Summary
Objective
Clot embolism remains a concern due to flushing of clot and thrombolytic agent centrally in the process of percutaneous pharmacomechanical thrombolysis (PMT) for a thrombosed prosthetic arteriovenous access (PAVA), which might be reduced by a modified technique.
Methods
We retrospectively review this modified technique that uses two balloon-catheters in crisscross fashion and occludes both ends of PAVA during thrombolysis. Underlying stenotic lesions were dilated simultaneously with balloon angioplasty when needed.
Results
Among the 23 patients treated, 21 (91.3%), 10 (43.5%), and seven (30.4%) presented significant stenosis at the outflow, intragraft, and inflow segments of PAVA, respectively. The median duration of follow-up was 310.0 (range, 288.0–327.0) days. Anatomic success was achieved in 12 out of 23 (52.2%). Clinical success for successful dialysis was achieved in all patients. The median primary patency and secondary patency were 126.0 days (range, 7.0–316.0) days and 308.0 days (range, 84.0–327.0), respectively.
Conclusion
We believe this method is safe and effective in dissolving PAVA thrombus as well as treating culprit stenosis. It may reduce concerns of flushing of clot and thrombolytic agent into the central circulation in the process of PMT.
Keywords
angioplasty;renal dialysis;thrombosis;thrombolytic therapy
1. Introduction
The outcome of prosthetic arteriovenous access (PAVA) rescue is very much dependant on both expediency to salvage procedure and attention to underlying factors that lead to graft failure. Surgical thrombectomy (ST) alone may not be adequate for ensuring long-term graft patency despite short-term success with just thrombus removal per se. 1 ; 2 Increasingly, there is a trend toward using endovascular technique for dialysis access salvage.3 Several pharmacomechanical thrombolysis (PMT) methodologies 4; 5; 6 ; 7 have been developed and demonstrated comparable outcome of PMT and ST for treatment of PAVA thrombosis. 8 ; 9 However, there remains a persistent concern with regards to whether flushing of lytic agent and clot into central vein during procedure will produce clinically significant complications.8 The purpose of this study was to describe a modified hydraulic macerating technique PMT using two occluding balloons, which allows for clot removal efficiently hence reducing clot dislodgement centrally during the salvage procedure.
2. Materials and methods
We obtained approval for the review study from the Far Eastern Memorial Hospital Research Ethics Committee (FEMH-97-023). Patients were fully informed of the operation. We followed the ethical standards of the Helsinki Declaration in obtaining informed consent and in conducting the surgical procedures. The exclusion criteria were contrast medium hypersensitivity, unstable general condition, graft infection, or ongoing systemic infection.
All salvage procedures were performed in the angio-suite under local anesthesia listed as “day-case.” In addition to the PMT, concomitant balloon angioplasty was performed when needed. A standardized leaflet containing detailed postoperative instructions was given to each patient. The techniques of PMT have been described.2 ; 10
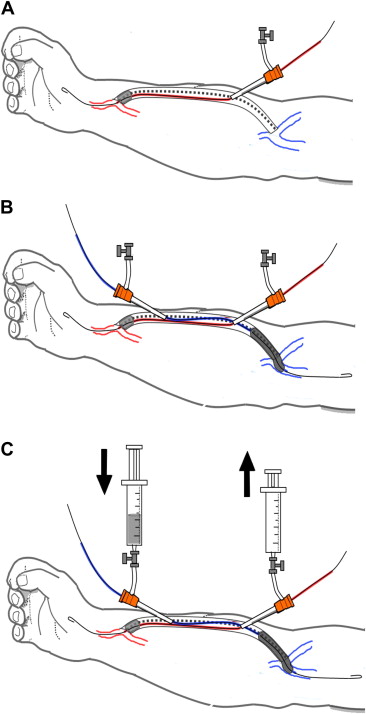
The modified technique of cross-balloon occlusive thrombolysis and angioplasty (CBOTA) is described as follows (Fig. 1). The midgraft position is marked on the surface of the graft and anesthetized locally with 2% Lidocaine. If possible, care is taken not to puncture sites where repeated needling has been performed for regular dialysis to avoid areas of possible intragraft stenosis related to repeated puncturing. Two sheaths are employed in a crisscrossing but preferably in a nonoverlapping fashion (Fig. 2). Specifically, following puncture with a 16-gauge needle near, the venous end and toward the arterial anastomosis, a guide wire is advanced under fluoroscopy, after which a 7-French sheath is inserted. A 0.025-in wire is then advanced under fluoroscopy well beyond the arterial anastomosis. A dual lumen 4-French Fogarty Thru-Lumen Embolectomy Catheter (Edwards Lifesciences, Irvine, CA, USA) is then advanced along the path of the wire. Location of the arterial anastomosis is then confirmed with antegrade angiogram followed by inflation