Abstract
Hecogenin is a sapogenin found in Agave species in high quantities and is responsible for the many therapeutic effects of these medicinal plants. In addition, this compound is also widely used in the pharmaceutical industry as a precursor for the synthesis of steroidal hormones and anti-inflammatory drugs. Despite Hecogenin being widely used, little is known about its toxicological properties. Therefore, the present study aimed to investigate the cytotoxic, genotoxic and mutagenic effects of Hecogenin on HepG2 cells. Cytotoxicity was analyzed using the MTT test. Then, genotoxic and mutagenic potentials were assessed by comet assay and cytokinesis-block micronucleus assay, respectively. Cytotoxic effect was observed only when cells were exposed to concentrations of Hecogenin equal or higher than 100 μM. Although a lower concentration of Hecogenin caused DNA damage, a reduction on nuclear mutagenic markers in HepG2 cells was observed. The results indicated that Hecogenin treatment generated DNA damage, but in fact it would be repaired, avoiding dissemination of the damage throughout the cell division. Further studies need to be performed to confirm the observed protective effect of Hecogenin against genomic instability.
Keywords
Hecogenin ; CBMN ; Genotoxicity ; Comet assay
1. Introduction
Sapogenins are the non-polar residue of amphipathic glycosides named Saponins [1] . The therapeutic properties of different classes of saponins such as analgesic, anti-inflammatory and anti-tumoral effects have already been demonstrated [2] . Furthermore, sapogenins are of great interest for the pharmaceutical industry as a source for the development of new drugs [3] .
Hecogenin is a sapogenin found in Agave sisalana species (commonly known as “sisal”), which are extensively spread throughout tropical and subtropical regions [4] . Brazil is one of the largest producers of sisal, representing 69% of worldwide production [5] . Plants of the Agave genus are used as therapeutic agents by Chinese Traditional Medicine in scabiosis, in reducing pain, treating different inflammatory conditions and even against cancer [6] .
Hecogenin has been indicated as the responsible agent for sisal therapeutic effect due to its beneficial properties involving anti-inflammatory, antioxidant, antifungal, hypotensive, anti-hyperalgesic and anti-nociceptive effects [7] , [8] and [9] . Moreover, Hecogenin is used in the pharmaceutical industry as a precursor of steroidal anti-inflammatory and steroidal hormone drugs [10] . Despite Hecogenin being widely used and its beneficial effects, information about its toxicity is still very scarce. Currently, the information available about Hecogenin toxicological effects has been tested using a high concentration of Hecogenin-rich extracts on animal models [11] . Unlike animal models, in vitro cell-based models provide mechanistic understanding and present some advantages such as simplicity, low cost, and reproducibility. They are commonly used in basic science for pharmaceutical research along with toxicological investigation [12] .
In the literature, studies aimed to investigate Hecogenin mechanisms of cytotoxicity have been reported using different cell lines such as A549 human lung cancer cell, human rheumatoid arthritis synovial cell and 1547 osteosarcoma cell lines [13] , [14] and [15] . These cell lines are interesting models to investigate cytotoxic and anti-tumoral properties of a diversity of substances, but since Hecogenin is used as a medicinal molecule, it is important to take into account toxicology in environments that allow metabolization, simulating what happens in live organisms [16] . For instance, the Human hepatoma cell line (HepG2) that contains endogenous metabolizing enzymes is an in vitro model that helps to assess xenobiotics that need a previous bioactivation [17] . Until now, there have been no reports of Hecogenin toxicity using this cell line. In addition, genetic toxicology helps understand the deleterious effect in a subclinical stage caused by exposure to different toxic substances [18] . Using genetic biomarkers on this cell model would broaden the scope of the toxicological assessment. Therefore, this work aimed to assess cytotoxic, genotoxic and mutagenic potentials of Hecogenin on HepG2 cells.
2. Materials and methods
2.1. Reagents and compounds
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cytochalasin B, cyclophosphamide, Giemsa dye, silver nitrate and SYBR Green were purchased from Sigma-Aldrich (St. Louis, MO, EUA). Normal-melting point (NMP) agarose, low-melting point (LMP) agarose, Dulbecco’s modified Eagle’s medium (DMEM), streptomycin/penicillin, fetal bovine serum and trypsin were acquired from Life Technologies (Carlsbad, CA, EUA).
