Abstract
Objective
The aim of this study was to evaluate the efficacy of gene enhanced tissue engineering followed mosaicplasty in a goat model.
Methods
An acute cylindrical defect 9 mm in diameter was created in the weight bearing area of the medial femoral condyle in a goat model. Thirty-six medial femoral condyles were divided into 6 groups using different proportion of gene enhanced tissue engineering and mosaicplasty to restore the defects. The specimen received gross and histology observation, which was evaluated by the histological grading scale of O'Driscoll, Keeley and Salter. Transmission electron microscope observation was also performed. Two factors analysis of variance and Student-Newman-Kewls test were used to compare the specimen.
Results
The gross and histology observation revealed that each defects of six groups had different restoration. The scores of the reparative tissue of three groups with gene enhancement were significantly higher than those in other three groups without gene enhancement (p > 0.05).
Conclusion
Gene enhanced tissue engineering followed mosaicplasty could restore a 9 mm diameter osteochondral defects in a goat model effectively. With the reduction of covering area of the graft, the advantages of the combined gene enhanced tissue engineering method can be better reflected.
Keywords
Osteochondral repair ; Tissue engineering ; Gene transduction ; Bone mesenchymal stem cells (BMSCs)
Introduction
Articular cartilage has a poor intrinsic capacity for healing. The lesions of cartilage may lead to early degenerative changes and subsequent osteoarthritis if left untreated.1 ; 2 For the treatment of chondral or osteochondral defects, Mosaicplasty is an effective method, which involves obtaining small-sized cylindrical osteochondral grafts from the minimal weight-bearing areas and transplanting them to prepared defect sites on the weight bearing surfaces.3 But as for autologous transplantation with limited donor area, mosaicplasty is not appropriate for large chondral or osteochondral defects. Regarding donor-site complications, the larger the defect, the higher the morbidity.
In the light of these advantages and disadvantages of mosaicplasty, the authors took into consideration whether something could be done to improve the effect of a larger osteochondral restoration based on the mosaicplasty. As it has been demonstrated that the gene enhanced tissue engineering enhanced the restoration of cartilage defects and Mosaicplasty associated with gene enhanced tissue engineering could solve the problem of the poor concrescence of the remnant defect and the integration of single mosaicplasty in authors' previous study,4 the authors designed the restoration of a 9 mm diameter osteochondral defect with gene enhanced tissue engineering followed mosaicplasty in a goat model. The reduction of the mosaicplasty coverage area and the increase of the area filled by tissue engineering materials were done. The ratio was studied in order to reach an optimal combination. The objective of this study was to evaluate the efficacy of this technique expecting to find a suitable method for the clinical larger osteochondral restoration.
Methods
Defects restored by different proportion of gene enhanced tissue engineering and mosaicplasty
Eighteen masculine goats with a mean weight of 22.5 kg were used in this study. The goats were randomized into one of three groups (Group I, Group II and Group III) with six in each group (Table 1 ). The Animal Research Committee of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine approved this investigation.
| Group | Defect position (knee) | Defect size (diameter/depth) | Graft quantity | Restorative procedure | Mosaic repair ratio |
|---|---|---|---|---|---|
| Group Il | Left | 9 mm/3 mm | 4 | Mosaicplasty | 44.44% |
| Group Ir | Right | 9 mm/3 mm | 4 | Mosaicplasty+ gene enhanced tissue engineering | 44.44% |
| Group IIl | Left | 9 mm/3 mm | 3 | Mosaicplasty | 33.33% |
| Group IIr | Right | 9 mm/3 mm | 3 | Mosaicplasty+ gene enhanced tissue engineering | 33.33% |
| Group IIIl | Left | 9 mm/3 mm | 2 | Mosaicplasty | 22.22% |
| Group IIIr | Right | 9 mm/3 mm | 2 | Mosaicplasty+ gene enhanced tissue engineering | 22.22% |
General anesthesia was administered. An acute cylindrical defect 9 mm in diameter and 3 mm in depth was created in the weight bearing area of the medial femoral condyle using a punch on the 36 legs of eighteen goats. The lesion thus produced was a deep defect extending through all chondral layers into the subchondral bone plate.
