Summary
Background/Objective
The concept of salvage pulmonary resection after definitive chemoradiotherapy (dCRT) is not yet commonly accepted in lung cancer treatment. We report our experience of eight patients in whom we performed salvage pulmonary resection for residual disease or isolated locoregional recurrence detected after dCRT.
Methods
Between 2005 and 2014, we performed salvage pulmonary resection for eight patients with N2 Stage-IIIA non-small cell lung cancer. The patients had initially received dCRT (radiation ≤ 60 Gy), but eventually underwent pulmonary resection with curative intent for residual disease or isolated locoregional recurrence. The postoperative complications, incidence of recurrence, and survival parameters were evaluated.
Results
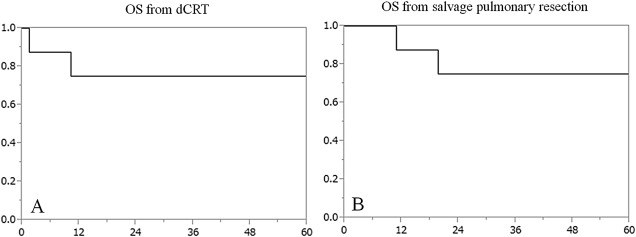
Salvage pulmonary resection was performed in four patients with residual disease and four patients with locoregional recurrence. Complete resection was successfully performed in all eight patients. Postoperative complications were observed in three patients, however, there were no postoperative mortalities. One patient developed local recurrence in a mediastinal lymph node and two patients died. Of the two fatalities, one was related to lung cancer. The estimated 5-year survival rate of the eight patients was 75.0%.
Conclusion
We report our experience of salvage pulmonary resection performed for residual disease or isolated locoregional recurrence diagnosed after dCRT in eight patients with locally advanced lung cancer. Although the postoperative complication rate was high, the survival data were favorable. A larger study is needed to confirm the safety and feasibility of salvage pulmonary resection after dCRT.
Keywords
chemotherapy;lung cancer;outcomes;radiation;salvage resection;surgery
1. Introduction
In patients with esophageal cancer, salvage esophagectomy is sometimes performed after definitive chemoradiation therapy (dCRT).1; 2 ; 3 Salvage esophagectomy can be defined in two ways: (1) esophagectomy performed for isolated locoregional recurrence developing after initial complete response to dCRT and (2) esophagectomy performed for residual disease detected after dCRT. Salvage esophagectomy carries definite risks, but could improve outcomes and is considered a valid therapeutic option in selected patients with isolated locoregional recurrence or residual disease detected after dCRT. The concept of salvage pulmonary resection is, however, not yet common in the area of lung cancer. We report our experience of salvage pulmonary resection performed in eight patients with N2 Stage-IIIA non-small cell lung cancer. The patients had initially been treated by dCRT (radiation ≤ 60 Gy), but eventually underwent pulmonary resection with curative intent for residual disease or isolated locoregional recurrence.
2. Methods
Salvage pulmonary resection was defined as follows in this study: pulmonary resection performed in patients with pathologically proven N2 Stage-IIIA non-small cell lung cancer who had received dCRT (radiation ≤ 60 Gy), which was not initially intended as an induction treatment for surgery, but who subsequently underwent pulmonary resection with curative intent for residual disease detected after dCRT or for isolated locoregional recurrence developing after initial complete response to dCRT. Residual disease in this study was defined as disease that showed good response to dCRT, but with a small amount of disease remaining at one or two sites. Locoregional recurrence was defined as disease developing after initial complete response to the dCRT. The candidates for salvage pulmonary resection were carefully evaluated by our Cancer Board for pulmonary function, performance status, comorbidity, and curability. Between 2005 and 2014, we performed salvage pulmonary resection in eight patients. The clinical data of the eight patients, including tumor histology, disease stage at diagnosis, treatments administered before the salvage resection, time interval from the first treatment to the salvage resection, surgical procedures employed, postoperative complications, and survival and recurrence data were analyzed. The 5-year survival rate was calculated using JMP, version 10 (SAS Institute Inc., Cary, NC, USA). Patients seen before 2009 were staged according to the sixth edition of the tumor, nodes, metastases (TNM) staging system and patients seen after 2010 were staged according to the seventh edition. This retrospective study was conducted with the approval of Shikoku Cancer Center hospital Internal Review Board.
