Summary
Background
We investigated the rate of early surgical complications after simultaneous pancreas–kidney transplantation (SPKT) and their impact on both grafts and recipient survival.
Materials and methods
The retrospective analysis of typical pancreas-related complications, different methods of correction, and their efficacy were performed. Data describing pancreas transplant recipients were drawn from our SPKT waiting list.
Results
The overall surgical complications rate was 37.5%. The 1-year pancreas graft survival was 82.5% and 1-year recipient survival was 90%. Surgical complications based on the graft loss rate did not exceed 2.5%. Direct surgical complications did not account for the loss of a single patient.
Conclusion
We conclude that the high rate of surgical complications is a major obstacle to widespread application of pancreas transplantation; early recognition and appropriate treatment of graft-related complications is fundamental for graft survival.
Keywords
outcomes;pancreas graft survival;pancreas transplantation;recipient survival rate;surgical complications
1. Introduction
Despite a large potential recipient pool, widespread application of pancreas transplants has been hampered by substantial graft failure caused by surgical complications.1; 2 ; 3 Although the islet cells account for < 2% of the overall pancreas graft mass, all surgical complications after pancreas transplants result from the remaining 98% of the tissue transplanted with the islets (i.e., vasculature, exocrine parenchyma, and, for whole organ pancreas grafts, the duodenum).1
Pancreas transplantation is associated with the highest complication rate of all of the routinely performed solid organ transplants.3; 4; 5 ; 6 Pancreatic grafts are susceptible to a unique set of surgical complications mostly related to exocrine secretions and the low microcirculatory blood flow of the gland.2 Surgical complications are also relevant, because they frequently result in graft loss (i.e., from vascular graft thrombosis and intra-abdominal infection).1 ; 3 In contrast to other surgical complications after pancreas transplants, graft thrombosis is, with rare exceptions, irreversible. It remains the leading cause of nonimmunologic graft failure after pancreas transplantation. Intra-abdominal infections lead to high rates of graft loss and substantial mortality.7 Post-transplant leaks still remain a significant risk factor for intra-abdominal infections.1 Graft pancreatitis is a major risk factor for graft thrombosis and is often associated with significant peripancreatitis and infection. Although the overall impact of bleeding on graft survival is comparatively benign, it is one of the most frequent indications for a relaparotomy after pancreas transplantation.1 ; 8 A higher incidence of graft loss and fatal outcome has characterized a group of patients in which a revised open surgery was performed to reduce surgical complications.3 ; 9 Thus, surgical complications requiring and leading to a high rate of repeat laparotomies, high risk of severe complications, and fatalities are in part a limiting factor in the widespread use of this method in clinical practice.5 ; 10 The purposes of this study were to evaluate the incidence of early surgical complications, and to analyze their structure and impact on pancreas graft and recipient survival.
2. Methods
The data for analysis were drawn from the simultaneous pancreas–kidney transplantation (SPKT) waiting list available as of August 05, 2014. From January 2008 to June 2014, 40 patients suffering from Type I diabetes complicated with end stage renal disease underwent SPKT. The age of the patients ranged from 25 years to 51 years and averaged 35.7 ± 6.36 years. The gender distribution was as follows: 19 women (47.5%) and 21 men (52.5%). A total of 20 recipients (50%) were blood type O, 14 recipients (35%) were blood type A, and six recipients (15%) were blood type B. There were no AB patients observed in our research. All patients had a long history of the disease—from 4 years to 39 years (mean duration 25.2 ± 7.6 years; Table 1). All of the patients were long-term disabled and suffered from multiple diabetic complications including severe retinopathy, microangiopathy and macroangiopathy, and some patients were lower extremity amputees. This category of patients is considered to be among the most severe cases amongst the group of patients requiring dialysis. Seventeen (42.5%) patients underwent pancreatic transplantation with intra-abdominal localization and the formation of the duodenojejunal anastomosis, 23 (57.5%) patients underwent retroperitoneal pancreatic transplantation with the formation of interduodenal anastomosis. In most of the patients (n = 31, 77.5%), venous drainage was directed into the vena cava inferior system, whereas in nine (22.5%) patients, the intravenous anastomosis was made with the portal vein system using a standard surgical technique.
