Abstract
Pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives were synthesized via a three component reaction of arylaldehydes, 4-phenylurazole and malononitrile in the presence of a catalytic amount of 2-hydroxyethylammonium formate and 2-hydroxyethylammonium acetate as effective mild basic ionic liquids, without using any additional co-catalyst, under solvent-free conditions at room temperature in good yields. Ionic liquids as catalysts were recovered and reused. In addition, the preparation of 2-amino-3-cyano-5,10-dioxo-4-phenyl-5,10-dihydro-4H-benzo[g]chromene derivatives from the reaction of arylaldehydes, malononitrile and 2-hydroxy-1,4-dihydronaphthalene-1,4-dione under solvent-free conditions at ambient temperature in the presence of mentioned catalysts is reported.
Keywords
2-hydroxyethylammonium formate ; 2-hydroxyethylammonium acetate ; Ionic liquids ; Catalyst
1. Introduction
Ionic liquids are salts consisting of ions, which exist in a liquid state at ambient temperature [1] , and show reasonably high ionic conductivity. The first ionic liquid, ethylammonium nitrate (mp 12 °C), was reported as early as 1914 [2] . On the other hand, Ionic Liquids (ILs), possessing advantages such as undetectable vapour pressure and excellent reusability, have been investigated extensively in organic transformations as solvents or catalysts [3] . Thus, various ILs have been used as reaction solvents as well as catalysts for green organic synthesis [4] . Ionic liquids, 2-hydroxyethylammonium formate and 2-hydroxyethylammonium acetate were simply prepared by a neutralization reaction of 2-hydroxyethylamine with formic acid or acetic acid, which have much lower cost, melting point, and viscosity [5] and [6] .
In continuation of our research on the application of catalysts in green organic synthesis [7] , [8] , [9] and [10] , we, herein, report some applications of 2-hydroxyethylammonium formate, [H3 N+ –CH2 –CH2 –OH][HCOO− ], and 2-hydroxyethylammonium acetate [H3 N+ –CH2 –CH2 –OH][C H3 COO− ] as recoverable ionic liquids for the synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione and 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo[g]chromene derivatives (Scheme 1 ).
Scheme 1.
The preparation of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione and 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo[g]chromene derivatives.
2. Results and discussion
In order to carry out the preparation of 7-amino-6-cyano-1,3-dioxo-2,5-diphenyl-1,2,3,5-tetrahydropyrazolo[1,2-a][1,2,4]triazole in a more efficient way, minimizing the time and amount of catalysts, the reaction of benzaldehyde (1.0 mmol), malononitrile (1.0 mmol), 4-phenylurazole (1.0 mmol) was selected as a model system. The different amounts of ionic liquid as a catalyst (0.17, 0.19, 0.24, 0.27, 0.30 mmol) were investigated. The best result was obtained with 2-hydroxyethylammoniumformate (0.27 mmol) and 2-hydroxyethylammoniumacetate (0.24 mmol) under ambient conditions (Table 1 ).
| Entry | Catalyst (mmol) | Time (min) | Yield (%)a | |||
|---|---|---|---|---|---|---|
| A | B | A | B | A | B | |
| 1 | 0.17 | 0.17 | 30 | 18 | 60 | 60 |
| 2 | 0.19 | 0.19 | 25 | 15 | 60 | 60 |
| 3 | 0.24 | 0.24 | 20 | 8 | 93 | 93 |
| 4 | 0.27 | 0. 27 | 10 | 8 | 93 | 93 |
| 5 | 0.30 | 0.30 | 10 | 8 | 93 | 93 |
a. Yields refer to pure isolated product.
Using this optimized reaction, the scope and efficiency of the reaction were explored for the synthesis of a wide variety of substituted pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives (Table 2 ). Interestingly, a variety of arylaldehydes, including electron withdrawing or releasing substituents (ortho -, meta -, and para -substituted), participated well in this reaction and gave the desired products in good to excellent yield. We examined aliphatic aldehyde, such as -heptanal and -octanal, instead of benzaldehyde in the reaction. All the starting materials in the reaction almost were intact, only a trace product was formed without any side products after 24 h (Table 2 , entries 11, 12).
