Summary
Objectives
Thymomas are relatively rare tumors. In this study, we investigated the clinical features of patients who underwent surgical resection for thymoma.
Patients and methods
This study clinicopathologically evaluated 54 consecutive patients who underwent a surgical resection of thymoma in our department between 1994 and 2006.
Results
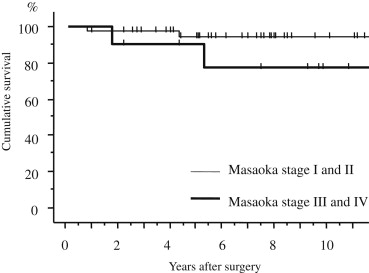
A complete resection was performed in 52 patients, while two patients underwent an incomplete resection due to pleural dissemination. Combined resection with adjacent organs was performed for the lung (n = 6), pericardium (n = 5), and large vessels (brachiocephalic vein in three, superior vena cava in two). The concomitant autoimmune diseases were observed in 20 patients (37%), and they included myasthenia gravis in 17 patients, macroglobulinemia in one, pemphigus vulgaris in one, and stiff person syndrome in one patient. The histologic types of the World Health Organization classification diagnosed as type A in four patients, type AB in 14, type B1 in eight, type B2 in 15, and type B3 in 11. There were 27, 17, eight, and two patients with Masaoka stages I, II, III, and IV, respectively. Four patients died, and the causes of death included recurrence of thymoma in two, gastric carcinoma in one, and respiratory failure due to myasthenia gravis in one patient. The overall survival rate at 10 years was 94.6% in patients with stages I and II disease and 77.1% in patients with stages III and IV disease.
Conclusions
Long-term survival can be expected not only for patients at early stages, as well as for patients with stages III and IV disease if surgical resection is completed macroscopically.
Keywords
autoimmune disease;mediastinal tumor;prognosis;surgical resection;thymoma
1. Introduction
Thymoma is a relatively rare tumor, with an incidence of 0.15 cases per 100,000. The neoplasm arises from thymic epithelial cells.1 The site of predilection for thymoma is usually in the thymus at the anterior mediastinum. This location accounts for 20%–40% of mediastinal tumors and is the most common tumor in the anterior mediastinum.2 ; 3 It is normally a slow-growing tumor, but it shows a locally invasive growth pattern. In addition, it sometimes leads to the development of pleural dissemination and distant metastasis.4 Surgery is the treatment of choice for this disease, and complete resection is the most important prognostic factor.4; 5 ; 6 The staging of thymomas is commonly performed based on the Masaoka classification, and the clinical staging also predicts the prognosis.7 However, no optimal treatment strategy has yet been established. In this study, we retrospectively reviewed the clinicopathologic characteristics of 54 consecutive patients who underwent surgical resection for thymoma.
2. Patients and methods
The hospital records of 54 consecutive patients who underwent a surgical resection of thymoma in the Second Department of Surgery, University of Occupational and Environmental Health (Kiatkyushu, Japan) between 1994 and 2006 were reviewed. The preoperative assessments included chest roentgenography and computed tomography (CT) of the chest, upper abdomen, and brain. Patient records, including clinical data, preoperative examination results, details of any surgeries, histopathologic findings, and Masaoka staging system classification were also reviewed.7 The histologic findings were classified according to the World Health Organization (WHO) criteria of cell types.8
Follow-up information was obtained from all patients through office visits or telephone interviews, either with the patients, with a relative, or with their primary physicians. The patients were evaluated every 3 months by chest roentgenography, and chest CT scans were performed every 6 months for the first 2 years after surgery and annually thereafter.
The Kaplan-Meier method was used to estimate the probability of survival, and survival differences were analyzed by the log-rank test. The categorical variables were compared using the chi-square test or Fishers exact test. Differences were considered to be statistically significant for p < 0.05. The data were analyzed using the StatView software package (Abacus Concepts, Inc., Berkeley, CA, USA).
