Abstract
Background
The Medtronic Evolut R (EVR) is a novel transcatheter heart valve designed to allow precise implantation at the intended position and to minimize prosthesis dysfunction as well as procedural complications. Our aim was to compare short-term functional and clinical outcomes of the new EVR with the established Medtronic CoreValve (CV) system.
Methods and results
Of 151 patients undergoing transfemoral transcatheter aortic valve implantation with a self-expanding valve at our institution between January 2013 and January 2016, 86 were treated with EVR and 65 with CV. Patients treated with EVR had a significantly lower rate of more-than-mild aortic regurgitation and a higher rate of device success. Recapture maneuvers to optimize valve deployment were performed in 22.1% of the EVR procedures. Transvalvular post-procedural gradients were slightly higher in the EVR group, while no differences were observed in the incidence of safety endpoints at 30 days, vascular complications, or need for permanent pacemaker implantation following asystole or complete atrioventricular block.
Conclusions
These initial single-center experience data on the short-term outcomes after EVR valve implantation show a substantially reduced rate of more-than-mild paravalvular regurgitation and higher device success, while 30-day safety outcomes were similar to the CV system. Clinical outcome data from long-term follow-up and larger scale multicenter experience are now necessary.
Keywords
Transcatheter aortic valve implantation ; CoreValve® ; Evolut R™ ; Paravalvular leak
1. Introduction
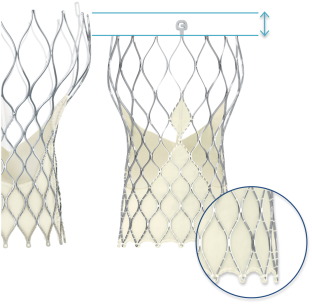
Transcatheter aortic valve implantation (TAVI) has emerged as the treatment of choice for symptomatic aortic stenosis in patients at high risk for conventional surgical valve replacement. The Medtronic CoreValve (CV), with leaflets made from porcine pericardium sutured into a self-expanding nitinol frame, was the first commercially available self-expanding TAVI system. The US Pivotal Trial showed excellent long-term outcomes after CV implantation in patients classified as high-risk for surgical aortic valve replacement [6] . Despite the generally low TAVI complication rates for such high-risk patient collective, several important and prognosis relevant issues including paravalvular leaks [9] , access site bleeding [3] or valve dislocation during deployment limited the procedural success of first generation TAVI prosthesis. To tackle these issues, the Evolut R (EVR) with the EnVeo R delivery catheter was introduced in 2014. This second generation prosthesis allows repositioning after implantation, has a lower delivery profile and has an extended sealing skirt to reduce the incidence of paravalvular leaks (Fig. 1 ).
|
|
|
Fig. 1. Compared to the traditional Medtronic CoreValve® prosthesis (left side), new features of the Medtronic Evolut R™ (right side) include a new design of the nitinol frame with a lower height and an extended sealing skirt (© Medtronic). |
We herein report a single-center experience with the EVR TAVI system in 86 patients, and compare short-term functional and clinical performance with this device with historical data using the established CV TAVI system.
2. Materials and methods
2.1. Patient characteristics and procedural planning
Between January 2013 and January 2016, a total of 151 consecutive patients received a self-expandable transcatheter aortic valve implantation in the native annulus with either the Medtronic CV or EVR prosthesis at our institution. Data were analyzed retrospectively. Non-self-expandable TAVI prostheses that were implanted during the same period included the Edwards Sapien XT/Sapien 3 (n = 226) and the Direct Flow Medical Valve (n = 59). Only patients who had symptomatic severe aortic valve stenosis, with an aortic valve area (AVA) of < 1.0 cm2 (confirmed by both echocardiography and invasive recordings) were treated. All patients were evaluated by our centers multidisciplinary Heart Team, and TAVI was generally recommended in the presence of additional risk factors contributing to increased risk for conventional surgical valve replacement according to current guidelines [10] . Demographic characteristics as well as clinical and procedural data and echocardiographic parameters were prospectively documented in our centers dedicated database as part of the national quality control requirements. Baseline demographics are shown in Table 1 . All patients had a diameter of the common femoral artery of > 6 mm (> 5 mm for EVR) and were considered suitable for transfemoral vascular access. Prior to valve replacement, coronary angiography was performed in all patients to rule out or treat relevant coronary artery disease.
