Summary
Background
Predicting the major complications after esophagectomy is important and may help in preselecting patients who are most likely to benefit from surgery, especially in locally advanced esophageal cancer patients who have poor prognosis.
Objective
To identify the factors associated with the development of pneumonia and anastomotic leakage complications, and the survival characteristics in locally advanced esophageal cancer patients.
Methods
A consecutive series of 232 locally advanced esophageal cancer patients (183 men and 49 women, median age 63 years) who underwent esophagectomy at Prince of Songkla University Hospital between 1998 and 2007 was analyzed.
Results
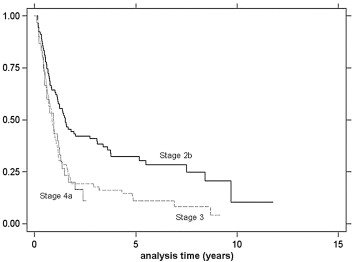
There were nine (3.8%) 30-day mortalities. Pneumonia occurred in 53 patients (22.8%) and anastomotic leakage in 37 patients (15.9%). Multivariate analyses showed that low body mass index was related to leakage (p = 0.015), while soft-diet dysphagia (p = 0.009), forced expiratory volume in 1 second <75% (p = 0.0005), type of surgery (McKeown technique) (p = 0.019), and long operative time (p = 0.006) were related to pneumonia. The median survival rate was 13.0 months. Stage 2b patients had longer survival than stages 3 and 4a patients (p = 0.0001).
Conclusion
Patient body mass index, dysphagia, spirometry, type of surgical technique, and operative time can help predict the likelihood of pulmonary or leak complications after esophagectomy. TNM (Tumor, Node, Metastasis) staging can help predict the overall survival after resection in locally advanced cases.
Keywords
complication;esophagectomy;leak;pneumonia;survival
1. Introduction
Esophageal carcinoma is the eighth most common cancer and the sixth most frequent cause of cancer deaths worldwide.1 Surgical techniques and multimodality treatments have improved over the years; however, improved rates of postoperative complications and survival after esophagectomy have not followed the treatment improvements, especially in locally advanced stages. The more common major postoperative complications of esophageal carcinoma include pneumonia and anastomotic leakage, both of which are correlated with mortality. TNM (Tumor, Node, Metastasis) staging has long served as the best tool to predict survival, but this system is based solely on the anatomic extent of invasion and metastasis and does not incorporate noncarcinoma variables such as patient characteristics, concomitant illnesses, or treatment.2
The aim of this study was to assess the prediction of postoperative esophagectomy pneumonia, anastomotic leakage complications, and survival characteristics in locally advanced esophageal cancer patients.
2. Patients and methods
The study was approved by the ethics committee of the Faculty of Medicine of Prince of Songkla University. Prospective data from 232 consecutive patients with Tany to operable T4, N1, and M0/M1a who underwent surgical resection for primary esophageal carcinoma at Prince of Songkla University Hospital between January 1998 and December 2007 were collected, including patient characteristics, blood chemistry and lung function test results, perioperative information, postoperative complications, and pathological findings.
The preoperative evaluations included a precise history and physical examination. Staging was based on chest X-ray, esophagogastroduodenoscopy with biopsy, bronchoscopy, endoscopic ultrasound, and computed tomography scans of chest and abdomen, and followed the sixth edition of the American Joint Committee on Cancer TNM staging system.3
In general, an Ivor–Lewis technique was performed for lower esophageal cancer and a McKeown technique for upper to lower parts. Two-field (mediastinal and abdominal stations) lymph node dissection was a routine procedure, and three-field (cervical, mediastinal, and abdominal stations) lymph node dissection was performed when cervical lymphadenopathy was found. A transhiatal or blunt technique was usually performed in cases of patients with a poor pulmonary function test.
