Abstract
Background
We have proposed a new scoring system (Anaphylaxis SCoring Aichi: ASCA) for a quantitative evaluation of the anaphylactic reaction that is observed in an oral food challenge (OFC). Furthermore, the TS/Pro (Total Score of ASCA/cumulative protein dose) can be a marker to represent the overall severity of a food allergy. We aimed to develop a prediction model for a severe allergic reaction that is provoked in a boiled egg white challenge.
Methods
We used two separate datasets to develop and validate the prediction model, respectively. The development dataset included 198 OFCs, that tested positive. The validation dataset prospectively included 140 consecutive OFCs, irrespective of the result.
A ‘severe reaction’ was defined as a TS/Pro higher than 31 (the median score of the development dataset). A multivariate logistic regression analysis was performed to identify the factors associated with a severe reaction and develop the prediction model.
Results
The following four factors were independently associated with a severe reaction: ovomucoid specific IgE class (OM-sIgE: 0–6), aged 5 years or over, a complete avoidance of egg, and a total IgE < 1000 IU/mL. Based on these factors, we made a simple scoring prediction model. The model showed good discrimination in a receiver operating characteristic analysis; area under the curve (AUC) = 0.84 in development dataset, AUC = 0.85 in validation dataset. The prediction model significantly improved the AUC in both datasets compared to OM-sIgE alone.
Conclusions
This simple scoring prediction model was useful for avoiding risky OFC.
Keywords
Anaphylaxis; Food allergy; Hens egg; Oral food challenge; Prediction model
Abbreviations
AUC, Area under the curve; OFC, Oral food challenge; OIT, Oral immunotherapy; NPV, Negative predictive value; PPV, Positive predictive value; ROC, Receiver operating characteristic; sIgE, Specific IgE
Introduction
Oral food challenges (OFC) have been performed for the definitive diagnosis of a food allergy and more frequently, for the discernment of tolerance to the allergen.1 In addition to these conventional purposes, OFCs are performed to determine the threshold dose of food allergens for individuals beginning oral immunotherapy (OIT) or to determine the threshold of minimal avoidance. More severe reactions tend to be provoked in these OFC settings.
Sampsons grade stratification2 is commonly used for an assessment of the severity of allergic reaction provoked in an OFC, and the Japanese Guideline for Food Allergy 2014 adopted it with minor modifications.3 This classification mostly aims to evaluate the severity of a reaction to decide the indications for therapy including intramuscular epinephrine injection. For that purpose, severity is judged based on the highest grade of symptoms, even if multi-organ reactions are provoked.
On the other hand, an evaluation of multi-organ symptoms can be an important severity marker in some purposes because the number of symptomatic organs involved in an allergic reaction affects the outcome of achieving tolerance afterward.4 and 5 For this purpose, we have developed an original scoring system named Anaphylaxis Scoring Aichi (ASCA) for a quantitative evaluation of multi-organ reactions provoked in OFCs.6 ASCA lists and sorts allergic symptoms according to five organ systems (respiratory, skin-mucosal, gastrointestinal, psycho-neurological, and cardiovascular). In the gastrointestinal symptoms, the degree of abdominal pain was expressed as face scale (Supplementary Fig. 1). Each symptom was given an organ system score of 0–60 points in accordance to the severity. The gradient of the points was set for the purpose that the sum of plural mild points does not exceed one severe organ system point. The organ system score of 40 points almost corresponds to grade 4 of Sampsons grade2 (Table 1).