| Entry | R | Time (min) | Yield (%)a | Found M.P(°C )/ [Lit. M.P (°C )] [Ref.] | ||
|---|---|---|---|---|---|---|
| A | B | A | B | |||
| 1 | 10 | 8 | 93 | 93 | >210/[>210] [11] | |
| 2 | 8 | 6 | 80 | 80 | >224/[>224] [11] | |
| 3 | 8 | 6 | 85 | 85 | >214/[>214] [11] | |
| 4 | 6 | 4 | 85 | 85 | >221/[>221] [11] | |
| 5 | 9 | 7 | 70 | 70 | >221/[>221] [11] | |
| 6 | 5 | 3 | 75 | 75 | >223/[>223] [11] | |
| 7 | 10 | 8 | 70 | 70 | >217/[>217] [11] | |
| 8 | 5 | 3 | 90 | 90 | 218/[>218] [11] | |
| 9 | 5 | 3 | 90 | 90 | 215/[>215] [11] | |
| 10 | 6 | 3 | 90 | 90 | 250–252 New product | |
| 11 | n-heptanal | 24 h | – | Trace | – | – |
| 12 | n-octanal | 24 h | – | Trace | – | – |
a. Yields refer to the isolated pure products. The desired pure products were characterized by comparison of their physical data (melting points, IR, 1 H and 13 C NMR) with those of known compounds.
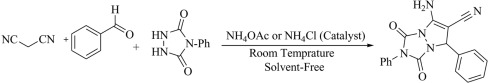
To expand our study, we used ammonium acetate (24 mol%) and ammonium chloride (24 mol%) as a catalyst instead of the mentioned ionic liquids in the preparation of 7-amino-6-cyano-1,3-dioxo-2,5-diphenyl-1,2,3,5-tetrahydropyrazolo[1,2-a][1,2,4]triazole (Scheme 2 ). The results showed us that the desired product was obtained at 28 and 30 min, with 75% and 73% yields, respectively.
Scheme 2.
The preparation of 7-amino-6-cyano-1,3-dioxo-2,5-diphenyl-1,2,3,5-tetrahydropyrazolo[1,2-a][1,2,4]triazole using ammonium acetate and ammonium chloride as catalysts under solvent-free at ambient conditions.
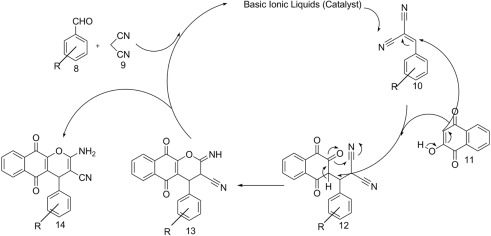
According to a literature survey [11] , the suggested mechanism for the formation of products is shown in Scheme 3 . First, the standard Knoevenagel condensation of malononitrile (1) and arylaldehydes (2) in the presence of the catalyst (ILs) was afforded benzylidenemalononitrile (3). Then, conjugated addition of 4-phenylurazole (4) to (3) creates an intermediate (5). Finally, cyclization and tautomerism affords the corresponding products. As shown in Scheme 3 , aliphatic aldehydes cannot form stable intermediates in the pathway of the mechanism. Thus, only a trace product was obtained.
Scheme 3.
Proposed mechanism for preparation of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione derivatives.
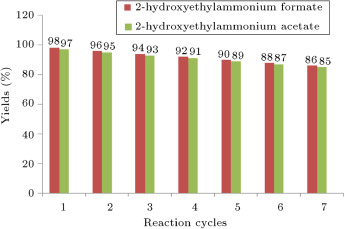
Recovery of the catalysts is important in green organic synthesis. Thus, for recycling the catalysts, after washing the solid products with water completely, the water containing ionic liquid (ILs is soluble in water) was evaporated under reduced pressure, and the ionic liquids were recovered and reused. The recovered catalysts were reused seven times without any loss of activity (Figure 1 ).
|
|
|
Figure 1. Investigation the recycling of the ionic liquids in the synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione. |
In order to show the accessibility of the present work in comparison with only one reported result in the literature [11] , we show that the present ionic liquids are efficient catalysts, with respect to reaction time and obtained product yield (Table 3 ). We also compare the results of ammonium acetate and ammonium chloride as a catalyst with the mentioned ionic liquids (Table 3 , entries 5, 6). We observed that the rate of reaction, and also yield, of the products are lower than of ionic liquids. In addition, NH4 OAc and NH4 Cl are not reusable catalysts. Triethylamine (20 mol%), as a catalyst, has been used in the synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-diones; solvent-free under ambient conditions (Table 3 , entry 2), but a trace product was obtained [11] .