Postoperative anastomotic leakage was defined as a leak requiring surgical treatment or contrast study documentation. Postoperative pneumonia was defined as a febrile illness plus the presence of pulmonary infiltration and leukocytosis. Neoadjuvant chemoradiation (5-FU plus cisplatin) was given to patients with a clinical resectable T4-tumor and adjuvant radiation or chemoradiation (5-FU plus cisplatin) was given to patients following microscopic residual (R1) or gross residual (R2) surgery. Patients were followed at 3-month intervals in the 1st year and at 6-month intervals in the 2nd and 3rd years, and then at 12-month intervals. Overall survival was calculated from the day of surgery.
2.1. Statistical analysis
We used the chi-square test for categorical comparison data. A p value of <0.05 was considered to indicate statistical significance. All tests were two-tailed with a 95% confidence interval (CI). Univariate and multivariate analysis were used. Stata version 10 statistical software (Stata Corp, College Station, TX, USA) was used for all statistical analyses.
3. Results
A total of 232 patients were identified (183 men, 49 women), with a median age of 63 years (range, 30–80). A total of 183 patients had a transthoracic resection (101 McKeown approaches and 82 Ivor–Lewis approaches), and 49 patients had a transhiatal resection. Moreover, 217 patients had squamous cell carcinoma and 15 patients had adenocarcinoma. Of the 232 primary tumors studied, 94 were classified as well, 97 as moderate, and 41 as poor histologic differentiation.
There were nine (3.8%) 30-day mortalities. The most frequent major morbidity was pneumonia, which occurred in 53 patients (22.8%), followed by anastomotic leakage in 37 patients (15.9%), and surgical site infection in 29 patients (12.5%).
The univariate analyses of pneumonia and leakage are shown in Table 1. Low body mass index (BMI) was the only significant risk factor for leakage, while soft-diet dysphagia, forced expiratory volume in 1 second (FEV1) <75%, forced vital capacity <75%, type of operation—McKeown technique, and long operative time were all found to be significant risk factors for pneumonia. The multivariate analyses of pneumonia and leakage are shown in Tables 2 and 3, and it was found that low BMI was related to leakage (p = 0.015), while soft-diet dysphagia (p = 0.009), FEV1 <75% (p = 0.0005), type of operation—McKeown technique (p = 0.019), and long operative time (p = 0.006) were related to pneumonia.
| Variable | Number | Leak | p | Pneumonia | p |
|---|---|---|---|---|---|
| Total | 232 (100%) | 37 (15.9%) | 53 (22.8%) | ||
| Age (y) | |||||
| <60 | 84 | 13 | 0.76 | 18 | 0.49 |
| 60–70 | 75 | 13 | 18 | ||
| >70 | 73 | 11 | 17 | ||
| Sex | |||||
| Male | 183 | 30 | 0.72 | 45 | 0.22 |