3. Results
There were 26 men and 28 women studied who had a mean age of 60.5 years and an age range of 19–79 years (Table 1). Fifty-two patients underwent complete resection, and two patients had pleural dissemination that resulted in an incomplete resection. Among the patients who underwent a complete resection, combined resection with adjacent organs was performed for the lung (n = 6), pericardium (n = 5), and vessels (brachiocephalic vein in three, superior vena cava in two). The histologic types based on the WHO classification included four patients with type A (7.7%), 14 with type AB (26.9%), eight with type B1 (15.4%), 15 with type B2 (28.8%), and 11 with type B3 (21.1%). The histologic types of two patients were not determined because the subjects had received preoperative radiation. In the Masaoka staging system, 27 patients (50.0%) were diagnosed to have stage I disease, 17 with stage II (31.5%), eight with stage III (14.8%), and two with stage IVa (3.7%). The youngest patients (a woman aged 19 years) was diagnosed at Masaoka stage III, and a complete resection was performed with combined resection of brachiocephalic vein.
| All | Without MGn = 37 | With MGn = 17 | |
|---|---|---|---|
| Average of age, y (range) | 60.5 (19–76) | 63.1 | 54.1* |
| Sex: male/female | 26/28 | 19/18 | 7/10 |
| WHO histologic classification | |||
| A | 4 | 3 (9) | 1 (6) |
| AB | 14 | 10 (29) | 4 (24) |
| B1 | 8 | 6 (17) | 2 (12) |
| B2 | 15 | 7 (20) | 8 (53)** |
| B3 | 11 | 9 (26) | 2 (12) |
| Masaoka stage | |||
| I | 27 | 17 (46) | 10 (59) |
| II | 17 | 12 (32) | 5 (29) |
| III | 8 | 6 (16) | 2 (12) |
| IVa | 2 | 2 (5) | 0 |
MG = myasthenia gravis.
- p = 0.019, the average age of patients with MG was significantly lower than that of patients without MG.
- p = 0.043, the prevalence of MG was observed significantly in type B2 thymoma compared with that in other types of thymoma.
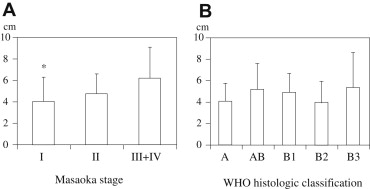
The correlation between Masaoka stage and WHO classification was revealed in Table 2. The patients with Masaoka stage I and II was classified into one of the five histologic types of WHO classification. However, the thymomas at Masaoka stage III and IVa corresponded to either B2 or B3 subtype, indicating more aggressive histology. The higher prevalence of early stages (Masaoka stages I and II) was observed in A and AB-type thymomas (18/18), while the proportion of Masaoka stages I and II was 76.5 % (26/34) in B1 through B3 thymomas (p = 0.039). The correlation between tumor size and Masaoka stage is indicated in Fig. 1A. The average tumor size in Masaoka stage I was significantly smaller than that in Masaoka stages III–IV. No significant correlation was observed between tumor size and WHO histologic classification (Fig. 1B). The concomitant autoimmune diseases were myasthenia gravis (MG) in 17 patients, macroglobulinemia in one, pemphigus vulgaris in one, and stiff person syndrome in one. In comparison between presence and absence of MG, the average age of patients with MG was significantly lower than that of patients without MG. In particular, four out of five patients younger than 40 years of age were diagnosed in association with MG. The prevalence of MG was significantly higher in type B2 thymoma than those in other types of thymoma.
| WHO histologic classification | |||||||
|---|---|---|---|---|---|---|---|
| A | AB | B1 | B2 | B3 | |||
| Masaoka stage | I | (n = 27) | 3 | 9 | 5 | 8 | 2 |
| II | (n = 17) | 1 | 5 | 3 | 6 | 2 | |
| III | (n = 6) | 0 | 0 | 0 | 1 | 5 | |
| IV | (n = 2) | 0 | 0 | 0 | 0 | 2 | |
WHO = World Health Organization.
