestinal hypoperistalsis syndrome.
4. Discussion
Almost all Japanese cases (∼ 98%) of ADHD in 10 years were collected in this nationwide survey. However, the number of cases in each institute was very small, and actually more than half of the institutes (53 institutes) had three cases or fewer. Therefore nation-wide survey is considered to be important.
Okamoto and Toyosaka5 published a multicenter study of ADHD in the Japanese literature in 1994. They classified ADHD into two categories based on pathology: (1) abnormal histology including IG, HG, hypogenesis, and IND; and (2) normal histology, including CIIP6 and MMIHS. We followed the classification of Okamoto and Toyosaka5 in order to compare our results to those of their survey. In addition to Okamoto and Toyosakas5 classification, SD and IASA are included in this study, referring to the literature.1 ; 3
The presence of immaturity and HG was confirmed and published by Taguchi et al.7 We proposed there were two types of HG, namely congenital and acquired.7Hypogenesis is considered to be the initial histological finding of congenital HG at neonatal period. The 4thInternational Symposium on Hirschsprungs disease and related neurocristopathies discussed that diagnosis of HG was difficult and the presence of HG was questionable. 8 However, our study shows that two types of HG do exist and congenital HG is one of the two main disorders of ADHD in Japan.
The 4thInternational Symposium on Hirschsprungs disease and related neurocristopathies discussed IND and reported as follows 8: (1) almost all the participants believe that IND does exist; (2) some believe in presently defined diagnostic criteria, whereas others suggest that these diagnostic criteria are not reliable enough; and (3) some participants question if IND is a truly separate entity or an acquired secondary phenomenon related to long-standing constipation or chronic obstruction. Therefore, we decided to include IND as one of ADHD.
The concept of chronic intestinal pseudo-obstruction (CIPO) including myopathy, neuropathy, collagenopathy (desmosis or fibrosis), and idiopathic.9 Some CIPO patients are reported to be adult onset.10 However, most myopathy and neuropathy types of CIPO cannot be diagnosed by conventional HE and AChE staining. Furthermore, abnormalities of Cajal cells shown by c-kit immunostaining were reported in some cases of CIIP.11 Therefore, so far, we decided to treat CIIP as a disorder that shows recurrent or persistent functional intestinal obstruction with normal histology by conventional staining by HE and AchE. Because the diagnosis of Hirschsprungs disease is generally obtained by HE and AchE staining in the most institutes of Japan, the criteria of ADHD are recommended to be based on the conventional histology so far. MMIHS has been considered to be the severe form of CIIP. However, MMIHS can be distinguished from CIIP by clinical characteristics.12 ; 13
The entity of SD was proposed by Swenson and Rathauser in 1959.14 The symptoms and signs of SD, especially sigmoid type of SD, resemble those of Hirschsprungs disease. Therefore, SD is included in ADHD in this study.
The entity of IASA has been considered to be synonymous of ultrashort-segment aganglionosis, which shows normal AchE staining but lacks rectoanal reflex.15 This entity was discussed in The 4thInternational Symposium on Hirschsprungs disease and related neurocristopathies and was reported to exist. 8 Therefore, we decided that IASA is included in ADHD.
The numbers of cases in each disorder are summarized and compared with Okamoto and Toyosakas5 study in Table 2. The distributions of each disorder are similar in these two studies. The numbers of patients as well as the answer rates of criteria were very small in acquired HG and IASA. The rarity of disease is considered to make criteria difficult.
For definitive pathological diagnosis of ADHD, immnunohistochemical staining has been reported to be useful using neuronal and muscular markers, such as: Bcl-2 for immature neurons; CD56 for the size of enteric ganglia; synaptophysin for neuromuscular innervation: S-100 protein for Schwann cells; c-kit for interstitial cells of Cajal; and smooth muscle actin for myopathy.16 The diagnosis of IG was easily obtained in several of our cases using Bcl-2 immunostaining (Figure 2).
In conclusion, almost all Japanese cases of ADHD for 10 years were collected in this study. Congenital HG and CIIP showed relatively high incidence, whereas acquired HG and IASA were extremely rare. Criteria of each institute were consisted with clinical signs, symptoms, and conventional histological examinations including AchE staining. Congenital HG, MMIHS, and CIIP showed poor survival rate. Further collection of precise data of each case is required to make guidelines for criteria and treatment strategies for ADHD.