| CoreValve | Evolut R | p -value | |
|---|---|---|---|
| Number of patients | 65 | 86 | |
| Age [years] | 84.2±0.5 | 82.9±0.8 | 0.19 |
| Female | 46 (70) | 59 (69) | 0.91 |
| log. EuroScore | 32±0.5 | 27.4±0.7 | 0.06 |
| Body mass index [kg/m2] | 27.3±0.6 | 26.9±0.7 | 0.7 |
| NYHA class | 0.25 | ||
| III | 48 (73) | 47 (55) | |
| IV | 9 (14) | 18 (21) | |
| Diabetes mellitus | 24 (36) | 28 (33) | 0.54 |
| End stage renal failure | 1 (1) | 2 (2) | 0.85 |
| Coronary artery disease | 38 (58) | 56 (65) | 0.69 |
| Previous myocardial infarction | 11 (17) | 14 (16) | 0.47 |
| Previous PCI | 11 (17) | 29 (34) | 0.03 |
| History of cardiac surgery | 8 (12) | 14 (16) | 0.61 |
| Peripheral artery disease | 7 (11) | 15 (17) | 0.57 |
| Neurological dysfunction | 8 (12) | 21 (24) | 0.13 |
| Pulmonary disease | 9 (14) | 11 (13) | 0.93 |
| Atrial fibrillation | 16 (24) | 28 (33) | 0.09 |
| Permanent pacemaker | 9 (14) | 8 (9) | 0.78 |
Values are mean ± standard error of mean or n (%).
Sizing was performed with ECG-triggered multislice CT scan with contrast infusion or alternatively (for patients who could not undergo contrast CT examination e.g. due to advanced stage renal impairment) with 3D transesophageal echocardiography. A dedicated software (3mensio) was used to analyze CT-derived annulus area and diameters, height of the coronary ostia and the degree of valvular/annular calcification. In order to assess the congruence between the native aortic valve annulus and the prosthesis, the “cover index” was calculated as 100 × ([prosthesis diameter − annulus diameter] / prosthesis diameter) [2] .
2.2. TAVI procedure
During the procedure, all patients were under general anesthesia and received transesophageal echocardiography for procedural guidance and immediate assessment of paravalvular leakages. In addition, hemodynamic assessment by simultaneous measurements of left ventricular (LV) and aortic root pressure was performed before and after valve implantation. All procedures were performed using the transfemoral route. To streamline the interventional treatment of possible vascular complications, a crossover maneuver was done in all patients with the positioning of a 0.018″ guidewire in the superficial femoral artery inserted from the contralateral side. Vascular access site closure was generally achieved with 2 Proglide devices (Abbott Vascular, Abbott Park (IL), USA).
2.3. Medtronic CoreValve and Evolut R transcatheter valve system
Both the CV and the EVR prosthesis feature a self-expandable nitinol frame with sutured leaflets made from porcine pericardium (Fig. 1 ). A sealing skirt slightly cranial to the native annulus was added to prevent paravalvular leak. When implanted in the correct position, the prosthetic leaflets are located in a supra-annular position. Major differences of the new generation EVR prosthesis include the option to recapture and reposition the valve, a smaller delivery profile (18F outer diameter for EVR when used with EnVeo R inline sheath; 22F outer diameter of the sheath generally required for the CV system), an extended sealing skirt, a more cylindrical shape of the lower part and more consistent radial force of the nitinol frame, as previously reported [5] . All EVR prostheses were implanted with the inline sheath and did not require the use of an additional 18F sheath.