of the contrast-filled Fogarty balloon within the artery just upstream of the arterial anastomosis. The inflated balloon can be seen to deform once it is pulled into the entrance of the graft hence dislodging the arterial plug into the graft lumen. A second 7-French sheath is then placed within the graft in the opposite direction toward the venous anastomosis. A scout angiogram is then performed by manipulating a 0.025-in wire centrally followed by a “pull-back” angiogram using an imaging catheter to ascertain the extent of clot formation, if any, beyond the graft-vein junction. An appropriately sized angioplasty balloon is then placed in the same fashion as described for the first Fogarty balloon and inflated within the graft just proximal to the venous anastomotic end. With the inflation of two balloons simultaneously, a sealed column is established within the prosthetic graft. A total of 1 million units of urokinase (Taiwan Green Cross Co., Taipei, Taiwan) dissolved in 40 ml of heparinized saline is then introduced slowly into the graft via the side port of one of the 7-French sheath. A hydraulic pressure is thus set up as the urokinase is pushed into the fixed volume confined with the graft. An empty syringe is attached to the side port of the second 7-French sheath as a column of clot/blood is forced into it as the urokinase is introduced into the graft. With the establishment of the hydraulic column, the clot/thrombus within the graft can be forced out via side ports into the syringes as the action of “emptying” from one syringe is complemented by “filling” the other syringe simultaneously. External compression along the graft could be performed to help macerate the thrombus. More heparinized saline could be used as many times as desired to flush out the clots using the syringes within the fixed column of graft material. The Fogarty balloon at the arterial end may be pulled further into the graft to mobilize any residual thrombus likewise for the angioplastic balloon at the venous end. Contrast can also be admixed into the final flush to visualize the completeness of thrombus removal using this modified PMT technique. The angioplastic balloon proximal to the venous anastomosis is then released and removed over the wire. With the other Fogarty balloon still inflated proximal to the arterial anastomosis, an angiogram is then performed to visualize the venous outflow. If residual thrombus or outflow stenosis is visualized, balloon angioplasty is performed accordingly. Once the graft outflow is deemed satisfactory, the Fogarty balloon at the arterial end is released and advanced upstream well beyond the arterial anastomosis to perform an antegrade angiogram. Any inflow stenosis would then be dealt with accordingly using balloon angioplasty.
|
|
|
Figure 1. (A) Drawing the steps of cross-balloon occlusive thrombolysis for a thrombosed straight forearm prosthetic arteriovenous access; (B) a 7-French sheath is placed near the venous end and towards the arterial end and, following a wire, a dual-lumen Fogarty balloon catheter is advanced to the arterial anastomosis and inflated. A second 7-French sheath is then placed within the graft in the opposite direction toward the venous anastomosis. An appropriately sized angioplasty balloon is then placed in the same fashion and inflated within the graft just proximal to the venous anastomotic end; (C) with the inflation of two balloons simultaneously, a sealed column is established within the prosthetic graft. Thrombolytic solution is then introduced slowly into the graft via the side port of one of the 7-French sheath. Two syringes are used to work alternatively in a push-pull fashion (arrows). |
|
|
|
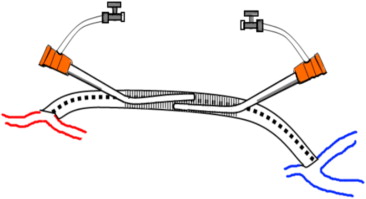
Figure 2. Drawing the ineffective zone when two sheaths are inserted deeply and overlapped. Treating the in-between segment (shaded area) will be difficult. |
The amount of thrombus in the PAVA was subjectively estimated by visual determination of contrast opacification versus intraluminal filling defect. Residual thrombus was macerated with either the embolectomy balloon or angioplasty balloon. Percutaneous balloon angioplasty was performed when stenoses were detected. Improved luminal diameter and flow were confirmed by completion digital subtract imaging.