3. Results
The characteristics of the eight patients who underwent salvage pulmonary resection are listed in Table 1. All eight patients were male and the median age was 61 years. The initial stage was N2 Stage IIIA in all eight patients, with N2 confirmed histologically in only two patients. The treatments administered before salvage resection were dCRT in six patients and dCRT followed by second-line chemotherapy in two patients. In four patients, the salvage resection was performed for residual disease, while in the remaining four patients the salvage resection was performed for isolated locoregional recurrence. A typical case of salvage pulmonary resection is shown in Figure 1. The primary tumor was located in the right upper lobe. Positron emission tomography-computed tomography (PET/CT) showed fluorine-18-deoxyglucose (FDG) accumulation in the primary tumor, #2R, #4R, and #10R. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) revealed adenocarcinoma in #4R. This patient was diagnosed as having T1N2M0 Stage IIIA disease and was treated with dCRT. PET/CT after completion of the dCRT revealed no evidence of disease in the primary tumor, #2R or #10R, but FDG accumulation was still observed in #4R. This patient was considered to have residual disease in #4R, and underwent salvage pulmonary resection.
| No. | Age (y) | Initial T | Initial N | Histological subtype | Treatments administered before salvage | Reason for salvage | Time interval from dCRT to salvage (mo) |
|---|---|---|---|---|---|---|---|
| 1 | 66 | 1a | 2 | Ad | CDDP + DOC + RT | Residual disease | 3.0 |
| 2 | 61 | 2a | 2 | SCC | CDDP + VNR + RT | Residual disease | 9.4 |
| 3 | 62 | 3 | 2 | SCC | CDDP + VNR + RT | Residual disease | 9.4 |
| 4 | 54 | 1 | 2 | SCC | CDDP + S-1 + RT → CDDP + GEM | Locoregional recurrence | 21.0 |
| 5 | 56 | 1 | 2 | SCC | CBDCA + PTX + RT → PTX | Locoregional recurrence | 49.8 |
| 6 | 61 | 1 | 2a | Ad | CDDP + DOC + RT | Locoregional recurrence | 19.7 |
| 7 | 57 | 1 | 2a | Ad | CDDP + DOC + RT | Residual disease | 3.2 |
| 8 | 67 | 2b | 2 | SCC | CDDP + DOC + RT | Locoregional recurrence | 14.5 |
| Extent of resection | Postoperative complication | Adjuvant chemotherapy | pT | pN | Recurrence | Alive or dead | OS from salvage (mo) |
|---|---|---|---|---|---|---|---|
| Lobectomy | Chylothorax (Grade 2) | No | 1a | 0 | No | Alive | 48.2 |
| Lobectomy + PA plasty | Pneumonia (Grade 3), Recurrent laryngeal nerve palsy (Grade 2) | No | 1b | 2 | No | Unknown death | 1.5 |
| Lobectomy | Pleural effusion (Grade 2) | No | 3 | 0 | Mediastinal lymph node | Lung cancer-related death | 10.3 |
| Lobectomy + PA plasty + bronchoplasty | None | No | 0 | 2 | No | Alive | 78.9 |
| Lobectomy + bronchoplasty | None | No | 2 | 0 | No | Alive | 80.0 |
| Lobectomy | None | CDDP + VNR | 1 | 2 | No | Alive | 64.1 |
| Lobectomy | None | CDDP + VNR | 1 | 2 | No | Alive | 60.9 |
| Pneumonectomy | None | No | 1a | 0 | No | Alive | 12.1 |
Ad = adenocarcinoma; CBDCA = carboplatin; CDDP = cisplatin; dCRT = definitive chemoradiotherapy; DOC = docetaxel; GEM = gemcitabine; OS = overall survival; PA = pulmonary artery; PTX = paclitaxel; RT = radiation; SCC = squamous cell carcinoma; VNR = vinorelbine.
a. N2 was confirmed histologically.
|
|
|
Figure 1. Images of positron emission tomography-computed tomography obtained before definitive chemoradiotherapy and before salvage resection in a typical case. dCRT = definitive chemoradiotherapy; OS = overall survival. |
The median time interval from initial treatment to salvage pulmonary resection was 9.4 months (3–50 months). Lobectomy was performed in four patients, lobectomy + pulmonary artery plasty in one patient, lobectomy + bronchoplasty in one patient, lobectomy + pulmonary artery plasty + bronchoplasty in one patient, and pneumonectomy in one patient. Complete resection (R0) was achieved in all eight patients. Postoperative complications were observed in three patients, however, there was no postoperative mortality. Adjuvant treatments were administered after the salvage resection in two patients. The median follow-up period after the salvage pulmonary resection was 48 months (range, 1.5–80 months). One patient developed mediastinal lymph node recurrence during the follow-up period. Two patients died during the study period, with one of these two patients dying of lung cancer 10 months after the salvage pulmonary resection and the other from an unknown cause. The estimated 5-year survival rate from the salvage pulmonary resection was 75.0%.