In Group I, mosaicplasty was done on the both legs. On the left knee which marked Il four recipient bone holes 5 mm in depth were drilled on the defect by the drill of 3 mm in diameter. Four cylindrical osteochondral grafts 5.5 mm in length and 3 mm in diameter were harvested at the medial femoral condyle periphery of the patellofemoral joint using tubular chisel of 3 mm in internal diameter. The delivery tamp was used to pull the cylindrical osteochondral grafts into the bone holes and compress the bone softly for 0.5 mm to make the cylindrical osteochondral grafts to be flush with the original articular surface. When all of the four grafts were seated, the knee was put through a range of motion and varus, valgus stressed to get to know that the grafts were stable. The operative wounds were irrigated with physiological saline before closing the incision. All goats were allowed to move freely in their pens after surgery.
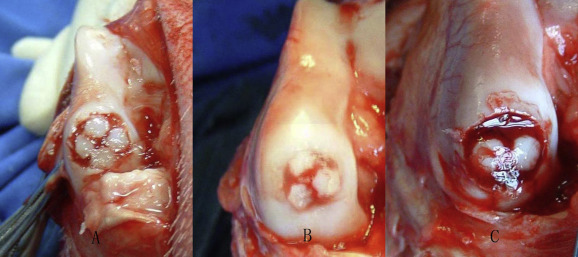
On the right knee which marked Ir of Group I, the acute defects creation and the restoration by mosaicplasty were the same as Il. Then, the dead space between the cylindrical grafts and the host cartilage were injected with the suspension of hTGF-β1 gene transduced autogenous BMSCs in sodium alginate with the density of 5 × 107 cells mL−1 . The description on the methods of hTGF-β1 gene transduced autogenous BMSCs could be found on the authors' previous study.4 Then, excessive 102 mmol/L CaCl2 was dropped in it. The CaCl2 and the sodium alginate crossed link to form calcium alginate gels. When gelation was complete, the excess of the calcium chloride gelling solution was removed (Fig. 1 ). The incision was closed. All goats were allowed to move freely in their pens after surgery.
|
|
|
Fig. 1. Using the filling of gels of gene transduced bone mesenchymal stem cells (BMSCs) in alginate followed mosaicplasty to restore the defect. A: Four cylindrical osteochondral grafts. B: Three cylindrical osteochondral grafts. C: Two cylindrical osteochondral grafts. |
In Group II, the acute defects creation was the same as Group I. But in the mosaicplasty procedure, three cylindrical osteochondral grafts with the same diameter and length were harvested and then seated in the defect area. For the left knee which marked IIl, only mosaicplasty was done. For the right knee which marked IIr, the filling of the gels of gene transduced BMSCs in alginate followed mosaicplasty was done (Fig. 1 ).
In Group III, the acute defects creation was the same as Group I. But in the mosaicplasty procedure, two cylindrical osteochondral grafts with the same diameter and length were harvested and then seated in the defect area. For the left knee which marked IIIl, only mosaicplasty was done. For the right knee which marked IIIr, the filling of the gels of gene transduced BMSCs in alginate followed mosaicplasty was done (Fig. 1 ).
Histopathological evaluation
At 24 weeks after the surgery, all goats were killed. The knees were examined grossly. The femoral condyles were excised and trimmed to include the filled defect and a thin rim of surrounding native bone and cartilage. Toluidine blue staining was used and transmission electron microscope was performed.