| Patient | Gender | Age (y) | Blood group | DM* duration | RRT** duration | Hemodialysis | CAPD | Preemptive | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | 0 (I) | A (II) | B (III) | AB (IV) | |||||||
| 1 | 1 | 45 | 1 | 34 | 16 | 1 | ||||||
| 2 | 1 | 30 | 1 | 30 | 2.5 | 1 | ||||||
| 3 | 1 | 38 | 1 | 26 | 3 | 1 | ||||||
| 4 | 1 | 33 | 1 | 19 | 1 | 1 | ||||||
| 5 | 1 | 32 | 1 | 20 | 1 | 1 | ||||||
| 6 | 1 | 35 | 1 | 22 | 3 | 1 | ||||||
| 7 | 1 | 30 | 1 | 27 | 9 | 1 | ||||||
| 8 | 1 | 36 | 1 | 29 | 1.5 | 1 | ||||||
| 9 | 1 | 35 | 1 | 26 | 9 | 1 | ||||||
| 10 | 1 | 28 | 1 | 24 | 1 | 1 | ||||||
| 11 | 1 | 49 | 1 | 39 | 1 | 1 | ||||||
| 12 | 1 | 39 | 1 | 23 | 10 | 1 | ||||||
| 13 | 1 | 38 | 1 | 4 | 1 | 1 | ||||||
| 14 | 1 | 30 | 1 | 20 | 1 | 1 | ||||||
| 15 | 1 | 31 | 1 | 22 | 3 | 1 | ||||||
| 16 | 1 | 30 | 1 | 20 | 1 | 1 | ||||||
| 17 | 1 | 40 | 1 | 34 | 1 | 1 | ||||||
| 18 | 1 | 26 | 1 | 14 | 6 | 1 | ||||||
| 19 | 1 | 39 | 1 | 30 | 2 | 1 | ||||||
| 20 | 1 | 28 | 1 | 17 | 1 | |||||||
| 21 | 1 | 34 | 1 | 20 | 1 | 1 | ||||||
| 22 | 1 | 42 | 1 | 33 | 1 | |||||||
| 23 | 1 | 36 | 1 | 26 | 7 | 1 | ||||||
| 24 | 1 | 31 | 1 | 20 | 2 | 1 | ||||||
| 25 | 1 | 40 | 1 | 27 | 1 | |||||||
| 26 | 1 | 27 | 1 | 19 | 1 | |||||||
| 27 | 1 | 47 | 1 | 34 | 4 | 1 | ||||||
| 28 | 1 | 32 | 1 | 26 | 4 | 1 | ||||||
| 29 | 1 | 37 | 1 | 27 | 5 | 1 | ||||||
| 30 | 1 | 34 | 1 | 18 | 4 | 1 | ||||||
| 31 | 1 | 51 | 1 | 29 | 2 | 1 | ||||||
| 32 | 1 | 33 | 1 | 11 | 2 | 1 | ||||||
| 33 | 1 | 39 | 1 | 32 | 1 | 1 | ||||||
| 34 | 1 | 33 | 1 | 30 | 1 | 1 | ||||||
| 35 | 1 | 45 | 1 | 35 | 9 | 1 | ||||||
| 36 | 1 | 30 | 1 | 22 | 1 | 1 | ||||||
| 37 | 1 | 38 | 1 | 25 | 1 | 1 | ||||||
| 38 | 1 | 43 | 1 | 39 | 2 | 1 | ||||||
| 39 | 1 | 25 | 1 | 18 | 1.5 | 1 | ||||||
| 40 | 1 | 40 | 1 | 36 | 1 | 1 | ||||||
| Mean | 35.7 | 6 | 0 | 25.175 | ||||||||
| SD | 6.4 | 7.578266834 | ||||||||||
| Median | 2 | |||||||||||
| 25th% | 1 | |||||||||||
| 75th% | 4 | |||||||||||
| Sum (%) | 19 (47.5) | 21 (52.5) | 20 (50) | 14 (35) | 6 (15) | 0 | 22 (55) | 14 (35) | 4 (10) | |||
CAPD - Chronic Ambulatory Peritoneal Dialysis; DM = diabetes mellitus; SD - standard deviation; RRT = renal replacement therapy.