| Female | 49 | 7 | 8 | ||
| Soft-diet dysphagia | |||||
| Yes | 210 | 35 | 0.65 | 52 | 0.004 |
| No | 22 | 2 | 1 | ||
| Weight loss ≥ 10% | |||||
| Yes | 130 | 21 | 0.92 | 30 | 0.92 |
| No | 102 | 16 | 23 | ||
| BMI (kg/m2) | |||||
| <17 | 64 | 18 | 0.02 | 14 | 0.76 |
| 17–20 | 90 | 12 | 19 | ||
| >20 | 78 | 7 | 20 | ||
| Tumor location | |||||
| Upper | 24 | 2 | 0.68 | 3 | 0.44 |
| Middle | 115 | 18 | 31 | ||
| Lower | 93 | 17 | 19 | ||
| Stage | |||||
| 2b | 96 | 20 | 0.91 | 23 | 0.43 |
| 3 | 105 | 13 | 24 | ||
| 4a | 31 | 4 | 6 | ||
| FEV1 (%) | |||||
| <75 | 27 | 5 | 0.99 | 14 | 0.002 |
| ≥75 | 174 | 29 | 33 | ||
| *(sensor) | (31) | (3) | (6) | ||
| FVC (%) | |||||
| <75 | 32 | 7 | 0.54 | 15 | 0.005 |
| ≥75 | 169 | 27 | 33 | ||
| *(sensor) | (31) | (3) | (5) | ||
| Hct (%) | |||||
| <35 | 92 | 15 | 0.73 | 26 | 0.23 |
| 35–40 | 85 | 15 | 15 | ||
| >40 | 55 | 7 | 12 | ||
| Albumin (g/dL) | |||||
| <3.5 | 57 | 10 | 0.92 | 15 | 0.77 |
| 3.5–4 | 79 | 12 | 17 | ||
| >4 | 96 | 15 | 21 | ||
| FBS (mg/dL) | |||||
| <100 | 97 | 15 | 0.79 | 24 | 0.12 |
| 100–110 | 53 | 10 | 16 | ||
| >110 | 82 | 12 | 13 | ||
| Cr clearance | |||||
| <50 | 101 | 16 | 0.96 | 23 | 0.98 |
| ≥50 | 131 | 21 | 30 | ||
| Treatment | |||||
| Surgery alone | 132 | 26 | 0.19 | 34 | 0.14 |
| Sx + XRT ± chemo | 100 | 11 | 19 | ||
| Surgery | |||||
| Ivor–Lewis | 82 | 16 | 0.56 | 12 | 0.009 |
| McKeown | 101 | 16 | 33 | ||
| Blunt | 49 | 5 | 8 | ||
| Blood loss (mL) | |||||
| <500 | 151 | 22 | 0.10 | 34 | 0.87 |
| 500–1000 | 59 | 8 | 13 | ||
| >1000 | 22 | 7 | 6 | ||
| Operative time (h) | |||||
| <5 | 82 | 15 | 0.75 | 9 | 0.006 |
| 5–6.5 | 99 | 15 | 28 | ||
| >6.5 | 51 | 7 | 16 | ||
FBS = fasting blood sugar; Hct = hematocrit; XRT = radiotherapy.
| Variable | Odds ratio | 95% CI | p |
|---|---|---|---|
| BMI (kg/m2) | |||
| <17 | 1 | 0.015 | |
| 17–20 | 0.651 | 0.572–2.949 | |
| >20 | 0.365 | 0.135–0.987 | |
| Variable | Odds ratio | 95% CI | p |
|---|---|---|---|
| Soft-diet dysphagia | |||
| No | 1 | 0.009 | |
| Yes | 6.810 | 1.532–30.261 | |
| FEV1 (%) | |||
| <75 | 1 | 0.0005 | |
| ≥75 | 0.196 | 0.056–0.451 | |
| Surgery | |||
| Ivor–Lewis | 1 | 0.019 | |
| McKeown | 2.617 | 1.168–5.859 | |
| Blunt | 1.988 | 0.630–6.268 | |
| Operative time (h) | |||
| <5 | 1 | 0.006 | |
| 5–6.5 | 3.010 | 1.231–7.359 | |
| >6.5 | 4.499 | 1.633–12.390 | |
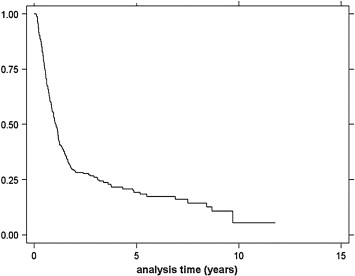
Follow-up times ranged from 3 to 38 months, and the median survival was 13.0 months (Fig. 1). The univariate analysis of survival at 1 year and 2 years is shown in Table 4. Univariate and multivariate analyses revealed that only stage of disease was related to survival, as stage 2b had significantly better survival compared to stages 3 and 4a (p = 0.0001) ( Table 5). Stage-specific survivals are shown in Fig. 2.