Statistical analysis
To evaluate the microscopic morphology, a histological grading scale described by O'Driscoll, Keeley and Salter was used5 (Table 2 ). The scored data were shown with average ± standard deviation. Two factors analysis of variance and Student–Newman–Kewls test were used to compare the specimen. The data disposal was processed by the SAS (6.12) statistics software.
| Category | Score |
|---|---|
| Nature of the predominant tissue | |
| Cell morphology | |
| Hyaline cartilage | 4 |
| Incompletely differentiated mesenchyme | 2 |
| Fibrous tissue or bone | 0 |
| Toluidine blue staining of the matrix | |
| Normal or nearly normal | 3 |
| Moderate | 2 |
| Slight | 1 |
| None | 0 |
| Structural characteristics | |
| Surface regularity | |
| Smooth and intact | 3 |
| Superficial horizontal lamination | 2 |
| Fissure-25 to 100% of the thickness | 1 |
| Severe disruption | 0 |
| Structural integrity | |
| Normal | 2 |
| Slight disruption, including cysts | 1 |
| Severe disintegration | 0 |
| Thickness | |
| 100% of normal adjacent cartilage | 2 |
| 50–100% of normal cartilage | 1 |
| 0–50% of normal cartilage | 0 |
| Bonding to the adjacent cartilage | |
| Bonded at both ends of graft | 2 |
| Bonded at one end, or partially at both ends | 1 |
| Not bonded | 0 |
| Freedom from cellular changes of degeneration | |
| Hypocellularity | |
| Normal | 3 |
| Slight | 2 |
| Moderate | 1 |
| Severe | 0 |
| Chondrocyte clustering | |
| No clusters | 2 |
| <25% of the cells | 1 |
| 25–100% of the cells | 0 |
| Freedom form degeneration changes in adjacent cartilage | |
| Normal cellularity, no clusters, normal staining | 3 |
| Normal cellularity, mild clusters, moderate staining | 2 |
| Mild or moderate hypocellularity, slight staining | 1 |
| Severe hypocellularity, poor or no staining | 0 |
Results
All of the goats could move freely after the surgery. There was no evidence of postoperative wound infection and all the wounds had healed uneventfully.
Gross findings and histological findings (Fig. 2 )
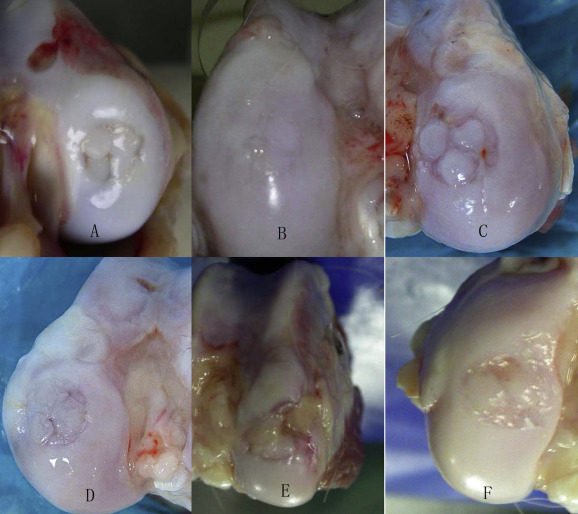
Each group had different gross findings and histological findings. The situation of the autologous cylindrical osteochondral grafts, the border and gaps were the main features in gross findings. Cartilage degeneration, newborn tissue, surface regularity and structural integrity were mainly described in histological findings. See Table 3 (see Fig. 3 ).