|
|
|
Figure 1. Tumor size according to Masaoka stage or WHO histologic classification. (A) Correlation between tumor size and Masaoka stage; (B) correlation between tumor size and WHO histologic classification. The results are expressed as the average values plus standard deviation. *p = 0.023, the tumor size in Masaoka stage I was significantly smaller than that in Masaoka stages III and IV. WHO = World Health Organization. |
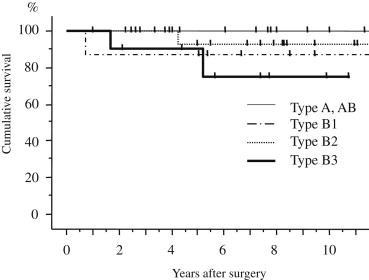
There was no postoperative mortality, and no morbidity. Radiation therapy was performed before surgery in four patients (one patient with stage I, three with stage III). Postoperative radiation therapy to the anterior mediastinum was performed in three patients with stage II disease, four with stage III, and one with stage IV disease. Two patients with stage IV and one patient with stage III disease underwent postoperative chemotherapy with carboplatin plus etoposide. Four patients died, and the causes of death included recurrence of thymoma in two, gastric carcinoma in one, and respiratory failure due to MG in one patient. The overall survival rate at 10 years was 94.6% in patients with stages I and II disease. The patients with stages III and IV disease showed a poorer prognosis (77.1% survival) than the patients with stages I and II disease (p = 0.129; Fig. 2). The overall survival rates at 10 years were 100% in WHO type A or AB patients, 87.5% in WHO B1, 92.0% in WHO B2, and 75.0% in WHO B3 patients (Fig. 3). The patients with type A or AB classifications showed no recurrent disease.
|
|
|
Figure 2. Overall survival curves after surgery according to the Masaoka stage. The overall survival rates at 10 years were 94.6% and 77.1% in patients with stages I and II and stages III and IV disease, respectively. The patients with stages III and IV disease showed a poorer prognosis than patients at an earlier stage (I and II; p = 0.129). |
|
|
|
Figure 3. Overall survival curves after surgery according to the WHO classification. The overall survival rates at 10 years were 100% in WHO types A or AB patients, 87.5% in WHO type B1, 92.0% in WHO type B2, and 75.0% in WHO type B3 patients. WHO = World Health Organization. |
4. Discussion
Thymoma is largely characterized by an indolent growth pattern and often shows very slow progression. However, it has malignant potential and sometimes invades surrounding structures and disseminates into the pleural cavity. The mainstay of therapy is complete surgical resection for early stage disease. It has been reported that complete resection of thymoma was significantly associated with a better prognosis.4; 5 ; 6 In the present study, 52 patients (96%) underwent a complete resection, and the combined resection of adjacent organs was performed in 16 patients (29.6%). In the patients with disseminated pleural nodules, we attempted to resect all of the disseminated nodules macroscopically. In spite of its local invasiveness, lymphogenous and hematogenous spread of thymoma is rare.4 We experienced only one patient who developed metastases in the lung, liver, and bone 15 months after surgery.
The Masaoka staging system is based on the degree of invasion of the tumor through the capsule into the surrounding structures, and is an important prognostic factor to determine the optimal therapeutic method for the patient.7 ; 9 The patients with stages III and IV disease showed a poorer prognosis than patients with stages I and II disease in the present study; however, there was no significant difference. It is thought to be due to small number of patients in each Masaoka stage. The average tumor size was correlated with Masaoka stage in the present study. The histologic grade of aggressiveness is also an important factor for predicting the prognosis. In 1999, the WHO classification was created, and thymic epithelial tumors are classified into types A, AB, B1, B2, B3, and C (thymic carcinoma) according to the WHO criteria. The criteria are useful for determining the treatment regimens and predicting patient survival. The WHO classification has a mild correlation with the Masaoka staging, indicating that there is a trend for more malignant WHO cell types was associated with more advanced Masaoka stages. All patients with WHO type A or AB had a diagnosis of either Masaoka stages I or II in the present study. However, the patients with Masaoka stage I included WHO classifications of types A, AB, B1, B2, and B3 in the present study. Several studies have demonstrated a consensus that the Masaoka staging, WHO histologic classification, and completeness of surgical resection were significant prognostic factors.10 ; 11