| Score organ | 0 | ① 1 point | ① 5 points | ② 10 points | ② 20 points | ③ 40 points | ④ 60 points |
|---|---|---|---|---|---|---|---|
| Respiratory (subjective) | None | Itchy nose | Laryngeal discomfort | Nasal congestionSuffocating breath | Speech disturbanceDifficulty in breathing | Loss of voice | |
| (objective) | Sneeze | Mild transient coughingRunny nose | Intermittent coughingMild wheezing | Frequent coughingApparent wheezingHoarseness | Continuous coughingStrong wheezingIntentional breathingInspiratory stridorRetraction | Weak breath soundsStrong retractionCyanosisSpO2 ≤ 90% | |
| Skin/Mucosal (subjective) | None | Itch (around mouth) Mild discomfort, Burning sensation | Itch (local and mild) | Itch (whole body) | unbearable itch | ||
| (objective) | <Peri-oral>Hives, Erythema, Swelling, Vesicle | <Local>Eye edema, BloodshotHives, Erythema, Swelling, Angioedema | <Multiple>Hives, Erythema, SwellingAngioedema | <Spreading, Generalized>Hives, Erythema, Swelling Angioedema | |||
| Gastrointestinal (subjective) | None | Oral or pharyngeal itch, Hot taste, Sore throat | Mild nausea, Abdominal pain (FS1) | Mild nausea, Abdominal pain (FS2) | Strong abdominal pain (FS3) | Unbearable abdominal pain (FS4) | |
| (objective) | Increased bowel sounds | Diarrhea, Vomiting | Recurrent vomiting | Dehydration by vomiting | |||
| Psycho-neurological | None | Refusal to eatMild excitement | Loss of activityIrritation | Sleep, Tendency to lay downMild excitement | Sleep (not usual)Agitating, Crying | Tend to fall unconsciousUncontrolled panic | Unconsciousness |
| Cardiovascular | None | Pale, TachycardiaCold extremities, Cold sweat | Bradycardia | ||||
| (Blood pressure) | Mild decrease of blood pressure<1 y: <70 mmHg1–10 y: <70+(2 x age) mmHg11–17 y: <90 | Low Blood Pressure<1 y: <50 mmHg1–10 y: <60 mmHg≥11 y: <70 mmHg |
Allergic symptoms are categorized into 5 organ systems, and each organ symptom score (0–60 points) is given based on the severity of the symptoms. Total score (TS) is defined as a sum of the highest organ symptom score observed throughout the course of allergic symptom (maximum 240 points).
FS, Face scale to express the degree of abdominal pain (Supplementary Fig. 1).
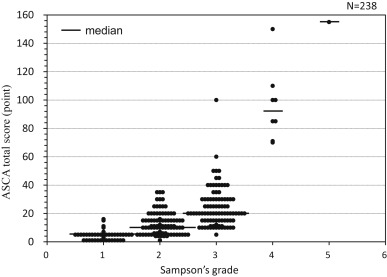
The total score (TS) was defined as the sum of 5 organ system scores (maximum 240 points) based on the highest organ system score observed throughout a course of OFC. We have already validated the TS to be correlated to Sampsons grade6 (Fig. 1).
|
|
|
Fig. 1. The correlation between total score of ASCA and Sampsons anaphylaxis grading. ASCA, Anaphylaxis scoring Aichi. Adapted from Ref. 6 with modification. |
Several studies have tried to predict the result of OFCs and their severity of outcome. Although a probability curve of specific Immunoglobulin E (sIgE) provides useful information for predicting a positive challenge7 and a severe reaction,8 another report suggested a limitation in its predictive accuracy.9 Component-resolved diagnostics for wheat (ω-5 gliadin)10 and peanut (Ara h 2)11 provided a promising prediction for the positive result of an OFC, but failed to predict the threshold dose and the severity of symptoms. Clinical backgrounds should also be considered to predict the outcome of an OFC including the severity of the provoked reactions.4, 12 and 13
A recent article suggested that a complex model incorporating both test results and a clinical history had a better predictive ability compared to simply relying on the sIgE and skin prick test (SPT) either alone or in combination with one another.4 For the purpose of identifying a severe allergic reaction, a complex model is thought to be a better predictor.12
The aim of this study was to identify the clinical factors contributing to a severe reaction provoked in an OFC and use them to develop a prediction model.
Methods
Oral food challenge (OFC)
An open OFC of 20 min-boiled egg white was performed according to the Japanese Guideline for Food Allergy 2014.3 We selected the consecutive 4 to 6 doses from 0.2, 0.5, 1, 2, 5, 10 and 20 g of boiled egg white depending on the age and estimated severity of the patient. Every dose was taken within 2 h (40–20 min intervals), but the challenge was stopped if the patient exhibited an objective allergic reaction corresponding to 5 point or more of ASCA TS.
Anaphylaxis Scoring Aichi (ASCA)6
The severity of provoked symptoms was scored using the TS of ASCA. Furthermore, we have developed a new indicator “TS/Pro” for the simultaneous expression of severity and threshold dose of antigen. “Pro” represents the cumulative protein dose of the allergen that provoked symptoms, and the TS/Pro was obtained by simply dividing the TS by Pro.
Dataset
The development dataset was obtained from OFCs to boiled egg white from April 2012 to May 2013. During this period, 450 OFCs to boiled egg white were conducted and 273 OFCs (60.1%) of those were positive. To minimize a selection bias, only positive results were chosen to develop a prediction model. The appropriate laboratory data (sIgE to ovomucoid (OM) and total IgE) that was collected during the 180 day period was missing in 75 of the cases. As a result, data was included from 198 cases.