2.4. Outcome measures
Overall patient outcome was subdivided in implantation data, procedural outcome and early safety clinical outcome. During the procedure, implantation depth was determined angiographically at the pre-specified optimal angulation with a perpendicular view of all 3 cusps of the native aortic valve. Hemodynamic assessment included recordings of the peak-to-peak gradient and calculation of the aortic regurgitation index [9] . Aortic regurgitation was additionally analyzed by aortic root angiography as described [8] .
Procedural outcome assessment included procedure-related complications such as coronary obstruction, annular rupture, major vascular complications, ventricular perforation and intraprocedural death. In addition, echocardiography was used to determine the post-procedural transvalvular gradient and degree of aortic regurgitation. Device success was defined according to the VARC-2 criteria [4] as the absence of procedural mortality, positioning of a single valve in the correct anatomic position, and proper valve performance (mean gradient < 20 mm Hg, no moderate or severe aortic regurgitation).
In order to assure reproducibility, all hemodynamic variables were consistently evaluated by a standardized protocol: pressure recordings were observed for at least 30 s until a stable signal without artifacts was obtained. Gradient analyses were done from at least 5 consecutive beats with simultaneous recordings of left-ventricular and aortic pressure after zero balance. With respect to angiographic and echocardiographic quantification of paravalvular aortic regurgitation, all recordings were independently analyzed by 2 interventional cardiologists with broad experience in echocardiography. If the grading of aortic regurgitation was not consistent, an additional cardiologist was consulted for final assessment.
Early safety outcome was a composite of all cause mortality, stroke, life-threatening bleeding, acute kidney injury stage 2–3, coronary artery obstruction, major vascular complication and valve-related dysfunction requiring repeat procedure within 30 days after the procedure. The incidence of postprocedural pacemaker implantations was categorized according to the indication (asystole, 2nd or 3rd degree atrioventricular block).
2.5. Statistical analysis
Continuous variables are presented as mean ± standard error of mean, and significance was tested by an unpaired t -test. Discrete variables are displayed as counts and percentages and were compared by using Pearsons Chi2 test. Significance was assumed for p -values < 0.05.
3. Results
3.1. Baseline characteristics
A total of 151 patients received implantation of either the Medtronic CV (n = 65) or Medtronic EVR (n = 86) at our institution between January 2013 and January 2016. Given the availability of the new EVR sizes (26 mm & 29 mm) in early 2015, these TAVI prostheses were implanted from February 2015 to January 2016, whereas the CV bioprostheses were implanted between January 2013 and February 2015. Other than for a difference in history of coronary intervention (CV 16.7% vs. EVR 33.7%; p = 0.03), patient characteristics including age, logistic EuroScore, body mass index and additional risk factors were comparable between both groups as shown in Table 1 .
The severity of aortic valve disease was comparable in both groups, including similar transvalvular gradients, left ventricular ejection fraction and pulmonary artery pressure in transthoracic echocardiography (Table 2 ).
| CoreValve | Evolut R | p -Value | |
|---|---|---|---|
| LVEF [%] | 48.0±1.3 | 50.5±1.0 | 0.12 |
| AV peak gradient [mmHg] | 70±3.3 | 68±3.1 | 0.68 |
| AV mean gradient [mmHg] | 45.6±2.2 | 42.9±2.1 | 0.41 |
| Aortic valve area [cm2] | 0.65±0.02 | 0.70±0.02 | 0.93 |
| Regurgitation (moderate/severe) | |||
| Aortic valve | 11 (17) | 18 (21) | 0.83 |
| Mitral valve | 23 (35) | 32 (38) | 0.60 |
| Tricuspid valve | 12 (18) | 27 (31) | 0.14 |
| Systolic pulmonary artery pressure [mmHg] | 40.5±1.8 | 43.9±1.5 | 0.18 |
| Annulus area (CT) [mm2] | 441.2 | 430.3 | 0.37 |
| Annulus diameter (CT, area derived) [mm] | 23.6±0.26 | 23.4±0.16 | 0.45 |
| Presence of annular calcification (CT) | 12 (35) | 13 (41) | 0.78 |
| Access site artery diameter (CT) [mm] | 8.0 | 6.8 | <0.0001 |
Values are mean ± standard error of mean or n (%).