Sheaths were removed, and hemostasis was achieved by manual compression and purse-string sutures for 1 hour postoperatively when needed. Procedure time was calculated from the first puncture to the completion of hemostasis. Restoration and maintenance of flow were confirmed with a bruit on auscultation or palpable thrill.
Anatomic success was defined as restoration of flow combined with a less than 30% maximal residual diameter stenosis for each segment. Clinical success was defined as resumption of at least one successful dialysis session.11 Clinical success was obtained by contact with patients, hemodialysis centers, dialysis nurses, or referring nephrologists. Primary patency was considered the time between CBOTA and subsequent intervention or graft failure.
Factors affecting the patency of an access and procedural complications were recorded and graded according to the reporting standards of the Society for Vascular Surgery and the American Association for Vascular Surgery.11
Data were presented as median (range) for continuous variables and number (percentage) for categorical variables. Events were defined as the development of any acute complications such as stenosis, thrombosis, or malfunction requiring intervention during the follow-up period. All statistical analyses were performed with IBM SPSS Statistics software (IBM Corp. Armonk, NY).
3. Results
In March and April 2008, we developed and treated 23 patients with acute PAVA thrombosis using the CBOTA method and their charts were retrospectively reviewed.
The demographics and factors related to outcomes are summarized in Table 1. There were 14 men and nine women. The median of age of patients was 72.0 years (range, 51.0–83.0). The median body mass index and body surface area were 24.8 kg/m (range, 18.4–34.9) and 1.7 m2 (range, 1.4–2.1), respectively. The approximate age of PAVA was known in 19 out of the 23 and was 18.0 months old (range 1.0–96.0).
| Characteristic | Dataa | |||
|---|---|---|---|---|
| Men:Women (number) | 9:14 | |||
| Age (yr) | 72.0 (51.0–83.0) | |||
| BMI (kg/m2) | 24.8 (18.4–34.9) | |||
| Body surface area (m–) | 1.7 (1.4–2.1) | |||
| Factors affecting outcomeb | Grade 1 | Grade 2 | Grade 3 | |
| Diabetes | 10/19 (52.6%) | 7 | 3 | 0 |
| Hypertension | 16/19 (84.2%) | 4 | 12 | 0 |
| Tobacco use | 2/19 (10.5%) | 2 | 0 | 0 |
| Outflow tract | 8/19 (42.1%) | 6 | 1 | 1 |
| Prior procedures on same limb | 18/18 (100.0%) | 3 | 7 | 8 |
| Other factors affecting outcome | ||||
| Prior thrombosis of same access | 12/19 (69.6%) | |||
| Age of graft (mo) | 18.0 (1.0–96.0) | |||
BMI = body mass index.
a. Data are presented as median (range) for continuous variables and number (percentage) for categorical variables.
b. Values indicate number of patients in each category. Grading of severity is presented according to reporting standards of the Society for Vascular Surgery/American Association for Vascular Surgery.
Among the 23 patients evaluated, four had incomplete factorial data. Out of the 19 patients, 10 (52.6%) had diabetes, 16 (84.2%) had hypertension, two (10.5%) used tobacco, eight (42.1%) had prior documented outflow tract problems, and 12 (69.6%) experienced prior events of intervention on the same access.
The clinical results are summarized in Table 2. The median procedure time was 80.0 minutes (range, 30.0–210.0). The median blood loss was 50.0 ml (range, 10.0–100.0). Out of 23 PAVAs, 21 (91.3%), 10 (43.5%), and seven (30.4%) presented significant stenosis at the outflow, intragraft, and inflow segments, respectively. The median duration of follow-up was 310.0 days (range, 288.0–327.0). Anatomically decreasing in severity of stenosis by completion of the graft gram was obtained in 21 out of 23 (91.3%) and anatomic success was achieved in 12 out of 23 (52.2%); Moderate residual stenosis in at least one segment was seen in 11 out of 23 (47.8%). Clinical success for successful dialysis was achieved in all patients. The median event-free PAVA survival time was 87.0 days (range, 5.0–316.0). The median primary patency and secondary patency were 126.0 days (range, 7.0–316.0) and 308.0 days (range, 84.0–327.0), respectively.