Thymomas are often associated with a number of immune- and nonimmune-mediated paraneoplastic syndromes. Almost 40% of patients with thymoma develop an autoimmune-paraneoplastic syndrome.12 MG is the most common of these syndromes. In the present study, 20 patients (37%) had parathymic autoimmune disease. The average age of patients with MG was significantly lower than that of patients without MG. In particular, four out of five patients younger than 40 years of age were diagnosed in association with MG. In a clinical review of 1089 patients with thymoma, Kondo et al6 reported a 25% incidence of MG. There was no overall survival difference between patients with or without MG. Hypogammaglobulinemia and pure red cell aplasia have also often been reported to be concomitant autoimmune diseases.13 Surgery alone is considered to be curative treatment for stage I thymoma, and it results in 10-year overall survival rates of almost 100%. However, there is little randomized evidence to guide the treatment for stages II–IV disease. There is still controversy regarding the value of postoperative prophylactic radiotherapy for patients with stage II disease.14; 15 ; 16 Furthermore, late local morbidities associated with mediastinal irradiation are well known (such as cardiac morbidities such as valve fibrosis, pericarditis with pericardial effusions, increased frequency of coronary artery disease, radiation pneumonia, chronic pulmonary fibrosis).17 For stage III thymomas, a one-stage operation is generally performed when a complete resection by combined resection of the lung and pericardium can be expected. Regarding postoperative radiotherapy, several studies have reported that it improved the prognosis or inhibited local recurrence.18 ; 19 Radiotherapy may also be useful as adjuvant therapy in cases of incomplete surgical resection with microscopic or macroscopic residual disease. Several reports demonstrated the effectiveness of chemotherapy as one arm of multidisciplinary treatment in patients with advanced thymoma.20 However, no sufficient evidence exists regarding the effectiveness of adjuvant chemotherapy, and there is currently no standardized regimen for chemotherapy. Nevertheless, chemotherapy is considered a valid option in selected patients with residual disease after local treatments, or as a neoadjuvant approach to improve the resectability in patients with Masaoka stages III or IV-a thymoma.21 ; 22
The most common recurrence pattern of stage III thymoma is pleural dissemination, but opinions are divided as to the clinical significance of the resection of recurrent lesions. Hanuda et al23 indicated that reoperation was not significantly effective for prolongation of the postrecurrence survival for patients with recurrent thymoma. Although pleuropneumonectomy for Masaoka stage IVa thymoma is associated with a high morbidity and mortality rate, pleuropneumonectomy or total resection of minimal pleural dissemination may provide good long-term survival in highly selected patients.24 ; 25
The WHO classification of thymoma has correlation with Masaoka stage, and the prevalence of MG was significantly higher in type B2 thymoma those that in other types of thymoma. Long-term survival can be expected not only for patients at early stages, but also for patients with stages III and IV disease if surgical resection is completed macroscopically. There have so far been only a few clinical trials regarding adjuvant chemotherapy or radiotherapy for thymoma because of its low incidence. Further accumulation of clinical experience with the disease and establishment of an effective treatment strategy are needed for improvement of the quality of life of patients because long-term survival can be expected not only for patients with early stage disease, but also for those with advanced disease.