In each case, access into the abdominal cavity was gained through the standard total median laparotomy, and the transplanted organ was placed bilaterally where the kidneys occupied the left-hand side. In three cases, the pancreas was transplanted first. However, knowing that the pancreas requires extended time during the operation, in an attempt to reduce the cold ischemia time (CIT) of the kidney, the first transplanted organ in other cases was the kidney. The median depicting nephrotransplant CIT was 7 (5–8) hours, whereas the median showing pancreas transplant CIT was 9 (8–10.75) hours.
The donors' ages varied from 18 years to 45 years (mean 28.2 ± 6.36 years). The gender distribution was 36 males (90%) and four females (10%). Head injuries are a major cause of death amongst the donors (n = 33, 82.5%), but in seven cases, the cause of death was acute irreversible cerebrovascular dysfunction (17.5%). Human leukocyte antigen (A, B, Dr) mismatch median was 5 (3, 5.5).
2.1. Immunosuppressive therapy
Induction immunosuppressive therapy was the following: 36 patients (90%)—basiliximab, three patients (7.5%)—Thymoglobulin, and one patient (2.5%)—daclizumab. Induction tacrolimus dose was 0.1 mg/kg/d and induction cyclosporine dose was 15 mg/kg/d.
The basic maintenance immunosuppression with tacrolimus was administered in 36 patients (90%; through levels, 8–12 ng/mL), cyclosporine—in four patients (through levels, 180–200 ng/mL), but in three cases the conversion to tacrolimus has been made in the early postoperative period.
Perioperative systemic anticoagulation therapy is not the routine practice in our clinic. In cases of patients' hypercoagulability state observed via thromboelastography, we used intravenous heparin at subtherapeutic doses.
The lifetime of the transplanted pancreas was determined as the period of total insulin independence. From the standpoint of statistics, the fatal outcome with a functioning graft was considered to be graft failure.
We classified all early surgical complications following the SKPT in accordance with the Clavien–Dindo classification system. Most often (33.3%) we encountered Grade IIIa complications, then Grade I and Grade II together (23.8%), and rarely, complications classified as Grade IIIb (19.1%).
2.2. Statistical analysis
The Kolmogorov–Smirnov one-sample test has been used to characterize the received data through comparison between the observed distributions and the hypothesized ones (normality). In the case of normal data distribution, the data is presented as the combination of mean value and average squared displacement. In cases where the data distribution is other than normal, the data are presented as Q1, median, and Q3.
This article provides an analysis of the following aspects: (1) frequency of surgical complications following SPKT; (2) pancreatic graft survival; and (3) recipient survival rate.
3. Results
We analyzed 21 surgical complications following SPKT in 15 patients (37.5%; Table 2).