To validate this model in an independent clinical setting, 140 consecutive OFCs to boiled egg white (95 positive and 45 negative) were analyzed from June 2013 to November 2013 in a prospective manner.
Selection of the risk factors
The factors analyzed as a candidate for contribution to the challenge result were age, sex, history of anaphylaxis to egg, history of atopic dermatitis or bronchial asthma, present state of egg avoidance, total IgE, egg white (EW)-sIgE, and OM-sIgE (Phadia AB, Uppsala, Sweden). Doctors and dietitians carefully took the patients dietary history to obtain information regarding egg avoidance because parents often serve some foods containing egg (for example, breads or cookies) unintentionally even if they declare that they are striving to follow a diet of “complete avoidance of egg”.
We divided patients in the development dataset into two groups (severe cases and non-severe cases) on the basis of the median value of the TS/Pro of the development dataset.
Development of the prediction model
For a univariate analysis, the Mann–Whitney U test was used to compare the sequential data and a chi-square analysis was used to compare the binary variables. The factors selected from the univariate analysis were then used for a multivariate regression analysis to identify the independent predictors of a severe allergic reaction. For measuring OM-sIgE, which has the nature of a logarithmic scale, the classification of sIgE (0–6: Supplementary Table 1) was applied. The values of OM-sIgE below 0.34 UA/mL (class 0) and above 100 UA/mL (class 6) were dealt as 0.34 UA/mL and 100 UA/mL, respectively. The values of OM-sIgE < 0.35 kUA/L and ≥100 kUA/L were determined to be 0.34 kUA/L and 100 kUA/L, respectively. For age and total IgE, the cutoff points were defined approximately in the upper quartile of the individual variables.
Based on the results, we constructed a logistic regression model using the independent factors with a probability value p < 0.05. We next created a simple scoring model by assigning the point scores of each variable. The discriminatory capacity of the model was assessed using the area under the receiver operating characteristics (ROC) curve. Comparing area under the curve of the ROC curve, we accepted the method proposed by DeLong et al. 14 The goodness of fit of the regression model was tested with the Hosmer–Lemeshow test, with p < 0.05 indicating a lack of deviation between the model and observed event rate.
This study was approved by the institutional review board of Aichi Childrens Health and Medical Center. All analyses were performed with the STATA (version 12.1 for Mac; STATA Inc, College Station, TX, USA) software program. For all analyses, a 2-sided probability value below 0.05 was considered to indicate statistical significance.
Results
Selection of the factors associated with the severe result of OFC
Characteristics of the development dataset are shown in Table 2 and Figure 2. The median age of the dataset was 3.95 years and 67.2% were males. The median TS was 15 points and the median cumulative dose of egg white was 3.7 g, thus resulting in a median TS/Pro of 31.2. According to this result, we defined the severe cases, as TS/Pro > 31, (n = 101) and non-severe cases (n = 97).
| Development dataset | Validation dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Severe (TS/Pro > 31) | Non-severe | p-value | Total | Severe (TS/Pro > 31) | Non-severe or negative result | p-value | |
| Number | 198 | 101 | 97 | 140 | 49 | 91 | ||
| Age | 3.95 (2.5–6) | 4.7 (3–6.3) | 3.3 (2.2–5.5) | 0.002 | 4.15 (2.6–6.1) | 5 (2.9–5.8) | 3.5 (2.2–6.4) | 0.30 |
| Male | 133 (67.2%) | 67 (66.3%) | 66 (68.0%) | 0.80 | 97 (69.3%) | 32 (65.3%) | 65 (71.4%) | 0.45 |