For procedural planning, multislice CT scans with contrast infusion (n = 118) or 3D transesophageal echocardiography (n = 33) were performed, showing similar geometry of the aortic annulus with no difference in the degree of annular calcification between groups (Table 2 ). Since the smaller profile of the EVR delivery catheter allowed transfemoral treatment of patients with smaller common femoral arteries, the diameter of the entry vessel was significantly smaller in EVR treated patients (CV 8.0 mm vs. EVR 6.8 mm; p < 0.0001).
3.2. Implantation data
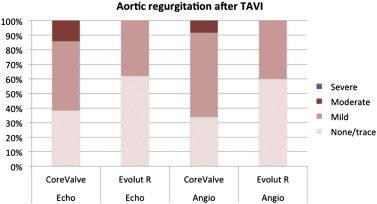
The percentage of predilation (CV 89.2% vs. EVR 86.0%; p = 0.98) as well as postdilation (CV 21.3% vs. EVR 16.5%; p = 0.39) was similar in both groups. When compared to CV, a key feature of the EVR is the option to fully recapture and to reposition the valve during deployment. This maneuver was performed in 22.1% of EVR treated patients, mostly due to an initially deep (ventricular) positioning of the valve. There were 2 cases of a supra-annular valve position in the CV treated patient group requiring the implantation of a second valve, and none in the EVR group. In addition, the option to recapture allowed a less ventricular implantation depth at the non-coronary cusp (CV 5.3 mm vs. EVR 4.0 mm, p = 0.03). Immediate hemodynamic and aortic root angiography as well as echocardiographic assessment was done in all patients, showing a lower incidence of mild to moderate aortic regurgitation in the EVR group (Tables 3 , 4 , Fig. 2 ), and an aortic regurgitation index of 21.3 for CV and 24.9 for EVR treated patients (p = 0.04). In contrast, transvalvular gradients by simultaneous invasive recordings after implantation were slightly higher in the EVR group (CV 4.9 mm Hg vs. EVR 8.9 mm Hg; p = 0.0003), possibly due to a higher degree of oversizing in the EVR group as indicated by an increased cover index (CV 15.3 vs. EVR 17.1; p = 0.01).
Table 3.
Implantation data.
| CoreValve | Evolut R | p -Value | |
|---|---|---|---|
| Valve size, mm | |||
| 23 | 2 (3) | 1 (1) | |
| 26 | 28 (42) | 22 (26) | |
| 29 | 33 (50) | 63 (73) | |
| 31 | 2 (3) | n.a. | |
| 0.001 | Aortic regurgitation by angiography | ||
| None/trace | 20 (34) | 48 (60) | |
| Mild | 34 (58) | 32 (40) | |
| Moderate | 5 (8) | 0 (0) | |
| Severe | 0 (0) | 0 (0) | |
| Balloon pre-dilation | 58 (89) | 74 (86) | 0.98 |
| Balloon post-dilation | 14 (22) | 14 (16) | 0.39 |
| Implantation depth NCC [mm] | 5.3±0.53 | 4.0±0.28 | 0.03 |
| Implantation depth LCC [mm] | 6.9±0.43 | 6.3±0.28 | 0.25 |
| Peak-to-peak gradient pre-TAVI [mmHg] | 49.8±4.3 | 51.8±3.2 | 0.73 |
| peak-to-peak gradient post-TAVI [mmHg] | 4.9±0.7 | 8.9±0.6 | 0.0003 |
| Implantation of >1 valve | 2 (3) | 0 (0) | |
| Aortic regurgitation index | 21.3±1.0 | 24.9±1.1 | 0.04 |
| Cover index | 15.3±0.56 | 17.1±0.33 | 0.01 |
| Contrast amount [ml] | 149.6±7.8 | 155.9±5.7 | 0.59 |
| Fluoroscopy time [min] | 29.0±1.8 | 25.3±1.2 | 0.06 |
| Recapture during valve implantation | n.a. | 19 (22) | |
| 1 full recapture | n.a. | 10 (12) | |
| 2 full recaptures | n.a. | 9 (10) | |
| Sheath to femoral artery ratio | 0.96 | 0.91 | 0.15 |
| Conversion to surgery | 0 (0) | 1 (3) | 0.62 |
Values are mean ± standard error of mean or n (%).