|
|
|
Figure 1. Kaplan–Meier analysis of overall survival. |
| Variable | 1 y (%) | 95% CI | 2 y (%) | 95% CI | p |
|---|---|---|---|---|---|
| Age (y) | |||||
| <60 | 48.91 | 38.38–58.62 | 23.91 | 15.79–32.99 | 0.06 |
| 60–70 | 51.39 | 39.34–62.18 | 25.00 | 15.72–35.39 | |
| >70 | 55.93 | 42.40–67.47 | 42.37 | 29.69–54.49 | |
| Sex | |||||
| Male | 49.71 | 42.11–56.85 | 26.86 | 20.53–33.57 | 0.20 |
| Female | 58.33 | 43.17–70.76 | 37.50 | 24.09–50.87 | |
| Soft-diet dysphagia | |||||
| Yes | 51.64 | 44.73–58.11 | 28.64 | 22.73–34.80 | 0.94 |
| No | 50.00 | 18.36–75.32 | 35.00 | 10.27–62.02 | |
| Weight loss ≥ 10% | |||||
| Yes | 50.82 | 41.64–59.27 | 26.23 | 18.80–34.25 | 0.37 |
| No | 52.48 | 42.32–61.66 | 32.67 | 23.77–41.86 | |
| BMI (kg/m2) | |||||
| <17 | 48.33 | 35.28–60.21 | 20.00 | 11.04–30.88 | 0.12 |
| 17–20 | 51.72 | 40.78–61.60 | 29.89 | 20.67–39.64 | |
| >20 | 53.95 | 42.14–64.35 | 35.53 | 24.99–46.19 | |
| Tumor location | |||||
| Upper | 50.00 | 15.20–77.49 | 12.50 | 00.66–42.27 | 0.53 |
| Middle | 48.21 | 38.71–57.08 | 28.57 | 20.54–37.10 | |
| Lower | 53.33 | 41.47–63.83 | 33.33 | 22.99–44.00 | |
| Tumor grading | |||||
| Well | 58.23 | 46.59–68.19 | 32.91 | 22.87–43.29 | 0.12 |
| Moderate | 31.25 | 16.38–47.34 | 21.88 | 09.65–37.24 | |
| Poor | 54.76 | 43.54–64.66 | 32.14 | 22.48–42.18 | |
| Cell type | |||||
| Squamous CA | 51.20 | 44.23–57.73 | 28.71 | 22.74–34.94 | 0.50 |
| Adeno CA | 63.64 | 29.69–84.52 | 36.36 | 11.18–62.68 | |
| Stage | |||||
| 2b | 64.44 | 53.63–73.36 | 43.33 | 32.98–53.24 | 0.0004 |
| 3 | 42.42 | 32.61–51.89 | 19.19 | 12.14–27.47 | |
| 4a | 43.33 | 25.56–59.89 | 20.00 | 08.12–35.64 | |
| FEV1 (%) | |||||
| <75 | 45.83 | 25.61–63.97 | 29.17 | 12.95–47.58 | 0.45 |
| ≥75 | 49.64 | 41.09–57.61 | 28.78 | 21.51–36.45 | |
| FVC (%) | |||||
| <75 | 50.00 | 27.13–69.19 | 30.00 | 12.25–50.14 | 0.86 |
| ≥75 | 48.95 | 40.54–56.83 | 28.67 | 21.51–36.21 | |
| Treatment | |||||
| Surgery alone | 50.81 | 41.71–59.20 | 29.84 | 22.05–38.00 | 0.46 |
| Sx + XRT ± chemo | 51.61 | 41.04–61.19 | 26.88 | 18.35–36.14 | |
| Surgery | |||||
| Ivor–Lewis | 54.88 | 43.51–64.88 | 26.83 | 17.79–36.69 | 0.22 |
| McKeown | 53.06 | 42.73–62.35 | 35.71 | 26.38–45.14 | |
| Blunt | 42.31 | 23.47–60.02 | 19.23 | 07.01–35.97 | |
| Blood loss (mL) | |||||
| <500 | 52.38 | 44.01–60.08 | 27.89 | 20.91–35.29 | 0.97 |
| 500–1000 | 53.57 | 39.75–65.55 | 30.36 | 18.96–42.54 | |
| >1000 | 40.00 | 19.28–60.05 | 35.00 | 15.66–55.19 | |
| Operative time (h) | |||||
| <5 | 52.50 | 41.05–62.73 | 28.75 | 19.32–38.86 | 0.88 |
| 5–6.5 | 50.53 | 40.10–60.05 | 28.42 | 19.76–37.67 | |
| >6.5 | 52.08 | 37.20–65.03 | 31.25 | 18.85–44.45 | |
| Variable | Odds ratio | 95% CI | p |
|---|---|---|---|
| Stage | |||
| 2b | 0.435 | 0.271–0.699 | 0.0001 |
| 3 | 0.831 | 0.531–1.300 | |
| 4a | 1 | ||
|
|
|
Figure 2. Kaplan–Meier analysis of overall survival by stage. |
4. Discussion