| SMA thrombosis | 5 (12.5%) |
| Venous thrombosis | 2 (5%) |
| Pancreas transplant arterial stenosis | 1 (2.5%) |
| Clinically significant pancreonecrosis | 6 (15%) |
| Duodenal leak | 1 (2.5%) |
| Asymptomic parapancreatic fluid collection | 21 (52.5%) |
| Parapancreatic external fistula | 1 (2.5%) |
| Intraabdominal infection | 2 (5%) |
| Bleeding | 3 (7.5%) |
| Relaparotomy rate | 3 (7.5%) |
| Transcutaneous drainage | 4 (10%) |
| Kidney transplant loss rate | 3 (7.5%) |
| Pancreas transplant loss rate | 2 (5%) |
| Due to surgical complications | 1 (2.5%) |
| Recipients' death rate | 4 (10%) |
| Due to surgical complications | 0 |
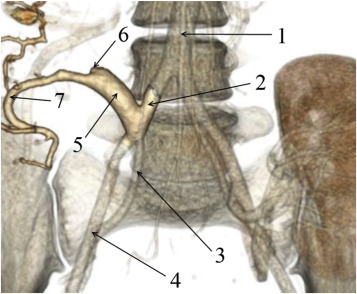
One patient (Patient 14) was diagnosed with partial nonocclusive thrombosis of both portal vein (up to 75% of the lumen) and splenic vein of the graft, and one patient (Patient 28) developed distal thrombosis of the transplant splenic vein. Ultrasound screening identified thrombosis of the upper mesenteric artery of the transplant in five patients (Patients 21, 34, 36, 37, and 39). This was subsequently confirmed by computed tomography (Figure 1).
|
|
|
Figure 1. Abdominal computed tomography (Patient 34): (1) aorta; (2) common iliac artery; (3) internal iliac artery; (4) external iliac artery; (5) pancreas transplant Y-graft; (6) filling defect in the region of the superior mesenteric artery of the pancreas transplant; (7) splenic artery. |
In one case (Patient 22), stenosis of splenic anastomosis exacerbated the vascular complications. It was successfully treated with emergency endovascular intervention (n = 1, 2.5%).
In the postoperative period, three patients (7.5%) developed bleeding of varying severity and localization. One female patient (Patient 1) developed life-threatening arrosive intra-abdominal bleeding due to a focal fungal infection at the sites of vascular anastomoses. Urgent relaparotomy and pancreas transplantectomy were performed to save the patients life. In addition to this, two patients (Patients 4 and 24) developed bleeding at the site of intestinal anastomosis: Patient 4—on Day 4 following the formation of duodenojejunal anastomosis; and Patient 24—on Day 37 following the formation of interduodenal anastomosis. In both cases, conservative drug therapy resolved the issues.
Clinically significant pancreonecrosis of the pancreas graft (macrofocal and microfocal foci of pancreatic necrosis) was diagnosed in six patients (Patients 1, 2, 5, 17, 25, and 37; 15%). In these patients, the pancreonecrotic processes were complicated by sequestration and hence required open surgery or transcutaneous drainage under ultrasound or X-ray guidance. In one case (Patient 1), a pancreatic necrosis was complicated by numerous parapancreatic abscesses and, furthermore, fungi disseminated across the vascular anastomoses which led to the dehiscence of the latter (see above).
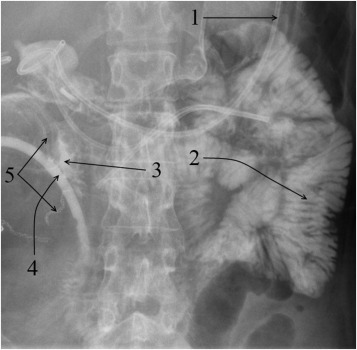
In one case, anastomotic dehiscence of the interduodenal anastomosis had caused the duodenal leakage with parapancreatic fluid collection formation in a female patient (Patient 25; Figure 2).
|
|
|
Figure 2. Gastrointestinal tract contrast X-ray radiography; (1) nasal-intestinal probe is inserted beyond the ligament of Treitz; (2) contrast-filled small-bowel loops; (3) contrast traveled into the drain through the interduodenal anastomosis dehiscence; (4) drain is inserted close to the vicinity of the interduodenal anastomosis; (5) visualized lines of staples sutures of the donor duodenum. |
Transcutaneous drainage of the duodenal leak was performed under ultrasound and x-ray guidance. Prolonged drainage of the concerned area yielded a positive outcome. During fistulography, sufficient closing of the duodenal fenestration was confirmed. Following the fistulography, closure of the drainage canal was completed by using a blend of Type 1 human collagen with cancellous bone chips.