| Entry | Catalyst (mol%) | Conditions | Time (min) | Yield (%) [Ref.] |
|---|---|---|---|---|
| 1 | Triethylamine (20 mol%) | Ultrasonic-irradiation conditions, 35 kHz, 70 W, EtOH as solvent, 50 °C | 45 | 93 [11]a |
| 2 | Triethylamine (20 mol%) | Solvent free, ambient conditions | 120 | Trace [11]a |
| 3 | 2-Hydroxyethylammonium formate (27 mol%) | Solvent-free, ambient conditions | 10 | 93b present work |
| 4 | 2-Hydroxyethylammonium acetate (24 mol%) | Solvent free, ambient conditions | 8 | 93b present work |
| 5 | Ammonium acetate (24 mol%) | Solvent free, ambient conditions | 28 | 75a |
| 6 | Ammonium chloride (24 mol%) | Solvent free, ambient conditions | 30 | 73a |
a. Triethylamine, ammonium acetate, and ammonium chloride aren’t reusable catalysts.
b. Ionic liquids, 2-hydroxyethylammonium formate and 2-hydroxyethylammonium acetate are reusable catalysts.
In continuation of our research on application of the mentioned ionic liquids, we tried to synthesize 2-amino-3-cyano-5,10-dioxo-4-phenyl-5,10-dihydro-4H-benzo[g]chromene derivatives. First, we optimized the amount of catalyst in the three component reaction between benzaldehyde (1.0 mmol), malononitrile (1.0 mmol), and 2-hydroxy-1,4-dihydronaphthalene-1,4-dione. The best result was obtained with 2-hydroxyethylammoniumformate (0.26 mmol) and 2-hydroxyethylammoniumacetate (0.23 mmol) under ambient conditions.
Next, the three-component condensation reaction of aromatic aldehydes, 2-hydroxynaphthalene-1,4-dione, and malononitrile, under optimized conditions, for preparation of 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo[g]chromene, was investigated (Table 4 ). The wide range of substituted and structurally diverse aldehydes synthesizes the corresponding products in high to excellent yields using the two basic ionic liquids as catalysts (Table 4 ). Then, we examined aliphatic aldehydes, such as -heptanal, instead of benzaldehyde in the reaction. All starting materials in the reaction almost were intact, only a trace product was formed without any side products after 24 h (Table 4 , entry 18).
| Entry | R | Time (min) | Yield (%)a | Found M.P (°C )/ [Lit. M.P (°C )] [Ref.] | ||
|---|---|---|---|---|---|---|
| A | B | A | B | |||
| 1 | 11 | 12 | 90 | 90 | 260–262 / [260–262] [12,13] | |
| 2 | 9 | 11 | 80 | 80 | 252–254 / [253–255] [12,13] | |
| 3 | 9 | 11 | 80 | 80 | 258–259 / [259–260] [12,13] | |
| 4 | 11 | 12 | 85 | 85 | 247–248 / [247–249] [12,13] | |
| 5 | 8 | 6 | 85 | 85 | 234–235 / [234–235] [12,13] | |
| 6 | 6 | 4 | 85 | 85 | 278–280 / [278–280] [12,13] | |
| 7 | 9 | 7 | 80 | 80 | 236–239 / [236–239] [12,13] | |
| 8 | 10 | 11 | 85 | 85 | 293–295 / [293–295] [12,13] | |
| 9 | 6 | 8 | 75 | 75 | 286–288 / [286–288] [12,13] | |
| 10 | 10 | 8 | 80 | 80 | 242–244 / [242–244] [12,13] | |
| 11 | 10 | 11 | 90 | 90 | 247–248 / [247–248] [12,13] | |
| 12 | 11 | 12 | 85 | 85 | 247–248 / [247–248] [12,13] | |
| 13 | 12 | 13 | 90 | 90 | 286–288 / [286–288] [12,13] | |
| 14 | 10 | 9 | 80 | 80 | 254–256 / [254–256] [12,13] | |
| 15 | 9 | 11 | 90 | 90 | 248–250 New product | |
| 16 | 8 | 10 | 85 | 85 | 278–280 New product | |
| 17 | 10 | 11 | 85 | 85 | 232–234 New product | |
| 18 | n-heptanal | 24 h | – | Trace | – | – |
a. Yields refer to the isolated pure products. The desired pure products were characterized by comparison of their physical data (melting points, IR, 1 H and 13 C NMR) with those of known compounds.
The formation of the product can be rationalized by an initial Knoevenagel condensation reaction between an aldehyde (8) and malononitrile (9). Then, the obtained intermediate (10) was attacked by the 2-hydroxynaphthalene-1,4-dione (11), which lead to the intermediate (12), and, subsequently, cyclization generates (13), which isomerizes to the desired product (14) (Scheme 4 ). Resonance structures and conjugation cause formation of stable intermediates using aromatic aldehydes, whereas these stable intermediates were not formed using aliphatic aldehydes (Scheme 4 ).