Esophagectomy has become the main treatment of esophageal cancer even in the locally advanced stage. However, there are often serious postoperative complications such as pneumonia and anastomotic leakage which increase the risk of mortality.4 ; 5
This study found post-esophagectomy pneumonia in 22.8% of our patients, compared to rates in other studies varying from 7.3% to 50%.6; 7; 8; 9; 10 ; 11 Laws et al7 reported the incidence of major pulmonary complication to be 15.9%, but these complications were responsible for 55% of patient deaths in their study. One study found that postoperative pulmonary complications occurred more frequently after transthoracic esophagectomy than transhiatal esophagectomy for esophageal cancer, and hypothesized that this greater rate was because the thoracotomy procedure itself tended to induce pulmonary complications.12 Unfortunately, the perhaps less harmful transhiatal approach is not suitable for curative surgery with lymph node dissection, so we performed this technique only in cases with a poor pulmonary function test. We also found that long operative time was related to post-esophagectomy pneumonia, and that the McKeown surgical approach took more time than the Ivor–Lewis and transhiatal approaches.
Preoperative spirometry should be done in every case with a history of smoking or respiratory symptoms.13 Avendano et al14 reported that a preoperative FEV1 <65% predicted was associated with an increased requirement for respiration support during the postoperative period. We found a forced vital capacity and an FEV1 <75% predicted were related to postoperative pneumonia. We also found that one of the predictors for pneumonia was soft-diet dysphagia, which might have been associated with preoperative repeated aspirations leading to poor lung parenchyma. Lundy et al15 reported that aspiration was detected by a modified barium swallow in 51.2% of their dysphagic patients.
Anastomotic leakage is a major complication in esophagectomy. Earlier studies have found an incidence of leakage varying from 8% to 15% in general esophagectomies, compared to 15.9% in this study, which focused on the locally advanced stage.12 There are various causes of such leakages, such as conduit ischemia, technical errors, and malnutrition.16 ; 17 Nutrition in patients with locally advanced stage of disease may be compromised, especially in cases of dysphagia, and correlated with an increased rate of anastomotic leakage. Patil et al18 reported a correlation between anastomotic leakage and an albumin level less than 3 g/dL. We did not observe this association in our patients; it may be that the preoperative serum albumin was not low because of dehydration. Our study also found preoperative low BMI to be related to postoperative anastomotic leakage. This may be explained by the relation between low BMI and some degree of malnutrition from dysphagia and cachexia.