|
|
|
Fig. 2. Macroscopic findings. A: Group Il. The autologous cylindrical osteochondral grafts were clear and intact. The border was distinct. Gaps around the implanted plugs were detected. B: Group Ir. The configuration of the autologous osteochondral cylinder was integrated. The dead space between the cylindrical grafts was replaced by the white analogous cartilage tissue. C: Group IIl. All of the grafts and the surrounding cartilage had obvious degeneration with dark color and rough surface. The hollow among the cartilages was obvious. Part tissue proliferated. D: Group IIr. The configuration of the autologous cylindrical osteochondral grafts was with clarity and integrity. The border was indistinct. Gaps were replaced by the white analogous cartilage tissue. The crackle existed among the neonatal tissue and the surrounding tissue. E: Group IIIl. The autologous osteochondral grafts existed with obvious degeneration. The surface was coarse without original color. The hollow among the cartilages was obvious with no filling. F: Group IIIr. The configuration of the autologous cylindrical osteochondral grafts was with integrity. Gaps were partly replaced by the proliferative tissue. The new tissue and peripheral tissue have obvious cracks. |
| Group | Grafts (Gross) | Border (Gross) | Gaps (Gross) | Cartilage degeneration (micro) | Newborn tissue (micro) | Surface regularity (micro) | Structural integrity (micro) |
|---|---|---|---|---|---|---|---|
| Group Il | Clear and intact | Distinct | Detected | A little | Incompletely differentiated cells | Fissure | Disruption |
| Group Ir | Integrated | Indistinct | Not obvious | No | Hyaline cartilage | Smooth, intact | Normal |
| Group IIl | Dark color, rough surface | Distinct | Obvious | Obvious | Fibrous tissue | Fissure | Disruption |
| Group IIr | Clear and intact | Indistinct | Not obvious | A little | Chondrocyte proliferation | Fissure | Slight disruption |
| Group IIIl | Obvious degeneration | Distinct | Obvious | Obvious | Fibrous tissue | Disruption | Disintegration |
| Group IIIr | Dark color, rough surface | Distinct | Obvious | Obvious | Fibrous tissue | Disruption | Disintegration |
|
|
|
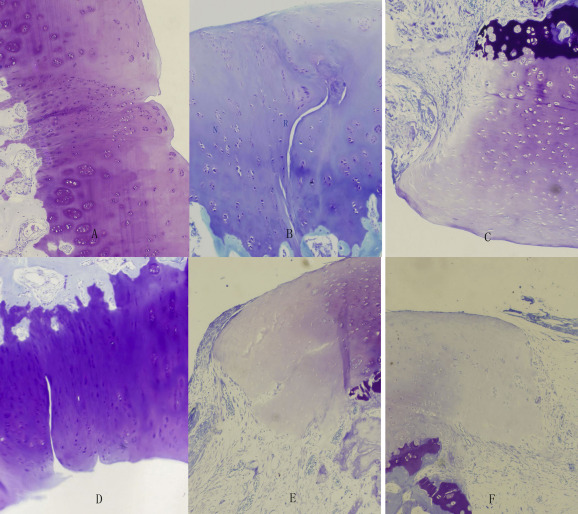
Fig. 3. Histological findings of Toluidine blue 100×. A: Group Il. The cartilage and the surrounding cartilage showed the degeneration. The number of cartilage cells got a small reduction. The gap around the implanted plugs was depressed. B: Group Ir. The graft cartilage and the surrounding cartilage preserved the characteristics of the original cartilage. There were newborn cartilages in the void areas of cartilage. The mature trabecular bone structure had been formed which was difficult to distinguish with the surrounding bone tissues. There were small fissures and proliferation of clustered chondrocytes. C: Group IIl. Autologous cartilage and surrounding cartilage had obvious degeneration. Gaps were detected among the surface cartilages. Fibrous tissue proliferated. D: Group IIr. The graft cartilage and the surrounding cartilage retained the characteristics of the original cartilage. There were gaps among the restoration tissue and the graft or normal cartilage, surrounded by a cluster of chondrocyte proliferation. E: Group IIIl. Autologous cartilage and surrounding cartilage had obvious degeneration. The hollow was obvious in the gap among the surface of cartilage. Partial fibrous tissue proliferated. F: Group IIIr. The reduction of cartilage cells appeared in the autologous graft cartilage and surrounding cartilage. The gap between the cartilages was hollow. Fibrous tissue proliferated. R: Reparative tissue, N: Normal cartilage. |
Electron microscope findings
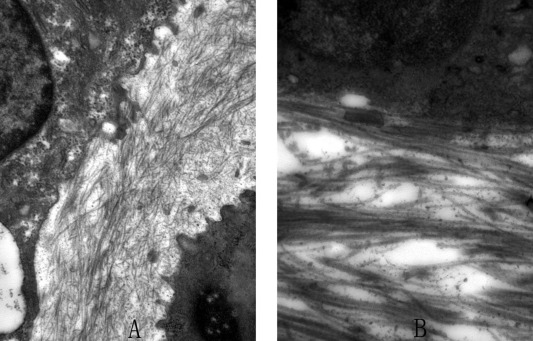
The reparative tissue among the cartilage of the Group Ir and Group IIr specimens were taken for transmission electron microscope examination (Fig. 4 ).