| CoreValve | Evolut R | p -Value | |
|---|---|---|---|
| Procedural mortality | 0 (0) | 0 (0) | |
| Coronary obstruction | 0 (0) | 0 (0) | |
| Annular rupture | 0 (0) | 0 (0) | |
| LV perforation | 0 (0) | 1 (3) | 0.62 |
| Aortic regurgitation by echocardiography | 0.0002 | ||
| None/trace | 24 (38) | 52 (62) | |
| Mild | 30 (48) | 32 (38) | |
| Moderate | 9 (14) | 0 (0) | |
| Severe | 0 (0) | 0 (0) | |
| AV peak gradient [mmHg] | 12.8±0.8 | 16.2±0.9 | 0.016 |
| AV mean gradient [mmHg] | 6.9±0.4 | 8.3±0.4 | 0.032 |
| Minor vascular complications | 8 (12) | 6 (7) | 0.35 |
| Implantation of covered stent | 5 (8) | 6 (7) | |
| Surgical repair | 3 (4) | 0 (0) | |
| Device success (VARC-2) | 54 (82) | 85 (99) | 0.0009 |
Values are mean ± standard error of mean or n (%).
|
|
|
Fig. 2. Degree of residual aortic regurgitation after TAVI as assessed by transthoracic echocardiography (left columns) or aortic root angiography (right columns). |
3.3. Procedural outcome
Despite our high-risk patient collective with a mean logistic EuroScore > 25% in both groups (Table 1 ), there was no procedural mortality in either group (Table 4 ). One patient in the EVR group developed a significant pericardial effusion after implantation. Pericardiocentesis showed hemorrhage due to left ventricular perforation by the guidewire, requiring conversion to open chest surgery. There were no other procedure related acute events in both groups (Table 4 ). Vascular complications occurred in 12.1% in CV treated vs. 7.0% in EVR treated patients (p = 0.35). In the EVR group, all of these were resolved by implantation of a covered stent and in the CV group 3 patients (4.5%) underwent surgical repair. The lower delivery profile of the EVR allowed transfemoral access despite challenging anatomies with smaller access site vessel diameter as compared to the CV group. As a consequence, the sheath to femoral artery ratio (SFAR) [3] was comparable in both groups (CV 0.96 vs. EVR 0.91).
Device success was significantly higher with EVR (CV 81.8% vs. EVR 98.8%; p = 0.0009), mainly driven by the implantation of a second valve (n = 2) and more cases of more-than-mild residual moderate aortic regurgitation (n = 6) in the CV treated patient group. In accordance with the invasive recordings, echocardiographic assessment of valvular function on days 2–5 showed higher transvalvular gradients in the EVR group (mean gradient CV 6.9 mm Hg vs. EVR 8.3 mm Hg; p = 0.032). Conversely, the presence of more-than-mild residual aortic regurgitation was lower in the EVR group (Table 4 ).
3.4. Early safety outcome
With respect to VARC-2 safety endpoints at 30 days, there were no significant differences between both valve types as shown in Table 5 . In particular, the incidence of new pacemaker implantations following asystole or third degree AV block was similar in both groups (CV 9.1% vs. EVR 12.8%), while more patients in the CV group had a previously implanted pacemaker (Table 1 ).