Mortality from esophagectomies has been steadily falling over recent decades, and in most current studies it is <5%.19; 20 ; 21 This improving outcome rate may result from improvements in multidisciplinary pre- and postoperative care, more refined patient selection, or the high-volume center effect. Some studies have reported that doctor/hospital volume was associated with better postoperative mortality rates.22; 23; 24 ; 25 Metzger et al26 found that institutions with more than 20 procedures per year were more likely to have lower mortality rates. Recently, in the Netherlands esophageal resections for cancer were banned from hospitals with less than 10 procedures annually.27
Chemoradiation followed by surgery is increasingly being used in patients with locally advanced esophageal cancer. A meta-analysis from Graham et al28 showed that survival of locally advanced esophageal cancer patients who underwent esophagectomy alone at 18 months and the relative risks (95% CI) of death for treatments compared with surgery were 0.87 (0.75–1.02) for neoadjuvant chemoradiation, 0.94 (0.82–1.08) for neoadjuvant chemotherapy, and 1.33 (0.93–1.93) for adjuvant chemoradiation. However, there was heterogeneity between the randomized controlled trials on neoadjuvant chemoradiation for esophageal cancer. Wijnhoven et al29 found that two of the six meta-analyses examined did not show a significant survival benefit in patients with resectable esophageal cancer. Moreover, besides survival outcome, toxicity should be considered in deciding whether neoadjuvant chemoradiation has more benefits than surgery alone.30 The National Comprehensive Cancer Network also recommends neoadjuvant chemoradiation for locally advanced esophageal cancer.31 We did not see the effect of adjuvant or neoadjuvant chemoradiation on survival rates in our study; however, this could be due to selective case bias in this nonrandom study.
In conclusion, we have demonstrated that preoperative BMI, dysphagia, pulmonary function test, type of surgical technique, and operative time may help to predict the chance of postoperative anastomotic leakage and pneumonia in locally advanced esophageal cancer patients, while stage of disease may help to predict survival.
References
- 1 D.M. Parkin, F. Bray, J. Ferlay, et al.; Global cancer statistics, 2002; CA Cancer J Clin, 55 (2005), pp. 74–108
- 2 P. Gaur, W.L. Hofstetter, B.N. Bekele, et al.; Comparison between established and the Worldwide Esophageal Cancer Collaboration staging systems; Ann Thorac Surg, 89 (2010), pp. 1797–1803
- 3 F.L. Greene; American Joint Committee on Cancer. American Cancer Society. AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual; (6th ed.)Springer, Chicago (2002)
- 4 M.K. Ferguson, T.R. Martin, L.B. Reeder, et al.; Mortality after esophagectomy: risk factor analysis; World J Surg, 21 (1997), pp. 599–603
- 5 L.W. Martin, S.G. Swisher, W. Hofstetter, et al.; Intrathoracic leaks following esophagectomy are no longer associated with increased mortality; Ann Surg, 242 (2005), pp. 392–399
- 6 W.J. Jiao, T.Y. Wang, M. Gong, et al.; Pulmonary complications in patients with chronic obstructive pulmonary disease following transthoracic esophagectomy; World J Gastroenterol, 28 (2006), pp. 2505–2509
- 7 S. Law, K.H. Wong, K.F. Kwok, et al.; Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer; Ann Surg, 240 (2004), pp. 791–800
- 8 W. Fang, H. Kato, Y. Tachimori, et al.; Analysis of pulmonary complications after three-field lymph node dissection for esophageal cancer; Ann Thorac Surg, 76 (2003), pp. 903–908
- 9 S.H. Bailey, D.A. Bull, D.H. Harpole, et al.; Outcomes after esophagectomy: a ten-year prospective cohort; Ann Thorac Surg, 75 (2003), pp. 217–222