|
|
|
Fig. 4. Electron microscope findings 14,500×. A: Group Ir. Collagen fibers composed the matrix arounding the chondrocytes in the reparative tissue. The fibers were in disorderly rows. Periodic cross striation could be seen. B: Group IIr. The fibers in the reparative tissue were in tight orderly rows. Periodic cross striation was obvious. |
Ir
Collagen fibers composed the matrix arounding the chondrocytes in the reparative tissue. The fibers were in disorderly rows. Periodic cross striation could be seen.
IIr
The fibers in the reparative tissue were in tight orderly rows. Periodic cross striation was obvious.
Statistical analysis
The restoration tissues in the dead space and the osteochondral grafts were scored (Table 4 ). The data are expressed as the standard deviation. The scored data processed by the SAS (6.12) statistics software showed that the scores of the reparative tissue of Group Il, Ir and IIr were significantly higher than those in Group IIl, IIIl and IIIr. But no statistical difference was detected among the Group Il, Ir and IIr.
| Group | Average ± standard deviation | Group Il | Group Ir | Group IIl | Group IIr | Group IIIl | Group IIIr |
|---|---|---|---|---|---|---|---|
| P value | |||||||
| Group Il | 18.667 ± 1.506 | – | |||||
| Group Ir | 20.167 ± 1.169 | >0.05 | – | ||||
| Group IIl | 8.167 ± 0.753 | <0.05 | <0.05 | – | |||
| Group IIr | 19.833 ± 1.169 | >0.05 | >0.05 | <0.05 | – | ||
| Group IIIl | 5.667 ± 1.211 | <0.05 | <0.05 | <0.05 | <0.05 | – | |
| Group IIIr | 6.333 ± 1.633 | <0.05 | <0.05 | <0.05 | <0.05 | >0.05 | – |
Discussion
Treatment of a large area of full-thickness cartilage defects and osteochondral defects which is more than 4 cm2 remains central to clinical orthopedics. The advantage of autologous osteochondral transplantation (mosaicplasty) was obvious including the simplicity of one-stage surgical procedure, low morbidity and cost, and a better clinical outcome.6 ; 7 However, as donor sites are limited and the morbidity ascribed to the donor sites must also be considered, it is not suitable for mosaicplasty to treat the large, deep and crater like defects which need a large number of grafts.8 ; 9 ; 10 ; 11 Clinical reports of showed that the success rate of mosaicplasty was low in the patient with the defects area over 4 cm2 or who had previous operation.8 The healing of the osteochondral graft and the integration of the autologous osteochondral grafts and the surrounding normal cartilage are also a potential factor to affect the long-term results. The use of large grafts can cause incongruity at the recipient site, which permanently alters the biomechanics of the joint. Therefore, the authors design the mosaicplasty combined with tissue engineering method to restore the osteochondral defects. The combination is not the simple sum of two kinds of treatment but the full embodiment of the superiority of the both to make it possible to get a better restoration effect.
The authors carried out in vitro amplification of a small amount of BMSCs, in accordance with the concentration of 5 × 107 /ml cells to restore. Then the authors transfected hTGF-β1. HTGF-β1 is the first choice growth factor for the cartilage tissue engineering, which has multiple biological effects.12 ; 13 ; 14 After the implantation of hTGF-β1 transfected BMSCs, the persistent hTGF-β1 expression in the defect made the BMSCs differentiate into cartilage cells and form cartilage tissue with the assistance of intra-articular hypoxia conditions, joint force and various active factors in the synovial fluid in the surface lay of defects. In the bottom of the defect adjacent to medullary cavity, due to rich blood supply and high oxygen pressure, the BMSCs continued to differentiate into bone cell and form trabecular bone.15 Thus, the restoration of compound defect of the bone and cartilage was acquired and favorable integration was obtained.