| CoreValve | Evolut R | p -Value | |
|---|---|---|---|
| Early safety endpoint at 30days (VARC-2) | 1 (1) | 1 (3) | 0.62 |
| All cause mortality | 1 (1) | 0 (0) | 0.86 |
| All stroke | 0 (0) | 0 (0) | |
| Life-threatening bleeding | 0 (0) | 0 (0) | |
| Acute kidney injury stage 2–3 | 0 (0) | 0 (0) | |
| Coronary artery obstruction | 0 (0) | 0 (0) | |
| Major vascular complication | 0 (0) | 1 (3) | 0.62 |
| Valve-related dysfunction requiring repeat procedure | 0 (0) | 0 (0) | |
| New pacemaker implantation by indication | |||
| Asystole | 1 (1) | 3 (3) | 0.85 |
| 2nd/3rd degree AVB | 5 (8) | 8 (9) | 0.97 |
| Postprocedural hospital stay [days] | 9.3±0.6 | 8.4±0.7 | 0.35 |
| Acute kidney injury stage 1 (VARC-2) | 2 (3) | 0 | 0.34 |
Values are mean ± standard error of mean or n (%).
4. Discussion
The Medtronic CV is the prototype of a self-expanding, transcatheter aortic bioprosthesis with excellent long-term clinical outcome [6] . However, a residual more than mild aortic regurgitation was more frequently observed in patients treated with CV than those treated with balloon-expandable valves [1] , with a reported impact on patient prognosis [9] . Therefore, the 2nd generation EVR valve was recently released with a new design and delivery system aimed to facilitate the implantation process and to reduce paravalvular leaks. Important new features of the EVR bioprosthesis include the option to recapture and reposition the valve during deployment, an extended sealing skirt, more consistent radial forces of the nitinol frame and a smaller insertion profile.
In our initial experience patient population, the option to recapture was used in 22.1% of the EVR implantation procedures, mainly due to a deep position of the prosthesis in the left ventricular outflow tract. This maneuver may prevent suboptimal valve deployment and allows delivery of the prosthesis at the intended position 3–5 mm below the native annulus. This was achieved in a real life scenario, as the average implantation depth at the non-coronary cusp was 4.0 mm for the EVR and slightly deeper with the CV prosthesis (average 5.3 mm). Even more important, supra-annular valve deployment happened in 2 patients treated with CV but was absent in the EVR group as a result of the recapture option.
As a possible consequence of less implantation depth, the EVR prosthesis had a lower incidence of moderate aortic regurgitation assessed by angiography, transthoracic echocardiography and aortic regurgitation index. Other features such as the extended sealing skirt or more consistent radial force may have contributed to this observation. In addition, the cover index was higher in the EVR as compared to the CV group, indicating a higher degree of prosthesis oversizing that may also increase the ability of the prosthesis to seal the native annulus.
On the other hand, oversizing may result in incomplete expansion and higher transvalvular gradients after implantation, which were indeed observed in EVR treated patients. The difference in postprocedural transvalvular gradients was particularly evident in the invasive recordings immediately after implantation. Since the nitinol frame of both prostheses exerts continuous radial force towards the annulus and adjacent structures, it may be that – particularly in patients with a more “oversized” annular-prosthetic ratio – smaller changes of prosthetic apposition occur during the first hours after implantation. This may explain why the gradients determined by echocardiography between days 2 and 5 after the procedure only show a smaller (albeit still statistically significant) difference. Most importantly, mean postprocedural gradients were within or even below the “normal” range of published mean TAVI transprosthetic gradients [11] (CV 6.9 ± 0.4 mm Hg, EVR 8.3 ± 0.4 mm Hg) and they were compatible with normal prosthetic function according to the VARC-2 criteria (mean gradient < 20 mm Hg). In addition, no events of annular rupture or other harmful effects possibly related to prosthesis oversizing were observed. To our knowledge, no data are available to date on whether differences in transvalvular gradients within the normal range may affect clinical outcomes. Since in our study the differences in hemodynamic gradients are small and were even less pronounced in postprocedural echocardiography, we believe that these findings are of minor clinical importance.