| Atopic dermatitis | 147 (74.2%) | 74 (73.3%) | 73 (75.3%) | 0.75 | 103 (73.6%) | 37 (75.5%) | 66 (72.5%) | 0.70 |
| Bronchial asthma | 58 (29.3%) | 33 (32.7%) | 25 (25.8%) | 0.29 | 44 (31.4%) | 23 (46.9%) | 21 (23.1%) | <0.01 |
| History of anaphylaxis | 31 (15.7%) | 18 (17.8%) | 13 (13.4%) | 0.39 | 20 (14.3%) | 11 (22.4%) | 9 (9.9%) | 0.04 |
| Complete avoidance | 162 (81.8%) | 95 (94.0%) | 67 (69.1%) | <0.001 | 104 (74.3%) | 46 (93.9%) | 58 (63, 7%) | <0.001 |
| Total IgE (IU/mL) | 439 (150–968) | 507 (197–1008) | 386 (110–922) | 0.07 | 504 (192–1031) | 590 (266–1115) | 432 (183–1021) | 0.25 |
| EW-sIgE (kUA/L) | 22.5 (7.9–44.4) | 32.6 (17.4–75) | 10.2 (4.3–25.7) | <0.001 | 15.3 (6.3–39.4) | 30.5 (14.2–59.1) | 9.24 (5.09–24.3) | <0.001 |
| OM-sIgE (kUA/L) | 11.45 (3.3–34) | 22.4 (11.3–44.8) | 3.49 (1.7–12.3) | <0.001 | 7.12 (2.2–21.0) | 18.5 (8.3–40.3) | 3.4 (1.1–9.7) | <0.001 |
| Total score of ASCA | 15 (10–30) | 25 (15–35) | 10 (5–15) | <0.001 | 5 (0–15) | 16 (15–30) | 1 (0–5) | <0.001 |
| Total amount of the boiled egg white (g) | 3.7 (1.7–8.8) | 1.7 (0.7–3.6) | 8.8 (8.5–18.5) | <0.001 | 3.7 (1.7–18) | 1.7 (0.7–1.7) | 8.7 (5.5–18.5) | <0.001 |
| TS/Pro | 31.2 (7.4–118) | 118 (59.8–265.5) | 7.4 (3.5–15.6) | <0.001 | 9.0 (0–69.5) | 104.1 (63.2–189.6) | 0.23 (0–5.3) | <0.001 |
Values are presented as medians (first and third quartiles) or number (proportion). Each p-value is calculated using the Mann–Whitney U test (sequential variables) or chi-square test (binary variables). EW, egg white; OM, omucoid; sIgE, specific IgE.
|
|
|
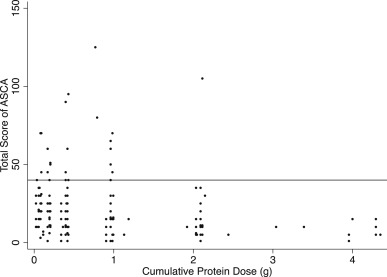
Fig. 2. Individual results of boiled egg white challenge. The scatter plot shows the distribution of cumulative protein dose (g of egg white protein) and the total score of ASCA in the development dataset (n = 198). The reference line indicates the total score 40 points. |
A univariate analysis identified 5 variables (EW-sIgE, OM-sIgE, complete avoidance, age, total IgE) as they showed a significant or marginally significant difference between the severe and non-severe groups (Table 2).
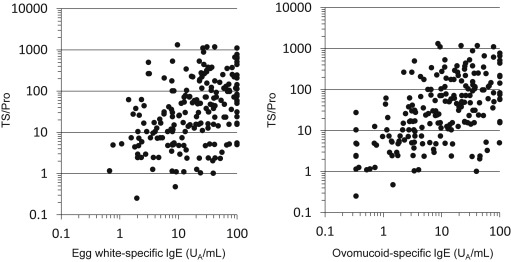
EW-sIgE and OM-sIgE showed a strong correlation (Spearmans R = 0.88) and they both showed statistically significant correlation to the TS/Pro (Spearmans R = 0.42 and 0.53, respectively, both p-value < 0.01, Fig. 3). Therefore, we selected OM-sIgE, but not EW-sIgE, as the candidate for the multivariable analysis to avoid multicovariance. To evaluate the validity of dealing with OM-sIgE (class) as numerical variables, the association between logistic coefficient (β) of OM-sIgE (class) and severe cases was confirmed.
|
|
|
Fig. 3. Correlation between specific IgE and the TS/Pro. Ovomucoid-specific IgE correlated to the TS/Pro (right, Rs = 0.53, p < 0.01) better than egg white-specific IgE (left, Rs = 0.42, p < 0.01). TS/Pro, Total Score of ASCA/cumulative protein dose. |
Development of the prediction model
Based on the results from the univariate analysis, the four variables, “OM-sIgE class (0–6)”, “complete avoidance”, “total IgE < 1000 IU/mL (upper quartile of the level)” and “5 years or more (upper quartile of age)” were included in the multivariate logistic regression analysis (Table 3). The result of the logistic regression analysis revealed these four factors to be independently associated with the result of OFC, which enabled us to construct a logistic regression model and a simple scoring model by approximating the logistic coefficients (β). The prediction score consisted of the base point of OM-sIgE (1 point/class), one point each for the total IgE < 1000 IU/mL and an age of 5 years or more, and 2 points for complete avoidance, resulting in a maximum of 10 points.