| Characteristic | Dataa | |||
|---|---|---|---|---|
| Procedure time (min) | 80.0 (30.0–210.0) | |||
| Blood loss (ml) | 50.0 (10.0–100.0) | |||
| Grading severity of access stenosis before angioplastyb | Grade 1 | Grade 2 | Grade 3 | |
| Outlfow stenosis | 21/23 (91.3%) | 0 | 13 | 8 |
| In-graft stenosis | 10/23 (43.5%) | 0 | 5 | 5 |
| Inflow stenosis | 7/23 (30.4%) | 0 | 5 | 2 |
| Maximal stenosis of whole access | 22/23 (95.7%) | 0 | 10 | 12 |
| Grading severity of residual stenosis on completionb | Grade 1 | Grade 2 | Grade 3 | |
| Completion outflow stenosis | 19/23 (82.6%) | 12 | 6 | 1 |
| Completion in-graft stenosis | 10/23 (43.5%) | 7 | 3 | 0 |
| Completion inflow stenosis | 7/23 (30.4%) | 5 | 2 | 0 |
| Maximal residual stenosis of whole access | 22/23 (95.7%) | 11 | 10 | 1 |
| Follow-up time (days) | 310.0 (288.0–327.0) | |||
| Duration to endpoint (days) | 308.0 (84.0–327.0) | |||
| Grading severity of complicationsc | Grade 1 | Grade 2 | Grade 3 | |
| Bleeding | 3/23 (13.0%) | 1 | 1 | 1 |
| Infection | 0/23 (0.0%) | 0 | 0 | 0 |
| Non-infectious fluid collection | 0/23 (0.0%) | 0 | 0 | 0 |
| Anastomotic complications | 1/23 (4.3%) | 1 | 0 | 0 |
| Mid-AV/Runoff vein complications | 19/23 (82.6%) | 17 | 2 | 0 |
| Access thrombosis | 12/23 (52.5%) | 10 | 1 | 1 |
| Access malfunction | 0/23 (0.0%) | 0 | 0 | 0 |
| Steal syndrome | 1/23 (4.3%) | 1 | 0 | 0 |
| Venous hypertension | 0/23 (0.0%) | 0 | 0 | 0 |
| Neuropathy | 0/23 (0.0%) | 0 | 0 | 0 |
a. Data are presented as median (range) for continuous variables and number (percentage) for categorical variables.
b. Values indicate number of patients in each category. Anatomic findings and results were determined by angiogram visually and scored in inflow, intra-graft, and outflow segments as grade 0 for no stenosis, grade 1 for less than 30% diameter reduction with no limitation of flow, grade 2 for equal or larger than 30% diameter reduction with limitation of flow, and grade 3 for severe residual stenosis or problem resulting in failure.
c. Values indicate number of patients in each category. Grading of severity is presented according to reporting standards of the Society for Vascular Surgery/American Association for Vascular Surgery.
Table 2 also presents the grading severity of complications. Among the 23 patients, three (13.0%) presented unexpected bleeding and one of them required suture repair and one (4.3%) had mild rupture that happened at the outflow segment by balloon dilatation but experienced no residual access malfunction.
4. Discussion
This study demonstrates that CBOTA was a feasible and efficacious option for treatment of PAVA thrombosis. Clinical success and primary patency of CBOTA achieved the patency goals of 85% of clinical success and 40% primary unassisted patency at 3 months.12 CBOTA produced reasonable hydraulic maceration and lacing forces helped to pulverize the clot effectively.
Clot embolism remains a concern although clot burden within the thrombosed PAVA is thought to be of low volume. A close system limited the potential showering of clot and lytic agents centrally. Experience has shown that the incidence of distant hemorrhage with locally administered thrombolytic therapy is negligible.7 The “lyse and wait” technique6 ; 7had been previously described a method of infusing low dose urokinase into the clotted graft. We believe the modified method of declotting described in our series serves to minimize the risk of arterial emboli within a truly sealed column using two balloons. In addition, the use of urokinase within sealed tubing also ensures minimal leakage of urokinase into the systemic circulation thereby reducing hemorrhagic complications. Although we did not measure the serum level of thrombolytic agent, most of the agent mixing with lytic clot in the close column of graft were pulled out during push-pull procedure and, therefore, was not infused systemically. No patient experienced systemic bleeding. Early CBOTA experience translated to a higher complication rate of post-procedure local bleeding but predicted noninferior clinical outcome. Three bleeding events happened locally on the sheath puncture site. Two of them happened in the postoperating room while preparing to leave the hospital because we did not place purse-string sutures when removing sheath in the beginning series; one of them experienced purse-string-related skin necrosis due to retention of one purse-string suture for more than one day accidentally. A close system procedure also limited blood loss with a median of 50.0 ml, which included the lytic clot.