Another important progress with EVR was the smaller insertion profile of 18F outer diameter (inline sheath of the EVR delivery system) as compared to 22F outer diameter for the 18F sheath generally used for CV implantation. Smaller sheath sizes will automatically decrease the sheath to the femoral artery ratio, which was associated with fewer vascular complications in a previous study [3] . However, the smaller insertion profile of the EVR allows transfemoral access in more challenging anatomies. In our patient subset, vascular complications and sheath/femoral artery ratio were comparable between groups despite a smaller entry vessel diameter in the EVR group, indicating that patients treated with EVR had a more challenging iliofemoral vessel anatomy. Whether the relatively low observed rate of vascular complications justifies the additional radiation and contrast exposure necessary for the routine placement of a crossover safety wire is open to discussion.
A major downside of TAVI is the frequent need for permanent pacing after the procedure, as valve expansion (required for a stable valve position) may lead to mechanically induced conduction disturbances. The CV system had higher rates of post-procedural permanent pacemaker implantations when compared to balloon-expandable valves, mostly owing to an unintended deep positioning of the self-expandable nitinol frame. Although the recapture option of the EVR prosthesis prevented deep valve deployment also in our study, this had no impact on the incidence of new permanent pacemaker implantation due to asystole or third degree AV block. However, it has to be noted that the implantation depth of the CV in our study was only 5.3 mm on average, which is clearly a higher implantation approach than the 9.9 mm reported in earlier studies [9] . Another factor that may affect pacemaker rate is the degree of oversizing [7] . Since EVR treated patients in our study had a higher cover index (Table 3 ), this may have increased the need for permanent pacing.
4.1. Limitations
The single-center observational retrospective analysis of 151 consecutive patients undergoing transfemoral self-expandable TAVI reported here cannot be considered as a true head-to-head comparison of the two TAVI systems. Albeit multimodal, the assessment of post-TAVI aortic regurgitation was not performed by core laboratory evaluation. The number of patients treated and the lack of longer-term follow-up to date do not allow for conclusions on clinical outcomes or the incidence of rare complications. However, in the evolving field of interventional heart valve therapy and inherent technical innovations, such observational data reflecting initial experience in “real world” patients provide valuable timely clinical information. Additional larger scale multi-center evaluation comprising a longer follow-up period will have to assess the potential clinical differences of EVR and CV observed in the present analysis.
5. Conclusions
The recapture option of the Medtronic EVR transcatheter bioprosthesis leads to a significantly higher rate of device success and a lower implantation depth as compared to the preceding CV system. Together with an extended sealing skirt and more consistent radial forces, this feature of EVR prevented the incidence of more than mild residual aortic regurgitation more efficiently in an all-comer clinical setting. The rates of vascular complications as well as post-procedural pacemaker implantation were similar and need to be addressed in larger scale clinical studies.
Abbreviations
CV
Medtronic CoreValve transcatheter aortic valve bioprosthesis
EVR
Medtronic Evolut R transcatheter aortic valve bioprosthesis
F
French (1/3 mm)
LBBB
left bundle branch block
LCC
left coronary cusp
NCC
non-coronary cusp
SFAR
sheath to femoral artery ratio
TAVI
transcatheter aortic valve implantation
VARC-2
Valve Academic Research Consortium-2
Conflicts of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
None.