BMSCs could be implanted into the defects with the carrier. Then, a large number of tissue engineered cartilage can be obtained, which can make up the shortage of the osteochondral transplantation. The calcium alginate gel was injectable material, which could used to restorate complex and irregular defect without residual.16 ; 17 So it was more likely to get a high degree of integration with surrounding tissue. Early load carrying capacity of the osteochondral transplantation could provide support and protection for the tissue engineered cartilage, improve the mechanical properties, and avoid the damage of the tissue engineered cartilage before it was formed. The common cartilage surface, which was formed by the osteochondral transplantation and tissue engineered cartilage, could make the defect area obtain a smooth and intact articular surface with the surrounding normal cartilage. Therefore, the authors believe that the method of the mosaicplasty associated with genes enhanced tissue engineering is suitable for the treatment of large area of bone defect.
The diameter of the femoral condyle of goats was about 12 mm, so the authors considered that the defect of the 9 mm was a quite large defect relative to the goat. In the study, four or three or two cylindrical osteochondral grafts with 3 mm diameter were transplanted. The percentage of the restoration area was 44.44%, 33.33%, 22.22%, respectively with theoretical calculation (The percentage of the restoration area % = n × π /π ). Each animals left knee was restored with single osteochondral transplantation, and the gap around the osteochondral cylinder in the right knee was with further tissue engineering material filling. Analysing the restored organization as a whole, it was found that the integration of the mosaicplasty associated with genes enhanced tissue engineering was better than the single mosaicplasty in the two groups with 44.44% mosaicplasty cover rates. The gap around the implanted plugs was restored with the immature cartilage tissue in 44.44% single group. But the overall score of the two 44.44% groups was not statistically different. The results showed at 24 weeks after surgery, if the mosaicplasty restoration area is enough large and the residual space is small, the tissues can be carried out satisfactory self-repair. The method of mosaicplasty associated with genes enhanced tissue engineering has no advantage. There was no statistical difference in the overall restoration effect among the 33.33% combined group and the two 44.44% groups. While the overall effect of the 33.33% single group was significantly worse than that of the 33.33% combined group and the two 44.44% groups. In the 33.33% single group, the gap around the osteochondral cylinder could not get a fibrous cartilage like tissue restoration. The formation of fibrous tissue, the existence of the depression, and abnormal stress subjected to the grafts and the surrounding cartilage resulted in the degeneration of the cartilage. Therefore, if the restoration area of autologous graft is more than 1/3 of the defect and the remaining defects are restored by gene enhanced tissue engineering method, a satisfactory restoration can also be obtained. Tissue engineering could solve the problem of the poor concrescence of the remnant defect followed the mosaicplasty. The overall restoration effects of the 22.22% single and combined groups were significantly worse than other groups. The degeneration of cartilage in the 22.22% simple group was even more obvious. The formation of tissue engineered cartilage failed in the 22.22% combined group. The space was partly filled with fibrous tissue, resulting in the degeneration of the grafts and the surrounding cartilage. The authors believe that the reason is that the calcium alginate has low strength and the biomechanical properties are weak. The limited number of grafts cannot provide protection to the calcium alginate, which leads to the inefficient formation of tissue engineered cartilage. In addition, the gap is large with a massive bleeding, resulting in the loss of calcium alginate gel.
Although the experiment object is the cartilage of goat, the structure and function of goats knee are similar to human. So it has some guidance for the treatment of acute human knee injury.