Scheme 4.
Proposed mechanism for preparation of 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo[g]chromene derivatives.
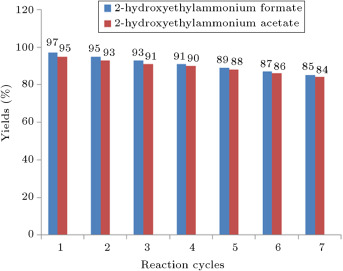
Recycling of the ionic liquids was studied as in the above procedure in the selected model. The recovered catalysts were reused seven times without any loss of activity (Figure 2 ).
|
|
|
Figure 2. Investigation the recycling of the ionic liquids in the synthesis of 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo[g]chromene. |
In order to show the accessibility of the present work in comparison with other results reported in literature [12] , [13] and [14] , we show that the present ionic liquids are efficient catalysts, with respect to reaction time and obtained product yield (Table 5 ). We also studied the synthesis of 2-amino-3-cyano-5,10-dioxo-4-phenyl-5,10-dihydro-4H-benzo[g]chromene derivatives using ammonium acetate (24 mol%), ammonium chloride (24 mol %), and triethylamine (20 mol%) as catalysts (Table 5 , entries 6–8). Triethylamine obtained a trace product in 120 min (Table 5 , entry 8).
| Entry | Catalyst (mol%) | Conditions | Time | Yield (%)a [Ref.] |
|---|---|---|---|---|
| 1 | Et3N (10 mol%) | CH3CN as Solvent Ambient temperature | 24 h | 86 [12] |
| 2 | TEBA (10 mol%) | Solvent free 85 °C | 4 h | 88 [13] |
| 3 | DBU (10mol%) | Water/Reflux | 60 min | 87 [14] |
| 4 | 2-Hydroxyethylammonium formate (26 mol%) | Solvent-free, ambient conditions | 10 | 90 Present work |
| 5 | 2-Hydroxyethylammonium Acetate (23 mol%) | Solvent free, ambient conditions | 8 | 90 Present work |
| 6 | Ammonium acetate (24 mol%) | Solvent free, ambient conditions | 25 | 85 |
| 7 | Ammonium chloride (24 mol%) | Solvent free, ambient conditions | 30 | 73 |
| 8 | Triethylamine (20 mol%) | Solvent free, ambient conditions | 120 min | Trace |
a. Based on the reaction of benzaldehyde (1.0 mmol), malononitrile (1.0 mmol), 2-hydroxy-1,4-dihydronaphthalene-1,4-dione (1.0 mmol).
3. Conclusion
We have developed a green and straightforward protocol for the synthesis of 7-amino-6-cyano-5-phenyl-1,3-dioxo-2-phenyl-1,2,3,5-tetrahydropyrazolo[1,2-a][1,2,4]triazoles and 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo [g]chromenes, using 2-hydroxyethylammonium formate and 2-hydroxyethylammonium acetate as reusable catalysts at room temperature under solvent free conditions. This procedure provides several advantages: cleaner reaction, easier workup, reduced reaction time and an eco-friendly promising strategy.
4. Experimental
All reagents were purchased from Merck and Aldrich and used without further purification. All yields refer to isolated products after purification. 2-Hydroxyethylammonium formate [5] and 2-hydroxyethylammonium acetate were synthesized according to the literature [6] . The NMR spectra were recorded on a Bruker Avance DPX 400 MHz instrument. The spectra were measured in DMSO-d6 relative to TMS (0.00 ppm). Elemental analyses for C, H, and N were performed using a Heraeus CHN-O-Rapid analyzer. FT-IR spectra were recorded on a JASCO FT-IR 460 plus spectrophotometer. TLC was performed on silica-gel Poly Gram SIL G/UV 254 plates.
4.1. General procedure for the synthesis of 7-amino-6-cyano-5-phenyl-1,3-dioxo-2-phenyl-1,2,3,5-tetrahydropyrazolo[1,2-a][1,2,4]triazoles under ambient conditions
A mixture of 4-phenylurazole (1 mmol), malononitrile (1 mmol), arylaldehydes (1 mmol) and (0.27 mmol) of 2-hydroxyethylammonium formate or (0.24 mmol) of 2-hydroxyethylammonium acetate as catalyst was stirred at room temperature. After completion of the reaction, the ionic liquids were dissolved in water. For recycling the catalysts, after washing the solid products with water completely, the water containing ionic liquid (ILs is soluble in water) was evaporated under reduced pressure and the ionic liquids were recovered and reused. The solid crude product(s) was recrystalized in ethanol and afforded the desired pure product(s).