Graft gram during CBOTA provided adequate evaluation of stenosis, which can be corrected by angioplasty in most instances.3 ; 13 Twenty-two out of 23 (95.7%) patients with PAVA thrombosis presented at least one significant stenosis in each access in this series. Anatomical improving was achieved by balloon dilatation during CBOTA procedure in 36 out of 38 (94.7%) diseased segments. One worsening happened because of rupture.
The limitations of this report include small numbers of cases, a retrospective single-arm study design, absence of blinded outcomes assessment, absence of test to see if we successfully reduced the possibility of pulmonary embolism, and variability in patient follow-up.
In conclusion, CBOTA technique achieved goals of clinical and anatomic success for PAVA thrombosis and provided adequate short-term patency. It may be an improvement over current techniques and may reduce concerns of flushing of clot and thrombolytic agent centrally in the process of percutaneous thrombolysis. Further follow-up studies to compare long-term results of varying approaches is needed.
References
- 1 J.F. Polak, M.F. Berger, H. Pagan-Marin, J.E. Aruny, M.F. Meyerovitz; Comparative efficacy of pulse-spray thrombolysis and angioplasty versus surgical salvage procedures for treatment of recurrent occlusion of PTFE dialysis access grafts; Cardiovasc Intervent Radiol, 21 (1998), pp. 314–318
- 2 L.D. Green, D.S. Lee, D.S. Kucey; A metaanalysis comparing surgical thrombectomy, mechanical thrombectomy, and pharmacomechanical thrombolysis for thrombosed dialysis grafts; J Vasc Surg, 36 (2002), pp. 939–945
- 3 A.M. Bakken, Galaria II, C. Agerstrand, et al.; Percutaneous therapy to maintain dialysis access successfully prolongs functional duration after primary failure; Ann Vasc Surg, 21 (2007), pp. 474–480
- 4 K. Valji, J. Bookstein, A. Roberts, G. Davis; Pharmacomechanical thrombolysis and angioplasty in the management of clotted hemodialysis grafts: early and late clinical results; Radiology, 178 (1991), pp. 243–247
- 5 G.A. Beathard; Mechanical versus pharmacomechanical thrombolysis for the treatment of thrombosed dialysis access grafts; Kidney Int, 45 (1994), pp. 1401–1406
- 6 J. Cynamon, P.S. Lakritz, S.I. Wahl, C.W. Bakal, S. Sprayregen; Hemodialysis graftdeclotting: description of the "lyse and wait" technique; J Vasc Interv Radiol, 8 (1997), pp. 825–829
- 7 Jr. Duszak R., D. Sacks; Dialysis graft declotting with very low dose urokinase: is it feasible to use "less and wait?"; J Vasc Interv Radiol, 10 (1999), pp. 123–128
- 8 T. Vesely; Thrombolysis versus surgical thrombectomy for the treatment of dialysis graft thrombosis: pilot study comparing costs; J Vasc Interv Radiol, 7 (1996), pp. 507–512
- 9 R. Uflacker, P.R. Rajagopala, J.B. Selby, C. Hannegan; Thrombosed dialysis access grafts: randomized comparison of the Amplatz thrombectomy device and surgical thromboembolectomy; Eur Radiol, 14 (2004), pp. 2009–2014
- 10 S.G. Cooper; Original report. Pulse-spray thrombolysis of thrombosed hemodialysis grafts with tissue plasminogen activator; AJR Am J Roentgenol, 180 (2003), pp. 1063–1066
- 11 A.N. Sidawy, R. Gray, A. Besarab, et al.; Recommended standards for reports dealing with arteriovenous hemodialysis accesses; J Vasc Surg, 35 (2002), pp. 603–610
- 12 NKF-K/DOQI; Clinical practice guidelines for vascular access: update 2000; Am J Kidney Dis, 37 (2001), pp. S137–S181
- 13 M.A. Cohen, D.A. Kumpe, J.D. Durham, S.C. Zwerdlinger; Improved treatment of thrombosed hemodialysis access sites with thrombolysis and angioplasty; Kidney Int, 46 (1994), pp. 1375–1380
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?