2.2. Hecogenin extraction from A. sisalana
Hecogenin was kindly provided by Prof. Dr. José Maria Barbosa-Filho, from the Pharmaceutical Sciences Department, UFPB. Protocol for the isolation, purification and identification performed for obtaining Hecogenin from A. sisalana is detailed in Cerqueira et al. [7] . First, 5 kg of A. sisalana leaves were extracted with ethanol using a soxhlet dispositive for 24 h. Afterward, solvent was removed under reduced pressure and the residue was hydrolyzed by refluxing with ethanolic hydrochloric acid for 4 h. After cooling and filtering, the acid-insoluble residue was extracted with hexane in a soxhlet apparatus for 12 h and the extract was re-crystallized with acetone and analyzed by MNR for identification. The Hecogenin was extracted by acetylation with an acetic anhydride/pyridine mixture, then the isolated Hecogenin was analyzed by HPLC and the purity reached 98%.
Hecogenin dry powder was suspended in acetone before use. The final acetone concentration in culture was less than 1%, as indicated by Burgess et al. [19] .
2.3. HepG2 cell culture
Human hepatoma cell lines (HepG2) were purchased from an American Culture Collection (ATCC, Rockville, MD). First, cells were cultivated in DMEM medium, supplemented with 10% fetal bovine serum, 1% streptomycin/penicillin and 2% l-glutamine at 37 °C and 5% CO2 .
2.4. Cell viability assessment (MTT test)
The MTT assay was performed to determine cell viability through the energetic cell metabolism. First, the MTT salt is reduced by succinic dehydrogenase in mitochondria to formazan, an insoluble violet crystal [20] . The MTT assay was performed following the protocol previously described by Mosmann [21] with minor modifications. Then, 104 cells per well were seeded in 96-well flat bottom culture plates and incubated overnight at 37 °C and 5% CO2 . After incubation, cells were exposed to concentrations of Hecogenin between 0 μM (negative control: medium + 1% vehicle), 10 μM, 50 μM, 100 μM, 150 μM and 200 μM for 24 h. MTT was added at a final concentration of 1 mg/mL and incubated for 4 h. Then, the medium was removed and 100% ethanol was added to dissolve the formed formazan crystals. Absorbance was determined at 570 nm in a spectrophotometer. The experiment was performed three times independently.
2.5. Genotoxic assessment (Comet assay)
Comet assay was performed to investigate the genotoxic effect of Hecogenin. This assay is based on the fact that damaged DNA loses its association with nuclear proteins while undamaged DNA does not. The resulting DNA fragments as consequence of the genetic damage can be observed microscopically after electrophoresis as “comets”, and the degree of DNA damage is estimated considering the tail size, the integrity of the nucleoid and the relationship of both [22] .
Comet assay was performed as previously described [22] with minor modifications. First, cells were seeded in 6-well plates and treated with four different concentrations of hecogenin, 0 μM (negative control: medium + 1% vehicle), 10 μM, 25 μM and 50 μM, and 30 μM of H2 O2 (positive control) for 24 h. Cell suspension was mixed with 1% (w/v) low-melting agarose and loaded onto slides pre-coated with 1.5% (w/v) normal melting agarose. After agarose solidification, the slides were submerged in lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris-HCl with 10% DMSO and 1% Triton X – 100 freshly added; pH 10.0; 3 h). Then, the nucleoids were submerged in electrophoresis buffer (10 M NaOH and 200 mM EDTA; pH 13.0, 4 °C) for 20 min for DNA denaturation. Electrophoresis was conducted for 30 min at 30 V and 400 mA. Afterward, the slides were washed with neutralization buffer (0.4 M of Tris-HCl buffer; pH 7.5) and absolute ethanol. Slides were stained with 0.02% silver nitrate solution, according to Nadin et al. [23] .