- 10 S.M. Griffin, I.H. Shaw, S.M. Dresner; Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: risk factors and management; J Am Coll Surg, 194 (2002), pp. 285–297
- 11 L. Gluch, R.C. Smith, C.P. Bambach, et al.; Comparison of outcomes following transhiatal or Ivor Lewis esophagectomy for esophageal carcinoma; World J Surg, 23 (1999), pp. 271–275
- 12 J.B. Hulscher, J.G. Tijssen, H. Obertop, et al.; Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis; Ann Thorac Surg, 72 (2001), pp. 306–313
- 13 E. Congedo, P. Aceto, R. Petrucci, et al.; Preoperative anesthetic evaluation and preparation in patients requiring esophageal surgery for cancer; Rays, 30 (2005), pp. 341–345
- 14 C.E. Avendano, P.A. Flume, G.A. Silvestri, et al.; Pulmonary complications after esophagectomy; Ann Thorac Surg, 73 (2002), pp. 922–926
- 15 D.S. Lundy, C. Smith, L. Colangelo, et al.; Aspiration: cause and implications; Otolaryngol Head Neck Surg, 120 (1999), pp. 474–478
- 16 B.P. Whooley, S. Law, A. Alexandrou, et al.; Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer; Am J Surg, 181 (2001), pp. 198–203
- 17 T. Lerut, W. Coosemans, G. Decker, et al.; Anastomotic complications after esophagectomy; Dig Surg, 19 (2002), pp. 92–98
- 18 P.K. Patil, S.G. Patel, R.C. Mistry, et al.; Cancer of the esophagus: esophagogastric anastomotic leak: a retrospective study of predisposing factors; J Surg Oncol, 49 (1992), pp. 163–167
- 19 G.G. Jamieson, G. Mathew, R. Ludemann, et al.; Postoperative mortality following oesophagectomy and problems in reporting its rate; Br J Surg, 91 (2004), pp. 943–947
- 20 R. Earlam, J.R. Cunha-Melo; Oesophageal squamous cell carcinoma: I. A critical review of surgery; Br J Surg, 67 (1980), pp. 381–390
- 21 J.M. Müller, U. Zieren, U. Wolters, et al.; Results of esophagectomy and gastric bypass for cancer of the esophagus; Hepatogastroenterology, 36 (1989), pp. 522–528
- 22 S.G. Swisher, L. Deford, K.W. Merriman, et al.; Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer; J Thorac Cardiovasc Surg, 119 (2000), pp. 1126–1132
- 23 M.W. Wouters, B.P. Wijnhoven, H.E. Karim-Kos, et al.; High-volume versus low-volume for esophageal resections for cancer: the essential role of case-mix adjustments based on clinical data; Ann Surg Oncol, 15 (2008), pp. 80–87
- 24 J.D. Birkmeyer, A.E. Siewers, E.V. Finlayson, et al.; Hospital volume and surgical mortality in the United States; N Engl J Med, 346 (2002), pp. 1128–1137
- 25 E.W. Gillison, J. Powell, C.C. McConkey, et al.; Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia; Br J Surg, 89 (2002), pp. 344–348
- 26 R. Metzger, E. Bollschweiler, D. Vallböhmer, et al.; High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality?; Dis Esophagus, 17 (2004), pp. 310–314
- 27 M.W. Wouters, P. Krijnen, S. Le Cessie, et al.; Volume- or outcome-based referral to improve quality of care for esophageal cancer surgery in The Netherlands; J Surg Oncol, 99 (2009), pp. 481–487
- 28 A.J. Graham, F.M. Shrive, W.A. Ghali, et al.; Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis; Ann Thorac Surg, 83 (2007), pp. 1257–1264
- 29 B.P. Wijnhoven, J.J. van Lanschot, H.W. Tilanus, et al.; Neoadjuvant chemoradiotherapy for esophageal cancer: a review of meta-analyses; World J Surg, 33 (2009), pp. 2606–2614
- 30 E.F. Courrech Staal, B.M. Aleman, H. Boot, et al.; Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer; Br J Surg, 97 (2010), pp. 1482–1496
- 31 NCCN Clinical Practice Guidelines in Oncology™. Esophageal Cancer; Version 1 (2011) Available at http://www.nccn.org [accessed 11.02.11]
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?