The spectroscopic data for a new compound are reported as below:
7-amino-6-cyano-1,2,3,5-tetrahydro-5-(4-cyanophenyl)-1,3-dioxo-2-phenylpyrazolo[1,2-a][1,2,4]triazole (Table 2 , Entry 10): yellow powder, mp=250–252 °C.; IR (KBr): , 2135, 2190, 1778, 1609, 1502, 1400, 1350, 754, 647 cm−1 .; 1 H NMR (400 MHz, DMSO-d6 ): (1H, s, CH), 7.50 (5H, m, arom), 7.73 (4H, t, Hz, arom), 7.95 (2H, d, Hz, NH2 ) ppm. 13 C NMR (100 MHz, DMSO-d6 ): , 111.9, 116.9, 119.1, 127.4 (2CH), 128.4 (2CH), 129.3, 129.5 (2CH), 131.4, 133.4 (2CH), 144.9, 150.7, 151.1, 154.2 ppm.; Anal. Calcd for C19 H12 N6 O2 : C, 64.04; H, 3.39; N, 23.58%. Found: C, 64.12; H, 3.42; N, 23.55%.
4.2. General procedure for the synthesis of 2-amino-3-cyano-5,10-dioxo-4-aryl-5,10-dihydro-4H-benzo[g]chromene
A mixture of arylaldehydes (1 mmol), malononitrile (1 mmol), 2-hydroxy-1,4-dihydronaphthalene-1,4-dione (1 mmol) and 2-hydroxyethylammonium formate (0.26 mmol) or 2-hydroxyethylammonium acetate (0.23 mmol) as catalyst was stirred at room temperature. After completion of the reaction, the ionic liquids were dissolved in water. For recycling the catalysts, after washing the solid products with water completely, the water containing the ionic liquid (ILs is soluble in water) was evaporated under reduced pressure and the ionic liquids were recovered and reused. The solid crude product(s) was recrystalized in ethanol and afforded the desired pure product(s).
The spectroscopic data for three new compounds are reported as below:
2-amino-3-cyano-4-(2,4-dimethoxyphenyl)-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene (Table 4 , Entry 15): Yellow powder; mp=248–250 °C. IR (KBr): , 1661, 1590 cm .; 1 H NMR (400 MHz, DMSO-d6 ): (3H, s, OMe), 3.77 (3H, s, OMe), 4.83 (1H, s, CH), 6.43–7.11 (3H, m, Ar), 7.17–8.06 (6H, m, H–Ar and NH2 ) ppm. C NMR (100 MHz, DMSO-d6 ): , 56.2, 57.2, 105.6, 119.2, 120.1, 122.4, 124.2, 126.2, 126.5, 130.3, 131.5, 134.5, 135.0, 149.7, 158.3, 159.7 (2C), 177.5, 183.0 (2C=O) ppm.; Anal. Calcd for C22 H16 N2 O5 : C, 68.04; H, 4.15; N, 7.21%. Found: C, 68.06; H, 4.12; N, 7.18%.
2-amino-3-cyano-4-(2,6-dichlorophenyl)-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene (Table 4 , Entry 16): Yellow powder; mp=278–280 °C. IR (KBr): , 2203, 1688, 1593 cm .; 1 H NMR (400 MHz, DMSO-d6 ): (1H, s, CH), 7.29–8.06 (9H, m, H–Ar and NH2 ) ppm. 13 C NMR (100 MHz, DMSO-d6 ): , 105.6, 119.3, 120.6, 124.2, 126.2, 126.4, 126.5, 129.3, 130.6, 130.8, 131.2, 134.8, 136.1, 150.2, 159.7, 177.1, 182.8 (2C=O) ppm.; Anal. Calcd for C H Cl N O : C, 60.48; H, 2.54; N, 7.05%. Found: C, 60.46; H, 2.50; N, 7.04%.