Fifty nucleoids per experiment were visually scored in an optical microscope (Olympus, Japan), totalizing 150 nucleoids per treatment, as described by Collins et al. [24] . Each comet was given an arbitrary unit of 0–4 (0–undamaged; 4–maximum damage). Damage score was thus assigned to each sample ranging from 0 (no damage: 50 cells x 0) to 200 (maximum damage: 50 cells x 4). Then, a mean was calculated for each treatment.
2.6. Mutagenicity assessment (CBMN assay)
DNA damage that overcame the repairing process can progress to chromosome abnormalities causing a mutagenic effect. Micronuclei (MN) characterized by lost genetic material from DNA double strand breaks acquiring the morphology of a small nucleus is one of resulting genetic consequence that can be seen in cells affected by mutagen substances and has been extensively used as a biomarker of mutagenicity. Moreover, other cytological characteristics help us understand the resulting genetic instability. For instance, amplified genes could be excluded from the nucleus, leaving a nucleoplasmic connection between it and the main nucleus, or could also be created from dicentric chromosomes that can form a continuous link between the nuclei in a binucleated cell. These clastogenic or aneugenic effects are characterized by the occurrence of nuclear buds (NBUDs), and nucleoplasmic bridges (NBRDs) [18] and [25] .
The CBMN assay was performed according to the protocol described by Vasquez et al. [26] , with minor modifications. First, HepG2 cells (5 × 105 ) were seeded in 6-well plates and allowed to attach overnight. Then, the cells were treated with 0 μM (negative control: medium + 1% vehicle), 10 μM, 25 μM and 50 μM of Hecogenin, and positive control (cyclophosphamide 0.2 mg/mL) for 24 h. Afterward, cytochalasin B was added (final concentration, 3.5 μg/mL) following 24 h of incubation. Cells were washed with hypotonic solution (0.075 M KCl, 4 °C, 3 min) and fixed with methanol and acetic acid (9:1). Slides were stained with 5% Giemsa solution and analyzed under light microscopy.
The Nuclear Division Index (NDI) is a useful parameter for comparing the cytostatic effects of substances evaluated in CBMN assay [25] . Five hundred viable cells were counted to determine NDI using the following formula [27] : NDI = (M1 + 2 M2 + 3 M3 + 4 M4 )/N, where M1 , M2,M3 and M4 are the number of cells with one, two, three and four (or more) nuclei and N is the number of assayed cells. For micronucleus (MN), nuclear bud (NBUD) and nuclear bridge (NBRD) quantification, 1000 binucleated cells were blind-scored in three independent experiments. The number of alterations in binucleated cells was determined following the examination criteria reported by Fenech [18] .
2.7. Statistical analysis
Comparison of the results among treatments was performed by Kruskal-Wallis test and the difference between groups was assessed by Mann-Whitney U test. Statistical differences were set for p ≤ 0.05. The statistical analysis was performed using SPSS software version 20.
3. Results
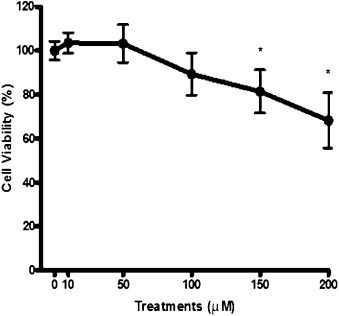
MTT assay was performed to assess the Hecogenin cytotoxicity. When treated with 10 μM and 50 μM of Hecogenin, the cells converted MTT to formazan in a quantity similar to negative control (culture medium + vehicle), revealing minimal changes in absorbance spectrum. Therefore, no cytotoxic effect was observed on HepG2 cells in this range of concentrations (Fig. 1 ). On the other hand, exposure to 100 μM of Hecogenin demonstrated a slight reduction in cell viability, although it did not reach statistical significance. In treatments over 100 μM, cell viability decreased significantly by about 30%.
|
|
|
Fig. 1. Cytotoxic effect of Hecogenin by MTT test. |
Comet assay was performed to evaluate genotoxic potential of Hecogenin. The results showed that all treatments of Hecogenin were capable of causing both overall DNA damage about two times the basal value observed in negative control, and also an increased frequency of high damage score (class 4) 4–6 times higher than the control (Table 1 ).