| Logistic Coefficient (β) | SE | OR (adjusted) | P-value | Score point | |
|---|---|---|---|---|---|
| OM-sIgE (class 0–6) | 1.18 | 0.20 | 3.26 | <0.001 | 1 |
| Complete avoidance | 1.69 | 0.57 | 5.42 | 0.003 | 2 |
| Total IgE < 1000 IU/mL | 1.58 | 0.51 | 4.80 | 0.002 | 1 |
| Aged 5 years or more | 0.94 | 0.39 | 2.55 | 0.02 | 1 |
OM, ovomucoid; sIgE: specific IgE.
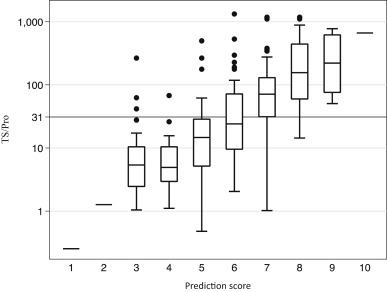
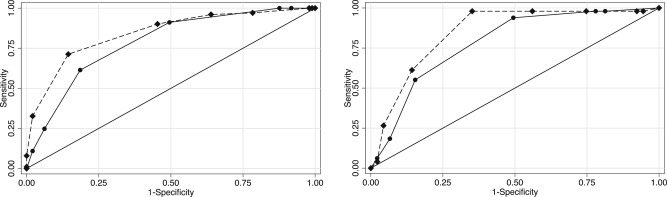
The prediction score showed a significant correlation to the TS/Pro (Fig. 4). The median score of the development dataset was 6 (ranging from 0 to 10). The area under the ROC curve was 0.84 (95% CI, 0.79 to 0.90) for the simple scoring model, which was almost identical to that obtained from the logistic regression model 0.86 (95% CI, 0.79 to 0.90). The Hosmer–Lemeshow statistic was not significant (probability value of 0.58 and 0.4 for the logistic model and the simple scoring model, respectively). The simple scoring model was significantly superior in its discriminatory ability for predicting severity (AUC = 0.84) in comparison to the prediction obtained using the OM-IgE class alone (AUC = 0.79) (p < 0.01, Fig. 5, left).
|
|
|
Fig. 4. Correlation between the prediction score and the TS/Pro. The box plots of TS/Pro in each prediction score are shown. The line at TS/Pro = 31 indicates the median level of the development dataset (n = 198). The prediction score significantly correlated to the TS/Pro (Rs = 0.65, p < 0.01). |
|
|
|
Fig. 5. A ROC analysis of the prediction score and specific IgE class. In the development dataset (left), the prediction score (diamond, dash line, AUC = 0.84) showed a better discrimination ability (p < 0.01) of a severe result of OFC than the ovomucoid-specific IgE class (Circle, Solid line, AUC = 0.79). The result was reproduced in the validation dataset (right); AUC = 0.85 and 0.78, respectively (p = 0.011). ROC, Receiver Operating Characteristic; AUC, area under the curve. |
Validation of the prediction model
The background characteristics of the validation dataset are shown in Table 2. Among 17 OFCs with a prediction score 8 and over, 13 OFCs (76.5%) resulted in a severe reaction (TS/Pro ≥ 31). On the other hand, 59/60 OFCs (98.3%) with a prediction score 5 and under presented with a non-severe reaction or negative results. The sensitivity and specificity of each cutoff is shown in Supplementary Table 2.
In this dataset, the area under the ROC curve was 0.85 for the simple scoring model, which was superior in its discriminatory ability in comparison to the OM-sIgE class alone (p = 0.011, Fig. 5, right) and the Hosmer–Lemeshow statistic was not significant (p = 0.063).
Discussion
We have developed the original severity score of allergic symptoms, named ASCA, and defined the overall severity indicator ‘TS/Pro’. By using this index, we identified the factors associated with severe results (low threshold and high symptom scores) in OFCs to boiled egg white. Furthermore, we developed a simple prediction model for a severe allergic reaction in OFCs. The model was constructed using the OFC positive dataset and the discriminative ability of this model was validated in another dataset including challenge-negative cases.