Acknowledgments
This study was supported by a grant from The Ministry of Health, Labor Sciences Research Grants for Research on intractable disease (H23-042, H24-037, H26-045). The authors thank all members of The Japanese Society of Pediatric Surgeons, The Japanese Society of Pediatric Nutrition, Gastroenterology, and Hepatology, and The Japanese Study Group of Pediatric Constipation. The authors thank Dr Bryan Quinn for reading the manuscript and also thank Ms Masutomi and Ms Yamazaki for their help in processing the data.
References
- 1 A. Holschneider, P. Puri; Hirschsprungs disease and allied disorders; (3rd ed.)Springer, New York (2008)
- 2 M.M. Ravitch; Pseudo Hirschsprungs disease; Ann Surg, 147 (1958), pp. 781–795
- 3 J.F.R. Bentley, H.H. Nixon, T.H. Ehrenpreis, B. Spencer; Seminar on pseudo-Hirschsprungs disease and related disorders; Arch Dis Child, 41 (1966), pp. 143–154
- 4 P. Puri, J.H. Gosemann; Variants of Hirschsprungs disease; Semin Pediatr Surg, 21 (2012), pp. 310–318
- 5 E. Okamoto, A. Toyosaka; Pseudo-Hirschsprungs disease. Research on the pathophysiology, diagnosis and treatment; Mie, Nagai-Shoten (1996) [In Japanese]
- 6 J.E. Maldonado, J.A. Gregg, P.A. Green, A.L. Brown; Chronic idiopathic intestinal pseudo-obstruction; Am J Med, 49 (1970), pp. 203–212
- 7 T. Taguchi, K. Masumoto, S. Ieiri, T. Nakatsuji, J. Akiyoshi; New classification of hypoganglionosis: congenital and acquired hypoganglionosis; J Pediatr Surg, 41 (2006), pp. 2046–2051
- 8 G. Martucciello, A. Pini Prato, P. Puri, et al.; Controversies concerning diagnostic guidelines for anomalies of the enteric nervous system: a report from the fourth International Symposium on Hirschsprungs disease and related neurocristopathies; J Pediatr Surg, 40 (2005), pp. 1527–1531
- 9 C.D. Rudolph, P.E. Hyman, S.M. Altschulter, et al.; Consensus report: diagnosis and treatment of chronic intestinal pseudo-obstruction in children; J Pediatr Gastroenterol Nutr, 24 (1997), pp. 102–112
- 10 H. Ohkubo, H. Iida, H. Takahashi, et al.; An epidemiologic survey of chronic intestinal pseudo-obstruction and evaluation of the newly proposed diagnostic criteria; Digestion, 86 (2012), pp. 12–19
- 11 V. Stanghellini, R.F. Cogliandro, R. De Giorgio, et al.; Natural history of chronic intestinal pseudo-obstruction in adults: a single center study; Clin Gastroenterol Hepatol, 3 (2005), pp. 449–458
- 12 J.H. Gosemann, P. Puri; Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome; Pediatr Surg Int, 27 (2011), pp. 1041–1046
- 13 W.E. Berdon, D.H. Barker, W.A. Blanc, et al.; Megacystis-microcolon-intestinal hypoperistalsis syndrome: A few cases of intestinal obstruction in the newborn. Report of radiologic findings in five newborn girls; AJR Am J Roentgenol, 126 (1976), pp. 957–964
- 14 O. Swenson, F. Rathauser; Segmental dilatation of the colon: a new entity; Am J Surg, 97 (1959), pp. 734–738
- 15 R. Doodnath, P. Puri; Long-term outcome of internal sphincter myectomy in patients with internal anal sphincter achalasia; Pediatr Surg Int, 25 (2009), pp. 869–871
- 16 S.H. Park, H. Min, J.G. Chi, K.W. Park, H.R. Yang, J.K. Seo; Immunohistochemical studies of pediatric intestinal pseudo-obstruction –Bcl2, a valuable biomarker to detect immature enteric ganglion cells; Am J Surg Pathol, 29 (2005), pp. 1017–1024
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?