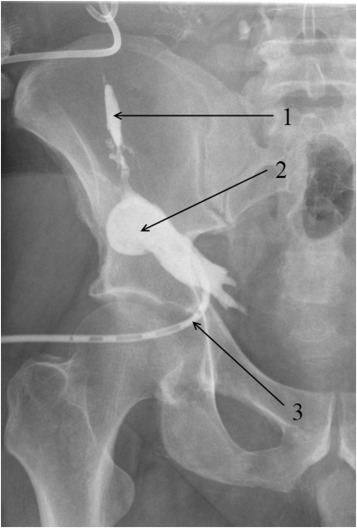
In another case (Patient 22), microfocal pancreonecrosis was complicated by the formation of two sinus tracts, one of which (external) produced an opening on the paraumbilical skin, whereas the second was in the subcutaneous fat in the right iliac fossa, thus forming an abscess (Figure 3).
|
|
|
Figure 3. Subcutaneous fat abscess fistulography: (1) sinus tract leading to the pancreas transplant; (2) abscess cavity in the subcutaneous fat; and (3) drain is in the abscess cavity. |
Transcutaneous drainage of the parapancreatic fluid collection and abscess cavity in the subcutaneous fat was performed. Following the fistulography, which failed to establish influx of the contrast into the donor duodenum, closure of all drainage canals was completed by use of a mix of Type 1 human collagen with cancellous bone chips. Asymptomatic parapancreatic fluid collections were observed in 20 patients (50%).
Two patients (Patients 1 and 2) were diagnosed with intra-abdominal infection in the form of localized infection (parapancreatic abscesses). Relaparotomy was made in order to drain the abscesses and eradicate the focal infection.
One patient (Patient 1) developed a surgical site infection secondary to the fungal infection of the pancreatic graft, which led to wound disruption and eventration. There was slow healing of the laparotomy wound by secondary intention following ointment dressings.
Thus, to correct the above mentioned surgical complications, the following procedures were completed:
- three laparotomies:
- a patient with an infected pancreonecrosis complicated with parapancreatic abscesses and arrosive intraperitoneal bleeding (Patient 1) secondary to the fungal infection of vascular anastomoses;
- two patients (Patients 2 and 5) with macrofocal or microfocal necrotizing.
- transcutaneous drainage under ultrasound and x-ray guidance:
- in two cases (Patients 17 and 37) of macrofocal pancreatic necrosis complicated with sequestration;
- in one case (Patient 22) of parapancreatic fluid collection complicated by two sinus tracts and subcutaneous tissue abscess formation;
- in one case (Patient 25) of the interduodenal anastomosis dehiscence.
- one emergency endovascular procedure;
- endoscopic hemostasis in case of interduodenal anastomotic bleeding (Patient 24);
- conservative hemostatic therapy in case of duodeno-jejunoanastomotic bleeding (Patient 4).
Overall, there were four deaths:
- three cases (Patients 3, 26, and 27) revealed leucopenia secondary to the therapy of rejection, which was complicated by pneumonia and, further, multiorgan dysfunction syndrome.
- severe diabetic nephropathy complicated with the sudden cardiopulmonary arrest 5 days following a successfully executed transplantation (Patient 23).