| Concentration | Number of cells in each comet class (mean ± SD) | Damage Score (mean ± SD) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| NC | 25.7±1.2 | 19.0±2.2 | 2.3±0.5 | 2.0±1.4 | 1.0±0.0 | 33.7±2.5 |
| PC | 0 * | 0 * | 9.0±3.5* | 23.0±4.3* | 18.0±1.3* | 159.0±17.0* |
| 10μM | 19.3±1.7* | 13.3±0.5* | 6.3±0.9* | 4.7±1.2 | 6.4±0.5* | 65.3±3.4* |
| 25μM | 16.0±1.4* | 20.8±2.7 | 4.7±1.2* | 4.0±1.4 | 4.5±0.7* | 56.7±9.3* |
| 50μM | 15.3±1.7* | 16.3±3.4 | 7.3±2.9* | 5.0±2.8 | 6.0±2.8* | 70.0±4.9* |
NC: Negative Control; PC: Positive Control (30 μM H2 O2 ).
- . Means p > 0.05.
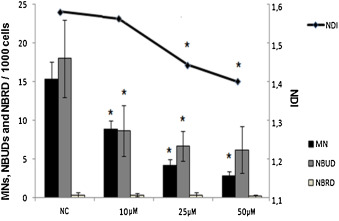
To assess mutagenic effect, CBMN assay was performed on HepG2 cells exposed to Hecogenin for 24 h. A reduction in NDI showed a significant cytostatic effect at Hecogenin concentrations of 25 μM and 50 μM. On the other hand, a reduction in MN and NBUD frequency was observed in every treatment assessed (Fig. 2 ).
|
|
|
Fig. 2. Mutagenic effect by CBMN test of Hecogenin. NC: Negative Control; MN: Micronucleus; NBUD: Nuclear Buds; NBRD: Nuclear Bridges. |
4. Discussion
Natural products used in traditional medicine are currently studied as potential sources of active principles for the treatment of different illness. Sapogenine-rich plants have shown many therapeutic properties [2] and [3] .
Nevertheless, the information in literature about cytotoxicity, genotoxicity and mutagenicity can have discrepancies, depending on the sapogenin or saponin studied. For instance, Corbiere et al. [28] , using an Osteosarcoma cell line found Hecogenin cytotoxic effect two-fold lower than that observed when testing diosgenin using the MTT test. In addition, the applied cellular model can show variability in the obtained results. For instance, Liagre et al. [14] reported a cytotoxic effect of Hecogenin through MTT assay in Human Rheumatoid Arthritis Fibroblasts-like synoviocyte cell line exposed to 40 μM, for 24 h. Also, Trouillas et al. [15] demonstrated a cytotoxic effect with a similar concentration and exposure time of Hecogenin in Human 1547 Osteosarcoma cells using the MTT test. In contrast, Fernández-Herrera et al. [29] , described low cytotoxicity from assayed Hecogenin derivatives using three different types of cancer cell lines.
In this work, Hecogenin cytotoxic effect was observed at concentrations equal or higher than 100 μM. The difference in the concentrations between the aforementioned studies and this work could be related to additional characteristics of the applied cellular model. For instance, in contrast with the other studies, Hecogenin was assessed on HepG2 cells, which have an endogenous capacity to biotransform xenobiotics [17] . A reduction in the Hecogenin toxicity could have taken place through a biotransformation process with a decrease in the resulting toxicity, as has been previously described for other natural products [30] . In addition, a cytostatic effect was observed. This fact was in agreement with a cell cycle arrest and an anti-proliferative effect on tumoral cells which have been previously described [28] , [29] and [31] .
The genotoxic and antigenotoxic effects of some saponins (the parent drug) have been described. For instance, Liu et al. [32] reported the ability of a mix of saponins extracted from plants of the Nuclea genus, widely used in African traditional medicine, to elicit DNA damage when tested on Chinese Hamster Ovary cells. On the other hand, the antigenotoxic effect of saponins has also been described. For instance, Zhao et al. [33] , reported protective effects of the saponin Dioscin against acetominophem genotoxicity in HepG2 cells, using a series of assays, including comet assay. Until now, no information in literature about genotoxic and/or mutagenic effects of Hecogenin has been described.