In the present study, OFCs were performed even for patients with a high probability for positive results, unless a recent anaphylaxis event was experienced with a small amount of allergen exposure. We aimed to find a safe dose of allergen ingestion, even among the challenge-positive patients, according to the basic strategy written in the Japanese guidelines.3 However, we needed to avoid dangerous and ineffective challenges with a low probability of finding the safe dose of allergen ingestion.
For the purpose of predicting such severe cases, we analyzed factors associated with severe cases and developed prediction model. We included only positive OFCs (60.7% of the total OFCs) to develop the prediction model. This was because the final dose was limited to a small amount for the safety of severely allergic patients and the true TS/Pro might not be zero if a larger amount was applied.
The developed model was validated in another dataset including both positive and negative results. Although sufficient discrimination ability was reproduced in the validation dataset, the Hosmer-Lemeshow goodness of fit test of the simple scoring model was marginally significant (p = 0.0629). This was partially due to the bias of our clinical policy, in which we tended to decrease the final dose of an OFC for any patient that was predicted to have a severe reaction, thus resulting in a negative result.
We selected the OM-sIgE titer instead of EW-sIgE as a specific IgE to predict a severe reaction. For identifying a positive OFC of boiled egg white, the OM-sIgE was reported to be superior to EW-sIgE.15 and 16 We have evaluated the severity of allergic reactions in positive challenges and found that OM-sIgE correlated to the TS/Pro better than EW-sIgE. In our data, sIgE values had an upper limit of 100 UA/mL [class6]. If the true values of >100 UA/mL could be applied, the Rs would be higher.
Nomura et al. reported a probability curve of OM-sIgE to predict a severe allergic reaction in an egg OFC, but the threshold dose was not examined. 8 As far as we know, this is the first article to present a correlation between OM-sIgE and the severity of reaction taking the threshold dose into account.
Low total IgE value (<1000 IU/mL) was identified as a risk factor for severe allergic reactions. Christensen LH et al. reported that non-specific or low-affinity IgE suppresses the specific IgE-mediated activation of basophils in vitro. 17 The low total IgE values were also reported to be associated with positive and severe reactions in a venom allergy18 and the results of OFCs.19
An older age was also one of the risk factors for a severe allergy. This result might not be ubiquitous in other settings. We have repeated OFCs to the challenge-positive patients almost once a year. As a result, the older patients represented the selection of patients with a refractory allergy.
“Complete avoidance” was identified as a risk factor for a severe allergic reaction. There could be two reasons for this finding. One is a screening effect of the patients who were already tolerant to a small amount of antigen. Another reason may be an immunotherapeutic effect from ingesting antigen. Studies of low-dose oral immunotherapy or sublingual immunotherapy have suggested the effect of ingesting a small amount of antigen to increase the threshold dose.20
For patients with food allergies, the only accepted management worldwide has been the complete avoidance of the allergen.21 Many studies have reported the efficacy of OIT,22, 23, 24, 25, 26, 27, 28, 29 and 30 but not all patient who performed an OIT could gain tolerance without difficulty. Vazquez-Ortiz et al. reported that patients with a low threshold in the beginning tended to exhibit severe reactions during an OIT and could not tolerate the allergen easily. 31
We usually instruct the patients with a low TS/Pro to start a small amount of ingestion.32 This dietary instruction is applied to a patient expected to ingest 2 g or more of boiled egg white safely. After determining the safety of the initial dose at the follow-up visit, we allow them to increase the dose slowly. In this practice, many patients achieved tolerance sooner than those remained on elimination diet.33
On the other hand, patients with higher TS/Pro generally need to continue complete avoidance. The cut-off level of TS/Pro at 31 was almost equivalent to our instruction policy of complete avoidance. For example, when a patient presented with multiple urticaria, intermittent cough and vomiting (TS = 30) after ingesting 8.7 g of boiled egg white (protein 0.98 g), the TS/Pro becomes 30.6.
We examined some other models using alternative cutoff value of TS/Pro or score point assigned to each factor to validate our prediction model. As a result, no other models showed superior discrimination ability with statistical significancy and suitability in the clinical use.
Based on the findings of the present study, we do not recommend performing an OFC for patients with a prediction score of 8 and over. If you decide to perform an OFC to the patient, you may better restrict the dose of antigen for a safer OFC. However, if an OFC is nevertheless performed on these patients, then a smaller dose of antigen should be used for safety reasons.