2-amino-3-cyano-4-(2-nitrophenyl)-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene (Table 4 , Entry 17): Yellow powder; mp=232–234 °C. IR (KBr): , 2207, 1666, 1631 cm .; 1 H NMR (400 MHz, DMSO-d6 ): (1H, s, CH), 7.46–8.07(10H, m, H–Ar and NH2 ) ppm. C NMR (100 MHz, DMSO-d6 ): , 119.3, 121.6, 122.4, 124.5, 126.4, 128.0, 130.3, 130.9, 131.4, 134.6, 135.0, 149.6, 158.2, 159.3, 177.3, 183.1 (2C=O) ppm.; Anal. Calcd for C20 H11 N3 O5 : C, 64.35; H, 2.97; N, 11.26%. Found: C, 64.32; H, 2.95; N, 11.25%.
Acknowledgments
We are grateful to the University of Sistan and Baluchestan Research Council for partial support of this research.
References
- [1] D. Zhao, M. Wu, Y. Kou, E. Min; Ionic liquids: applications in catalysis; Catal. Today, 74 (2002), pp. 157–159
- [2] W. Lu, A.G. Fadaev, B. Qu, E. Smela, B.R. Mattes, J. Ding, G.M. Spinks, J. Mazurkiewicz, D. Zhou, G.G. Wallace, D.R. MacFarlane, S.A. Forsyth, M. Forsyth; Use of ionic liquids for pi-conjugated polymer electrochemical devices; Science, 297 (2002), pp. 287–983
- [3] W.W. Miao, T.H. Chan; Ionic-liquid-supported synthesis: a novel liquid-phase strategy for organic synthesis; Acc. Chem. Res., 39 (2006), pp. 897–908
- [4] A.G. Ying, X.Z. Chen, W.D. Ye, D.F. Zhang, L. Liu, J.H. Chen; DBU drived ionic liquids and their application in organic synthetic reactions; Prog. Chem., 20 (2008), pp. 1642–1650
- [5] N. Bicak; A new ionic liquid: 2-hydroxyethylammonium formate; J. Mol. Liq., 116 (2005), pp. 15–18
- [6] M.A.P. Martins, C.P. Frizzo, D.N. Moreira, Nilo. Zanatta, H.G. Bonacorso; Ionic liquids in heterocyclic synthesis; Chem. Rev., 108 (2008), pp. 2015–2050
- [7] H.R. Shaterian, H. Yarahmadi, M. Ghashang; Silica supported perchloric acid (HClO4 –SiO2 ): an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols ; Tetrahedron, 64 (2008), pp. 1263–1269
- [8] H.R. Shaterian, M. Honarmand, A.R. Oveisi; Multicomponent synthesis of 3,5-diaryl-2,6-dicyanoanilines under thermal solvent-free conditions; Monatsh. Chem., 560 (2010), pp. 141–557
- [9] H.R. Shaterian, A.R. Oveisi; A simple green approach to the synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives catalyzed by 3-hydroxypropanaminium acetate (HPAA) as a new ionic liquid; J. Iran. Chem. Soc., 8 (2011), pp. 545–552
- [10] H.R. Shaterian, A. Hossienian, M. Ghashang; Ferric hydrogen sulfate as an efficient heterogeneous catalyst for environmentally friendly greener synthesis of 1,8-Dioxo-octahydroxanthenes; Turk. J. Chem., 2 (2009), pp. 233–240
- [11] D. Azarifar, R. Nejat Yami; Ultrasonic-assisted one pot synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-diones; Heterocycles, 81 (2010), pp. 2063–2073
- [12] A. Shaabani, R. Ghadari, S. Ghasemi, M. Pedarpour, A.H. Rezayan, A. Sarvary, S. Weng Ng; Novel one-pot three- and pseudo-five-component reactions: synthesis of functionalized benzo[ ]- and dihydropyrano[2,3- ]chromene derivatives ; J. Comb. Chem., 11 (2009), pp. 956–959
- [13] Y. Changsheng, Y. Chenxia, L.I. Tuanjie, T. Shujiang; An efficient synthesis of 4H-benzo[ ]chromene-5,10-dione derivatives through triethylbenzylammonium chloride catalyzed multicomponent reaction under solvent-free conditions ; Chin. J. Chem., 27 (2009), pp. 1989–1994
- [14] J.M. Khurana, B. Nand, P. Saluja; DBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H-benzo[g]chromenes in aqueous medium; Tetrahedron, 66 (2010), pp. 5637–5641
Document information
Published on 01/01/2017
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?
![The preparation of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione and ...](/wd/images/3/32/Draft_Content_831018676-1-s2.0-S1026309813000710-sc1.jpg)
![Proposed mechanism for preparation of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione ...](/wd/images/6/6c/Draft_Content_831018676-1-s2.0-S1026309813000710-sc3.jpg)