In this work, a genotoxic effect of Hecogenin in the whole range of concentrations tested was observed. However, the capability of inducing DNA damage was not related with the mutagenic pattern shown. In fact, a reduction in nuclear instability was observed. A dose-response reduction in MN and NBUD frequency was described. By taking these facts together, it is possible to speculate a protective capacity of Hecogenin. The observed DNA damage could be repaired, avoiding the occurrence of mutations, and also reducing the basal frequency of MNs and NBUDs. These facts could be related to the stimulation of the cellular repair complex to correct the elicited damage cause by Hecogenin exposure. These observations could be interpreted as a protective capability of Hecogenin, preventing the dissemination of the DNA damage in cell division. This genotoxic and protective profile has already been highlighted by other studies. Alves et al. [34] described extracts from saponin-rich medicinal plants which could cause DNA damage when analyzed separately and, at the same time, be a protective agent when tested against mutagenic drugs in mice. Furthermore, Fernández-Herrera et al. [29] and [31] have demonstrated the role of Hecogenin on caspase activation, preventing the proliferation of the damaged cells by apoptosis.
In this work, Hecogenin showed genotoxicity, but also a protective role against mutagenic effect on HepG2. These facts were in agreement with the aforementioned studies, reinforcing the idea that the apoptotic mechanism is triggered by Hecogenin to avoid proliferation of genetic damage. More studies will have to be performed to confirm this hypothesis.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- [1] V.L. Challinor, J.J. De Voss; Open-chain steroidal glycosides, a diverse class of plant saponins; Nat. Prod. Rep., 30 (2013), pp. 429–454 http://dx.doi.org/10.1039/c3np20105h
- [2] I. Kostova, D. Dinchev; Saponins in Tribulus terrestris – Chemistry and bioactivity; Phytochem. Rev., 4 (2005), pp. 111–137 http://dx.doi.org/10.1007/s11101-005-2833-x
- [3] D.J. Newman, G.M. Cragg; Natural products as sources of new drugs over the 30 years from 1981 to 2010; J. Nat. Prod., 75 (2012), pp. 311–335 http://dx.doi.org/10.1021/np200906s.Natural
- [4] J. Eskander, C. Lavaud, D. Harakat; Steroidal saponins from the leaves of Agave macroacantha; Fitoterapia, 81 (2010), pp. 371–374 http://dx.doi.org/10.1016/j.fitote.2009.11.002
- [5] J. Santos, I. Vieira, R. Braz-Filho, A. Branco; Chemicals from Agave sisalana biomass: isolation and identification; Int. J. Mol. Sci., 16 (2015), pp. 8761–8771 http://dx.doi.org/10.3390/ijms16048761
- [6] G.M. Hocking; A Dictionary of Natural Products; Plexus Publishing, Inc, Medford, NJ (1997)
- [7] G.S. Cerqueira, G.S. Silva, E.R. Vasconcelos, A.P.F. Freitas, B.A. Moura, D.S. MacEeo, A.L. Souto, J.M. Barbosa Filho, L.K.A. Leal, G.A.C. Brito, C. Souccar, G.S.B. Viana; Effects of hecogenin and its possible mechanism of action on experimental models of gastric ulcer in mice; Eur. J. Pharmacol., 683 (2012), pp. 260–269 http://dx.doi.org/10.1016/j.ejphar.2012.02.043
- [8] K.B. Gama, J.S.S. Quintans, A.R. Antoniolli, L.J. Quintans-Júnior, W.A. Santana, A. Branco, M.B.P. Soares, C.F. Villarreal; Evidence for the involvement of descending pain-inhibitory mechanisms in the antinociceptive effect of hecogenin acetate; J. Nat. Prod., 76 (2013), pp. 559–563 http://dx.doi.org/10.1021/np3007342