On the other hand, pediatric allergists may generally perform an OFC for patients with a prediction score 5 and under, because severe reactions might be unexpected. As a result, the ingestion of a small amount of allergen may be given safely.
One limitation of the current study is that it was conducted only at one center specialized for food allergy. The indication of an OFC in our department might have affected proportion of positive result in OFCs, and the cut-off level of a severe allergic reaction (TS/pro > 31). This prediction model can be applied only to an OFC using 20 min boiled egg white because we have not evaluated the data with other egg products or other food allergens. We are now creating the corresponding prediction model for cows milk and wheat allergens.
In conclusion, our prediction model showed a good discrimination of severe allergic reaction based on the TS/Pro. It may therefore be clinically effective for many pediatric allergists to avoid a high-risk OFC and instead encourage a low-risk OFC.
Acknowledgment
This study was partially supported by the Practical Research Project for Allergic Disease and Immunology from Japan Agency for Medical Research and Development, AMED (15ek0410019h0101).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1 S.H. Sicherer; Food allergy; Lancet, 360 (2002), pp. 701–710
- 2 H.A. Sampson; Anaphylaxis and emergency treatment; Pediatrics, 111 (2003), pp. 1601–1608
- 3 A. Urisu, M. Ebisawa, K. Ito, Y. Aihara, S. Ito, M. Mayumi, et al.; Japanese guideline for food allergy 2014; Allergol Int, 63 (2014), pp. 399–419
- 4 A. DunnGalvin, D. Daly, C. Cullinane, E. Stenke, D. Keeton, M. Erlewyn-Lajeunesse, et al.; Highly accurate prediction of food challenge outcome using routinely available clinical data; J Allergy Clin Immunol, 127 (2011), pp. 633–639 e1–3
- 5 A. DunnGalvin, L.M. Segal, A. Clarke, R. Alizadehfar, J.O. Hourihane; Validation of the Cork-Southampton food challenge outcome calculator in a Canadian sample; J Allergy Clin Immunol, 131 (2013), pp. 230–232
- 6 A. Hino, T. Maeda, Y. Haneda, T. Kobayashi, M. Yasui, N. Kando, et al.; [Establishment of “Anaphylaxis Scoring Aichi (ASCA),” a new symptom scoring system to be used in an oral food challenge (OFC)]; Arerugi, 62 (2013), pp. 968–979 (in Japanese)
- 7 T. Komata, L. Soderstrom, M.P. Borres, H. Tachimoto, M. Ebisawa; The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age; J Allergy Clin Immunol, 119 (2007), pp. 1272–1274
- 8 T. Nomura, Y. Kanda, T. Kato, T. Sobajima, T. Morishita, S. Sugiura, et al.; Probability curves focusing on symptom severity during an oral food challenge; Ann Allergy Asthma Immunol, 112 (2014), pp. 556–557 e2
- 9 T.T. Perry, E.C. Matsui, M.K. Conover-Walker, R.A. Wood; Risk of oral food challenges; J Allergy Clin Immunol, 114 (2004), pp. 1164–1168
- 10 K. Ito, M. Futamura, M.P. Borres, Y. Takaoka, J. Dahlstrom, T. Sakamoto, et al.; IgE antibodies to omega-5 gliadin associate with immediate symptoms on oral wheat challenge in Japanese children; Allergy, 63 (2008), pp. 1536–1542
- 11 M. Ebisawa, R. Movérare, S. Sato, M.P. Borres, K. Ito; The predictive relationship between peanut- and Ara h 2-specific serum IgE concentrations and peanut allergy; J Allergy Clin Immunol Pract, 3 (2015), pp. 131–132
- 12 A. Cianferoni, J.P. Garrett, D.R. Naimi, K. Khullar, J.M. Spergel; Predictive values for food challenge-induced severe reactions: development of a simple food challenge score; Isr Med Assoc, 14 (2012), pp. 24–28
- 13 J.M. Spergel, J.L. Beausoleil, J.M. Fiedler, J. Ginsberg, K. Wagner, N.A. Pawlowski; Correlation of initial food reactions to observed reactions on challenges; Ann Allergy Asthma Immunol, 92 (2004), pp. 217–224