Acknowledgments
This study was supported in part by a University of Occupational and Environmental Health (UOEH) Research Grant for the Promotion of Occupational Health and Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1 E.A. Engels, R.M. Pfeiffer; Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies; Int J Cancer, 105 (2003), pp. 546–551
- 2 B.V. Duwe, D.H. Sterman, A.I. Musani; Tumors of the mediastinum; Chest, 128 (2005), pp. 2893–2909
- 3 R. Sakata, Y. Fujii, H. Kuwano; Thoracic and cardiovascular surgery in Japan during 2008: annual report by The Japanese Association for Thoracic Surgery; Gen Thorac Cardiovasc Surg, 58 (2010), pp. 356–383
- 4 C.R. Thomas, C.D. Wright, P.J. Loehrer; Thymoma: state of the art; J Clin Oncol, 17 (1999), pp. 2280–2289
- 5 A. Kurup, P.J. Loehrer Sr.; Thymoma and thymic carcinoma: therapeutic approaches; Clin Lung Cancer, 6 (2004), pp. 28–32
- 6 K. Kondo, Y. Monden; Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan; Ann Thorac Surg, 79 (2005), pp. 219–224
- 7 A. Masaoka, Y. Monden, K. Nakahara, T. Tanioka; Follow-up study of thymomas with special reference to their clinical stages; Cancer, 48 (1981), pp. 2485–2492
- 8 World Health Organization (WHO); WHO Classification of Tumors: Pathology and Genetics of Tumors of Lung, Pleura, Thymus and Heart; International Agency for Research on Cancer (IARC) Press, Lyon, France (2004)
- 9 A. Masaoka; Staging system of thymoma; J Thorac Oncol, 5 (2010), pp. S304–S312
- 10 K. Kondo, K. Yoshizawa, M. Tsuyuguchi, et al.; WHO histologic classification is a prognostic indicator in thymoma; Ann Thorac Surg, 77 (2004), pp. 1183–1188
- 11 O. Rena, E. Papalia, G. Maggi, et al.; World Health Organization histologic classification: an independent prognostic factor in resected thymomas; Lung Cancer, 50 (2005), pp. 59–66
- 12 A. Marx, P. Hohenberger, H. Hoffmann, et al.; The autoimmune regulator AIRE in thymoma biology: autoimmunity and beyond; J Thorac Oncol, 5 (2010), pp. S266–S272
- 13 J. Chen, Y. Yang, D. Zhu, et al.; Thymoma with pure red cell aplasia and Goods syndrome; Ann Thorac Surg, 91 (2011), pp. 1620–1622
- 14 S. Singhal, J.B. Shrager, D.I. Rosenthal, V.A. LiVolsi, L.R. Kaiser; Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation; Ann Thorac Surg, 76 (2003), pp. 1635–1641
- 15 O. Rena, E. Papalia, A. Oliaro, et al.; Does adjuvant radiation therapy improve disease-free survival in completely resected Masaoka stage II thymoma?; Eur J Cardiothorac Surg, 31 (2007), pp. 109–113
- 16 Y. Kundel, A. Yellin, A. Popovtzer, et al.; Adjuvant radiotherapy for thymic epithelial tumor: treatment results and prognostic factors; Am J Clin Oncol, 30 (2007), pp. 389–394
- 17 G. Kleikamp, U. Schnepper, R. Korfer; Coronary artery disease and aortic valve disease as a long-term sequel of mediastinal and thoracic irradiation; J Thorac Cardiovasc Surg, 45 (1997), pp. 27–31
- 18 J.H. Chang, H.J. Kim, H.G. Wu, J.H. Kim, Y.T. Kim; Postoperative radiotherapy for completely resected stage II or III thymoma; J Thorac Oncol, 6 (2011), pp. 1282–1286
- 19 K. Ogawa, T. Uno, T. Toita, et al.; Postoperative radiotherapy for patients with completely resected thymoma: a multi-institutional, retrospective review of 103 patients; Cancer, 94 (2002), pp. 1405–1413
- 20 K. Yokoi, H. Matsuguma, R. Nakahara, et al.; Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone; J Thorac Oncol, 2 (2007), pp. 73–78
- 21 L. Spaggiari, M. Casiraghi, J. Guarize; Multidisciplinary treatment of malignant thymoma; Curr Opin Oncol, 24 (2012), pp. 117–122
- 22 E.S. Kim, J.B. Putnam, R. Komaki, et al.; Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report; Lung Cancer, 44 (2004), pp. 369–379
- 23 M. Haniuda, R. Kondo, H. Numanami, A. Makiuchi, E. Machida, J. Amano; Recurrence of thymoma: clinicopathological features, re-operation, and outcome; J Surg Oncol, 78 (2001), pp. 183–188
- 24 D. Fabre, E. Fadel, S. Mussot, et al.; Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma; Eur J Cardiothorac Surg, 39 (2011), pp. e133–e138
- 25 J.F. Regnard, F. Zinzindohoue, P. Magdeleinat, L. Guibert, L. Spaggiari, P. Levasseur; Results of re-resection for recurrent thymomas; Ann Thorac Surg, 64 (1997), pp. 1593–1598
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?