- [9] J.S.S. Quintans, R.S.S. Barreto, W. De Lucca Júnior, C.F. Villarreal, C.M. Kaneto, M.B.P. Soares, A. Branco, J.R.G.S. Almeida, A.G. Taranto, A.R. Antoniolli, R.M. Freitas, L.J. Quintans-Júnior; Evidence for the involvement of spinal cord-inhibitory and cytokines-modulatory mechanisms in the anti-hyperalgesic effect of hecogenin acetate, a steroidal sapogenin-acetylated, in mice; Molecules, 19 (2014), pp. 8303–8316 http://dx.doi.org/10.3390/molecules19068303
- [10] L.J. Serafini, M.R. Quintans, J.S.S. Antoniolli, Â.R. Santos, M.R.V. Quintans-Junior; Mapeamento de Tecnologias Patenteáveis com o uso da Hecogenina; Rev. Geintec., 2 (2012), pp. 427–435
- [11] M.B. Botura, G.D. Silva, H.G. Lima, J.V.A. Oliveira, T.S. Souza, J.D.G. Santos, A. Branco, E.L.T. Moreira, M.A.O. Almeida, M.J.M. Batatinha; In vivo anthelmintic activity of an aqueous extract from sisal waste (Agave sisalana Perr.) against gastrointestinal nematodes in goats; Vet. Parasitol., 177 (2011), pp. 104–110 http://dx.doi.org/10.1016/j.vetpar.2010.11.039
- [12] S. Shafaie, V. Hutter, M.T. Cook, M.B. Brown, D.Y.S. Chau; In vitro cell models for ophthalmic drug development applications ; Biores. Open Access, 5 (2016), pp. 94–108 http://dx.doi.org/10.1089/biores.2016.0008
- [13] J. Gasparotto, N. Somensi, A. Kunzler, C.S. Girardi, M.A. de, B. Pasquali, V.M. Ramos, A. Simoes-Pires, L.J. Quintans-Junior, A. Branco, J.C.F. Moreira, D.P. Gelain; Hecogenin acetate inhibits reactive oxygen species production and induces cell cycle arrest and senescence in the A549 human lung cancer cell line; Anticancer Agents Med. Chem., 14 (2014), pp. 1128–1135 http://dx.doi.org/10.2174/1871520614666140408151751
- [14] B. Liagre, P. Vergne-Salle, D.Y. Leger, J.-L. Beneytout; Inhibition of human rheumatoid arthritis synovial cell survival by hecogenin and tigogenin is associated with increased apoptosis, p38 mitogen-activated protein kinase activity and upregulation of cyclooxygenase-2; Int. J. Mol. Med., 20 (2007), pp. 451–460
- [15] P. Trouillas, C. Corbiere, B. Liagre, J.-L. Duroux, J.-L. Beneytout; Structure −function relationship for saponin effects on cell cycle arrest and apoptosis in the human 1547 osteosarcoma cells: a molecular modelling approach of natural molecules structurally close to diosgenin; Bioorg. Med. Chem., 13 (2005), pp. 1141–1149 http://dx.doi.org/10.1016/j.bmc.2004.11.031
- [16] C.J. Omiecinski, J.P. Vanden Heuvel, G.H. Perdew, J.M. Peters; Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities; Toxicol. Sci., 120 (2011) http://dx.doi.org/10.1093/toxsci/kfq338
- [17] S. Knasmüller, V. Mersch-Sundermann, S. Kevekordes, F. Darroudi, W.W. Huber, C. Hoelzl, J. Bichler, B.J. Majer; Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge; Toxicology, 198 (2004), pp. 315–328 http://dx.doi.org/10.1016/j.tox.2004.02.008
- [18] M. Fenech; Cytokinesis-block micronucleus cytome assay; Nat. Protoc., 2 (2007), pp. 1084–1104 http://dx.doi.org/10.1038/nprot.2007.77
- [19] L.C. Burgess, E. Rice, T. Fischer, J.R. Seekins, T.P. Burgess, S.J. Sticka, K. Klatt; Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an in vitro system with doses across the physiological range; Toxicol. In Vitro, 22 (2008), pp. 1297–1300