- 14 E.R. DeLong, D.M. DeLong, D.L. Clarke-Pearson; Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach; Biometrics, 44 (1988), pp. 837–845
- 15 H. Ando, R. Moverare, Y. Kondo, I. Tsuge, A. Tanaka, M.P. Borres, et al.; Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy; J Allergy Clin Immunol, 122 (2008), pp. 583–588
- 16 Y. Haneda, N. Kando, M. Yasui, T. Kobayashi, T. Maeda, A. Hino, et al.; Ovomucoids IgE is a better marker than egg white-specific IgE to diagnose boiled egg allergy; J Allergy Clin Immunol, 129 (2012), pp. 1681–1682
- 17 L.H. Christensen, J. Holm, G. Lund, E. Riise, K. Lund; Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge; J Allergy Clin Immunol, 122 (2008), pp. 298–304
- 18 G.J. Sturm, A. Heinemann, C. Schuster, M. Wiednig, A. Groselj-Strele, E.M. Sturm, et al.; Influence of total IgE levels on the severity of sting reactions in Hymenoptera venom allergy; Allergy, 62 (2007), pp. 884–889
- 19 K. Horimukai, K. Hayashi, Y. Tsumura, I. Nomura, M. Narita, Y. Ohya, et al.; Total serum IgE level influences oral food challenge tests for IgE-mediated food allergies; Allergy, 70 (2015), pp. 334–337
- 20 C.A. Keet, P.A. Frischmeyer-Guerrerio, A. Thyagarajan, J.T. Schroeder, R.G. Hamilton, S. Boden, et al.; The safety and efficacy of sublingual and oral immunotherapy for milk allergy; J Allergy Clin Immunol, 129 (2012), pp. 448–455 e1–5
- 21 H.A. Sampson; Food allergy. Part 2: diagnosis and management; J Allergy Clin Immunol, 103 (1999), pp. 981–989
- 22 R.A. Wood; Food-specific immunotherapy: past, present, and future; J Allergy Clin Immunol, 121 (2008), pp. 336–337
- 23 A. Nowak-Wegrzyn, H.A. Sampson; Future therapies for food allergies; J Allergy Clin Immunol, 127 (2011), pp. 558–573 quiz 74–5
- 24 U. Staden, C. Rolinck-Werninghaus, F. Brewe, U. Wahn, B. Niggemann, K. Beyer; Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction; Allergy, 62 (2007), pp. 1261–1269
- 25 J.M. Skripak, S.D. Nash, H. Rowley, N.H. Brereton, S. Oh, R.G. Hamilton, et al.; A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cows milk allergy; J Allergy Clin Immunol, 122 (2008), pp. 1154–1160
- 26 K. Beyer, U. Wahn; Oral immunotherapy for food allergy in children; Curr Opin Allergy Clin Immunol, 8 (2008), pp. 553–556
- 27 A. Buchanan, T. Green, S. Jones, A. Scurlock, L. Chrisite, K. Althage, et al.; Egg oral immunotherapy in nonanaphylactic children with egg allergy; J Allergy Clin Immunol, 119 (2007), pp. 199–205
- 28 A.W. Burks, S.M. Jones, R.A. Wood, D.M. Fleischer, S.H. Sicherer, R.W. Lindblad, et al.; Oral immunotherapy for treatment of egg allergy in children; N Engl J Med, 367 (2012), pp. 233–243
- 29 P. Meglio, P.G. Giampietro, R. Carello, I. Gabriele, S. Avitabile, E. Galli; Oral food desensitization in children with IgE-mediated hens egg allergy: a new protocol with raw hens egg; Pediatr Allergy Immunol, 24 (2013), pp. 75–83
- 30 P. Ojeda, I. Ojeda, G. Rubio, F. Pineda; Home-based oral immunotherapy protocol with pasteurized egg for children allergic to hens egg; Isr Med Assoc J, 14 (2012), pp. 34–39
- 31 M. Vazquez-Ortiz, M. Alvaro-Lozano, L. Alsina, M.B. Garcia-Paba, M. Piquer-Gibert, M.T. Giner-Munoz; Safety and predictors of adverse events during oral immunotherapy for milk allergy: severity of reaction at oral challenge, specific IgE and prick test; Clin Exp Allergy, 43 (2013), pp. 92–102
- 32 K. Ito; Diagnosis of food allergies: the impact of oral food challenge testing; Asia Pac Allergy, 3 (2013), pp. 59–69
- 33 T. Kobayashi, N. Kando, Y. Haneda, M. Yasui, T. Maeda, A. Hino, et al.; [Diet instructions to increase the tolerated dose in patients with positive egg challenge test results (2nd report)]; [Jpn J Pediatr Allergy Clin Immunol], 27 (2013), pp. 692–700 (in Japanese)
Document information
Published on 05/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?