4. Discussion
The concept of salvage pulmonary resection in the area of lung cancer treatment is not yet commonly accepted and the definitions of salvage pulmonary resection vary among investigators. While several investigators have reported and refer to pulmonary resection after stereotactic radiotherapy as salvage resection,4 ; 5 other investigators refer to pulmonary resection after chemotherapy or Iressa as salvage resection.6; 7 ; 8 In the field of esophageal cancer treatment, salvage esophagectomy is defined as esophagectomy with curative intent for residual disease or isolated locoregional recurrence diagnosed after dCRT.1; 2; 3 ; 9 In this study, we applied a similar definition and evaluated the outcomes of salvage pulmonary resection.
Diagnosis of residual disease or recurrence should be made carefully, because it is critical to determine the indications of salvage pulmonary resection appropriately. In general, transbronchial lung biopsy, EBUS-TBNA, or CT-guided needle biopsy are performed to obtain histological confirmation during the process of lung cancer diagnosis. Histological confirmation is, however, often difficult in patients with suspected residual disease or recurrence diagnosed after dCRT. In such cases, diagnosis of residual disease or recurrence is usually made based on imaging findings. Recently, it has been reported that PET/CT is useful for prediction of response to chemotherapy and/or radiation and that PET-CT findings are correlated with survival.10 ; 11 PET/CT is also considered to be useful to detect recurrence or residual disease, but its accuracy and specificity in this regard remain unproven.12 ; 13 In this study, the diagnosis of residual disease or recurrence was made based on PET/CT findings in all patients and no histological confirmation was obtained before salvage pulmonary resection. Postoperative histological examination revealed viable cells in all the lesions detected by PET/CT. Diagnosis of residual disease or locoregional recurrence, however, should be carefully made.
Baumann et al14 reported on a series of 24 cases of salvage pulmonary resection performed after definitive radiation with or without chemotherapy. They reported postoperative mortality, morbidity, and 3-year survival rates after salvage pulmonary resection of approximately 4%, 58%, and 47%, respectively, and concluded that while salvage pulmonary resection entailed some risk, it could potentially offer long-term survival. Albain et al15 reported a phase III study of induction chemoradiotherapy followed by resection. They reported a good local control rate of as low as 10% and a 5-year survival rate of 27%, while the perioperative mortality rate was 8%, which was ∼4–8-times as high as that of ordinary lobectomy for lung cancer.16; 17 ; 18 They concluded that pulmonary resection after induction chemoradiotherapy improved overall survival, with a lower incidence of local recurrence and acceptable mortality. By contrast, a standard treatment for residual disease or locoregional recurrence after dCRT is chemotherapy. The reported response rate to chemotherapy in patients with a previous history of chemotherapy is only ∼10%, and the efficacy of this treatment is limited.19 ; 20 Cure can rarely be expected from chemotherapy treatment in patients with residual disease or isolated locoregional recurrence diagnosed after dCRT. Considering these issues, salvage pulmonary resection in patients with residual disease or locoregional recurrence after dCRT could be taken into consideration, provide good local control, and potentially offer longer-term survival.
Regarding locoregional recurrence, salvage pulmonary resection was performed in those patients who had initially shown complete response to dCRT and developed only locoregional recurrence without distant metastasis. The tumor behavior in such patients was considered to be less aggressive and had a tendency to remain localized, therefore, the outcome of salvage resection in this patient population was expected to be good. In patients with residual disease, salvage pulmonary resection was performed only in patients who showed good response to dCRT and had residual disease in only one or two sites. In studies of induction chemoradiotherapy followed by surgery, patients with good response, stable disease, and progressive disease have been evaluated together based on the rule of intent to treatment. Outcomes, however, would be better if only patients who showed good response to induction chemoradiotherapy were selected and evaluated. The patients with residual disease in this study are considered to be similar to patients who showed good response to induction chemoradiotherapy. These characteristics may explain the good 5-year survival rate of salvage pulmonary resection observed in this study.
This study has some distinct limitations. Diagnosis of N2 was confirmed histologically in only two patients and was determined based on imaging findings, including findings using PET/CT, in the remaining six patients. A doubt was raised in this study concerning whether the initial N2 diagnosis was accurate. The study was retrospective in nature, based on clinical records, and this limitation is always involved. The other issue might involve the small sample size of eight patients. Many of our patients who underwent dCRT for N2 Stage-IIIA disease developed distant metastasis after dCRT, with only a few developing locoregional recurrences without distant metastasis. Regarding residual disease, salvage pulmonary resection was performed in this study only in patients who showed good response to dCRT, with residual disease remaining in only one or two sites. Moreover, salvage pulmonary resection was performed only in healthy patients and not in patients with a poor performance status and organ dysfunction, even if the patients had only resectable disease. Thus, candidates for salvage pulmonary resection are considered to be very limited and only eight patients were, therefore, identified as suitable candidates for salvage pulmonary resection during the 10-year period. It is difficult to evaluate the safety and feasibility of salvage pulmonary resection after dCRT from the results of this study, since this study was retrospective in nature and the sample size was too small. Therefore, a large and ideally prospective study is needed to confirm our findings.