Conclusion
Gene enhanced tissue engineering followed mosaicplasty could restore a 9 mm diameter osteochondral defects in a goat model effectively. With the reduction of covering area of the graft, the advantages of the combined gene enhanced tissue engineering method can be better reflected.
References
- 1 T.Y. Emre, T. Ege, O. Kose, D. Tekdos Demırcıoglu, B. Seyhan, M. Uzun; Factors affecting the outcome of osteochondral autografting (mosaicplasty) in articular cartilage defects of the knee joint: retrospective analysis of 152 cases; Arch Orthop Trauma Surg, 133 (2013 Apr), pp. 531–536
- 2 Á. Berta, Z. Duska, F. Tóth, L. Hangody; Clinical experiences with cartilage repair techniques: outcomes, indications, contraindications and rehabilitation; Eklem Hast Cerrahisi, 26 (2015), pp. 84–96
- 3 L. Hangody, G. Kish, Z. Kárpáti, I. Udvarhelyi, I. Szigeti, M. Bély; Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice; Orthopedics, 21 (1998 Jul), pp. 751–756
- 4 J. Sun, X.K. Hou, X. Li, et al.; Mosaicplasty associated with gene enhanced tissue engineering for the treatment of acute osteochondral defects in a goat model; Arch Orthop Trauma Surg, 129 (2009 Jun), pp. 757–771
- 5 S.W. O'Driscoll, F.W. Keeley, R.B. Salter; Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year; J Bone Jt Surg Am, 70 (1988 Apr), pp. 595–606
- 6 L. Hangody, P. Füles; Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience; J Bone Jt Surg Am, 85-A (2003), pp. 25–32
- 7 H. Robert; Chondral repair of the knee joint using mosaicplasty; Orthop Traumatol Surg Res, 97 (2011 Jun), pp. 418–429
- 8 J.D. Harris, R.A. Siston, X. Pan, D.C. Flanigan; Autologous chondrocyte implantation: a systematic review; J Bone Jt Surg Am, 92 (2010 Sep 15), pp. 2220–2233
- 9 L. Hangody, G. Vásárhelyi, L.R. Hangody, et al.; Autologous osteochondral grafting–technique and long-term results; Injury, 39 (2008 Apr), pp. S32–S39
- 10 L. Hangody, J. Dobos, E. Baló, G. Pánics, L.R. Hangody, I. Berkes; Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study; Am J Sports Med, 38 (2010 Jun), pp. 1125–1133
- 11 R.P. Jakob, T. Franz, E. Gautier, P. Mainil-Varlet; Autologous osteochondral grafting in the knee: indication, results, and reflections; Clin Orthop Relat Res, 401 (2002 Aug), pp. 170–184
- 12 A.F. Steinert, U. Nöth, R.S. Tuan; Concepts in gene therapy for cartilage repair; Injury, 39 (2008 Apr), pp. S97–S113
- 13 W.S. Khan, D.S. Johnson, T.E. Hardingham; The potential of stem cells in the treatment of knee cartilage defects; Knee, 17 (2010 Dec), pp. 369–374
- 14 R.K. Elmallah, J.J. Cherian, J.J. Jauregui, T.P. Pierce, W.B. Beaver, M.A. Mont; Genetically modified chondrocytes expressing TGF-β1: a revolutionary treatment for articular cartilage damage?; Expert Opin Biol Ther, 15 (2015 Mar), pp. 455–464
- 15 E. Potier, J. Noailly, K. Ito; Directing bone marrow-derived stromal cell function with mechanics; J Biomech, 43 (2010 Mar 22), pp. 807–817
- 16 H. Chajra, C.F. Rousseau, D. Cortial, et al.; Collagen-based biomaterials and cartilage engineering. Application to osteochondral defects; Biomed Mater Eng, 18 (2008), pp. S33–S45
- 17 S.J. Bidarra, C.C. Barrias, P.L. Granja; Injectable alginate hydrogels for cell delivery in tissue engineering; Acta Biomater, 10 (2014 Apr), pp. 1646–1662
Document information
Published on 31/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?