References
- [1] M. Abdel-Wahab, J. Mehilli, C. Frerker, F.J. Neumann, T. Kurz, R. Tolg, D. Zachow, E. Guerra, S. Massberg, U. Schafer, M. El-Mawardy, G. Richardt; Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial; JAMA, 311 (2014), pp. 1503–1514 http://dx.doi.org/10.1001/jama.2014.3316
- [2] D. Detaint, L. Lepage, D. Himbert, E. Brochet, D. Messika-Zeitoun, B. Iung, A. Vahanian; Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence; JACC Cardiovasc. Interv., 2 (2009), pp. 821–827 http://dx.doi.org/10.1016/j.jcin.2009.07.003
- [3] K. Hayashida, T. Lefevre, B. Chevalier, T. Hovasse, M. Romano, P. Garot, D. Mylotte, J. Uribe, A. Farge, P. Donzeau-Gouge, E. Bouvier, B. Cormier, M.C. Morice; Transfemoral aortic valve implantation new criteria to predict vascular complications; JACC Cardiovasc. Interv., 4 (2011), pp. 851–858 http://dx.doi.org/10.1016/j.jcin.2011.03.019
- [4] A.P. Kappetein, S.J. Head, P. Genereux, N. Piazza, N.M. van Mieghem, E.H. Blackstone, T.G. Brott, D.J. Cohen, D.E. Cutlip, G.A. van Es, R.T. Hahn, A.J. Kirtane, M.W. Krucoff, S. Kodali, M.J. Mack, R. Mehran, J. Rodes-Cabau, P. Vranckx, J.G. Webb, S. Windecker, P.W. Serruys, M.B. Leon; Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2); Eur. J. Cardiothorac. Surg., 42 (2012), pp. S45–S60 http://dx.doi.org/10.1093/ejcts/ezs533
- [5] N. Piazza, G. Martucci, K. Lachapelle, B. de Varennes, L. Bilodeau, J. Buithieu, D. Mylotte; First-in-human experience with the Medtronic CoreValve Evolut R; EuroIntervention, 9 (2014), pp. 1260–1263 http://dx.doi.org/10.4244/EIJV9I11A215
- [6] J.J. Popma, D.H. Adams, M.J. Reardon, S.J. Yakubov, N.S. Kleiman, D. Heimansohn, J. Hermiller Jr., G.C. Hughes, J.K. Harrison, J. Coselli, J. Diez, A. Kafi, T. Schreiber, T.G. Gleason, J. Conte, M. Buchbinder, G.M. Deeb, B. Carabello, P.W. Serruys, S. Chenoweth, J.K. Oh; Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery; J. Am. Coll. Cardiol., 63 (2014), pp. 1972–1981 http://dx.doi.org/10.1016/j.jacc.2014.02.556
- [7] T. Schroeter, A. Linke, M. Haensig, D.R. Merk, M.A. Borger, F.W. Mohr, G. Schuler; Predictors of permanent pacemaker implantation after Medtronic CoreValve bioprosthesis implantation; Europace, 14 (2012), pp. 1759–1763 http://dx.doi.org/10.1093/europace/eus191
- [8] R.D. Sellers, M.J. Levy, K. Amplatz, C.W. Lillehei; Left retrograde cardioangiography in acquired cardiac disease: technic, indications and interpretations in 700 cases; Am. J. Cardiol., 14 (1964), pp. 437–447
- [9] J.M. Sinning, C. Hammerstingl, M. Vasa-Nicotera, V. Adenauer, S.J. Lema Cachiguango, A.C. Scheer, S. Hausen, A. Sedaghat, A. Ghanem, C. Muller, E. Grube, G. Nickenig, N. Werner; Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation; J. Am. Coll. Cardiol., 59 (2012), pp. 1134–1141 http://dx.doi.org/10.1016/j.jacc.2011.11.048
- [10] A. Vahanian, O. Alfieri, F. Andreotti, M.J. Antunes, G. Baron-Esquivias, H. Baumgartner, M.A. Borger, T.P. Carrel, M. De Bonis, A. Evangelista, V. Falk, B. Iung, P. Lancellotti, L. Pierard, S. Price, H.J. Schafers, G. Schuler, J. Stepinska, K. Swedberg, J. Takkenberg, U.O. Von Oppell, S. Windecker, J.L. Zamorano, M. Zembala; Guidelines on the management of valvular heart disease (version 2012); Eur. Heart J., 33 (2012), pp. 2451–2496 http://dx.doi.org/10.1093/eurheartj/ehs109
- [11] J. Wohrle, B. Gonska, C. Rodewald, U. Trepte, S. Koch, D. Scharnbeck, J. Seeger, S. Markovic, W. Rottbauer; Transfemoral aortic valve implantation with the repositionable Lotus valve compared with the balloon-expandable Edwards Sapien 3 valve; Int. J. Cardiol., 195 (2015), pp. 171–175 http://dx.doi.org/10.1016/j.ijcard.2015.05.139
Document information
Published on 19/07/16
Licence: CC BY-NC-SA license
Share this document
claim authorship
Are you one of the authors of this document?