In conclusion, we report the eight cases of salvage pulmonary resection performed for residual disease or isolated locoregional recurrence diagnosed after dCRT. The postoperative complication rate was high, although the survival data were favorable. A larger study is needed to evaluate the feasibility of salvage pulmonary resection after dCRT.
References
- 1 S. Borghesi, M.A. Hawkins, D. Tait; Oesophagectomy after definitive chemoradiation in patients with locally advanced oesophageal cancer; Clin Oncol (R Coll Radiol), 20 (2008), pp. 221–226
- 2 X.B. D'Journo, P. Michelet, L. Dahan, et al.; Indications and outcome of salvage surgery for oesophageal cancer; Eur J Cardiothorac Surg, 33 (2008), pp. 1117–1123
- 3 W.L. Hofstetter; Salvage esophagectomy; J Thorac Dis, 6 (Suppl. 3) (2014), pp. S341–349
- 4 F. Chen, Y. Matsuo, A. Yoshizawa, et al.; Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients; J Thorac Oncol, 5 (2010), pp. 1999–2002
- 5 P.E. Van Schil; Salvage surgery after stereotactic radiotherapy: a new challenge for thoracic surgeons; J Thorac Oncol, 5 (2010), pp. 1881–1882
- 6 K. Takamochi, K. Suzuki, H. Sugimura, et al.; Surgical resection after gefitinib treatment in patients with lung adenocarcinoma harboring epidermal growth factor receptor gene mutation; Lung Cancer, 58 (2007), pp. 149–155
- 7 H. Uramoto, F. Tanaka; Salvage thoracic surgery in patients with primary lung cancer; Lung Cancer, 84 (2014), pp. 151–155
- 8 T. Hishida, K. Nagai, T. Mitsudomi, et al.; Salvage surgery for advanced non–small cell lung cancer after response to gefitinib; J Thorac Cardiovasc Surg, 140 (2010), pp. e69–e71
- 9 J.L. Marks, W. Hofstetter, A.M. Correa, et al.; Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma; Ann Thorac Surg, 94 (2012), pp. 1126–1133
- 10 T. Berghmans, M. Dusart, M. Paesmans, et al.; Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project; J Thorac Oncol, 3 (2008), pp. 6–12
- 11 N. Al-Sarraf, K. Gately, J. Lucey, et al.; Clinical implication and prognostic significance of standardised uptake value of primary non-small cell lung cancer on positron emission tomography: analysis of 176 cases; Eur J Cardiothorac Surg, 34 (2008), p. 892
- 12 C. Poettgen, D. Theegarten, W. Eberhardt, et al.; Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer; Oncology, 73 (2007), pp. 316–323
- 13 J.-S. Ryu, N.C. Choi, A.J. Fischman, T.J. Lynch, D.J. Mathisen; FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology; Lung Cancer, 35 (2002), p. 179
- 14 J.E. Bauman, M.S. Mulligan, R.G. Martins, B.F. Kurland, K.D. Eaton, D.E. Wood; Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes; Ann Thorac Surg, 86 (2008), p. 1632
- 15 K.S. Albain, R.S. Swann, V.W. Rusch, et al.; Radiotherapy plus chemotherapy with or without surgical resection for Stage III non-small-cell lung cancer: a phase III randomised controlled trial; Lancet, 374 (2009), pp. 379–386
- 16 R.J. Ginsberg, L.V. Rubinstein; Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung cancer study group; Ann Thorac Surg, 60 (1995), pp. 615–622 discussion 622–613
- 17 G. Myrdal, G. Gustafsson, M. Lambe, L.G. Horte, E. Stahle; Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity; Eur J Cardiothorac Surg, 20 (2001), pp. 694–699
- 18 H. Asamura, T. Goya, Y. Koshiishi, et al.; A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers; J Thorac Oncol, 3 (2008), pp. 46–52
- 19 F.V. Fossella, R. DeVore, R.N. Kerr, et al.; Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 non-small cell lung cancer study group; J Clin Oncol, 18 (2000), pp. 2354–2362
- 20 N. Hanna, F.A. Shepherd, F.V. Fossella, et al.; Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy; J Clin Oncol, 22 (2004), pp. 1589–1597
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?