4. Discussion
Most transplant surgeons consider pancreas graft thrombosis, duodenal leakage, and intra-abdominal (or retroperitoneal) infection to be general surgical complications after pancreas transplantation. Consequently, they frequently result in graft loss and therefore restrict widespread application of pancreas transplantation. Their timely diagnosis and prompt treatment are necessary options for pancreas transplant salvage and decreasing rates of pancreas graft loss. In our practice, we observed no case of pancreas graft thrombosis; duodenal leakage and most intra-abdominal/retroperitoneal infection cases were treated without pancreas transplant loss. In case of pancreonecrosis, abscesses, duodenal leaks, and parapancreatic fluid collections after retroperitoneal pancreas transplantation, abnormal focus compartmentalize in the right retroperitoneal space and do not contact with abdominal cavity. Benefits of minimal invasive therapy in complications correction enable avoidance of relaparotomy, therefore, transcutaneous drainage under ultrasound or x-ray guidance becomes the preferable correction modality. Formation of interduodenal anastomosis following retroperitoneal pancreas transplantation enables visual inspection of duodenal mucosa and endoscopic hemostasis in cases of anastomotic bleeding. We consider retroperitoneal placement of pancreas transplant to be a simple, more safe physiologic technique.
5. Conclusion
Surgical complications developed in 15 patients (37.5%). Relaparotomy (n = 3), draining (n = 4), emergency endovascular surgery (n = 1), and a conservative and/or endoscopic hemostasis (n = 1) were employed in order to correct surgical complications. Relaparotomy rate was 7.5%. Relaparotomy was indicated only in patients with intra-abdominal pancreas transplantation. The pancreas graft loss rate due to surgical complications was 2.5%. Having considered the cases of pancreas transplant loss due to immunological factors in the early postoperative period (irreversible antibody-mediated rejection in Patient 29) and in the late postoperative period (untreated cellular rejection due to noncompliance of Patient 18) as well as patients death with a functioning graft, the 1-year graft survival rate was 82.5% and the 1-year patient survival rate was 90%. Patients loss rate due to surgical complications was 0%. Patients' death cases were induced by complications of immunosuppressive therapy (n = 3) and the initially severe condition of the patient (n = 1).
References
- 1 C. Troppmann; Surgical complication; R.W.G. Gruessner, D.E.R. Sutherland (Eds.), Transplantation of the Pancreas, Springer, New York (2004), pp. 206–237
- 2 N.S. Hakim, R.J. Stratta, D. Gray, P. Friend, A. Coleman (Eds.), Pancreas, Islet, and Stem Cell Transplantation for Diabetes (2nd ed.), United States by Oxford University Press Inc., New York (2010), pp. 179–189
- 3 C. Troppmann, A.C. Gruessner, D.L. Dunn, D.E.R. Sutherland, R.W.G. Gruessner; Surgical complications requiring early relaparotomy after pancreas transplantation: a multivariate risk factor and economic impact analysis of the cyclosporine era; Ann Surg, 227 (1998), pp. 255–268
- 4 R.J. Corry, R. Shapiro (Eds.), Pancreat Transplant, Informa Healthcare, New York (2007), pp. 159–170
- 5 A. Humar, R. Kandaswamy, D. Granger, R.W. Gruessner, A.C. Gruessner, D.E. Sutherland; Decreased surgical risks of pancreas transplantation in the modern era; Ann Surg, 231 (2000), pp. 269–275
- 6 G. Michalak, J. Czerwiński, A. Kwiatkowski, et al.; Surgical complications observed is simultaneous pancreas-kidney transplantation: thirteen years of experience of one center; Transpl Proc, 34 (2002), pp. 661–662
- 7 N. Berger, R. Wirmsberger, R. Kafka, et al.; Infectious complications following 72 consecutive enteric-drained pancreas transplants; Transpl Int, 19 (2006), pp. 549–557
- 8 J.L. Chen, R.C. Lee, Y.M. Shyr, et al.; Imaging spectrum after pancreas transplantation with enteric drainage; Korean J Radiol, 15 (2014), pp. 45–53
- 9 L. Martins, A.C. Henriques, L. Dias, et al.; Pancreas-kidney transplantation: complications and readmissions in 9-years of follow-up; Transpl Proc, 42 (2010), pp. 552–554
- 10 T. Grochowiecki, Z. Gałazka, S. Frunze, et al.; Influence of simultaneous pancreas and preemptive kidney transplantation on severity of postoperative complications; Transpl Proc, 43 (2011), pp. 3102–3104
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?