- [20] B. Kosmider, E. Zyner, R. Osiecka, J. Ochocki; Induction of apoptosis and necrosis in A549 cells by the cis-Pt (II) complex of 3-aminoflavone in comparison with cis-DDP; Mutat. Res., 563 (2004), pp. 61–70 http://dx.doi.org/10.1016/j.mrgentox.2004.05.018
- [21] T. Mosmann; Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays; J. Immunol. Methods, 65 (1983), pp. 55–63
- [22] A. Hartmann, E. Agurell, C. Beevers, S. Brendler-Schwaab, B. Burlinson, P. Clay, A. Collins, A. Smith, G. Speit, V. Thybaud, R.R. Tice; Recommendations for conducting the in vivo alkaline Comet assay; Mutagenesis, 18 (2003), pp. 45–51
- [23] S.B. Nadin, L.M.V. Roig, D.R. Ciocca; A silver staining method for single-cell gel assay; J. Histochem. Cytochem., 49 (2001), pp. 1183–1186 http://dx.doi.org/10.1177/002215540104900912
- [24] A. Collins, M. Dusinská, M. Franklin, M. Somorovska, H. Petrovska, S. Duthie, L. Fillion, M. Panayiotidis, K. Raslova, N. Vaughan; Comet assay in human biomonitoring studies: reliability, validation, and applications; Environ. Mol. Mutagen., 30 (1997), pp. 139–146
- [25] M. Fenech; The in vitro micronucleus technique; Mutat. Res., 455 (2000), pp. 81–95
- [26] M.I. Vasquez, M. Garcia-Käufer, E. Hapeshi, J. Menz, K. Kostarelos, D. Fatta-Kassinos, K. Kümmerer; Chronic ecotoxic effects to Pseudomonas putida and Vibrio fischeri, and cytostatic and genotoxic effects to the hepatoma cell line (HepG2) of ofloxacin photo(cata)lytically treated solutions; Sci. Total Environ., 450–451 (2013), pp. 356–365 http://dx.doi.org/10.1016/j.scitotenv.2012.05.096
- [27] D. Eastmond, J. Tucker; Kinetochore localization in micronucleated cytokinesis-blocked Chinese hamster ovary cells: a new and rapid assay for identifying aneuploidy-inducing agents; Mutat. Res., 224 (1989), pp. 517–525
- [28] C. Corbiere, B. Liagre, A. Bianchi, K. Bordji, M. Dauça, P. Netter, J.L. Beneytout; Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids hecogenin and tigogenin, on human 1547 osteosarcoma cells; Int. J. Oncol., 22 (2003), pp. 899–905
- [29] M.A. Fernández-Herrera, H. López-Muñoz, J.M.V. Hernández-Vázquez, L. Sánchez-Sánchez, M.L. Escobar-Sánchez, B.M. Pinto, J. Sandoval-Ramírez; Synthesis and selective anticancer activity of steroidal glycoconjugates; Eur. J. Med. Chem., 54 (2012), pp. 721–727 http://dx.doi.org/10.1016/j.ejmech.2012.06.027
- [30] S. Maisanaba, A.I. Prieto, M. Puerto, D. Gutiérrez-Praena, E. Demir, R. Marcos, A.M. Cameán; In vitro genotoxicity testing of carvacrol and thymol using the micronucleus and mouse lymphoma assays; Mutat. Res. Toxicol. Environ. Mutagen., 784–785 (2015), pp. 37–44 http://dx.doi.org/10.1016/j.mrgentox.2015.05.005
- [31] M.A. Fernández-Herrera, J. Sandoval-Ramírez, L. Sánchez-Sánchez, H. López-Muñoz, M.L. Escobar-Sánchez; Probing the selective antitumor activity of 22-oxo-26-selenocyanocholestane derivatives; Eur. J. Med. Chem., 74 (2014), pp. 451–460 http://dx.doi.org/10.1016/j.ejmech.2013.12.059
- [32] W. Liu, C. Di Giorgio, M. Lamidi, R. Elias, E. Ollivier, M.P. De Méo; Genotoxic and clastogenic activity of saponins extracted from Nauclea bark as assessed by the micronucleus and the comet assays in Chinese Hamster Ovary cells; J. Ethnopharmacol., 137 (2011), pp. 176–183 http://dx.doi.org/10.1016/j.jep.2011.05.005
- [33] X. Zhao, X. Cong, L. Zheng, L. Xu, L. Yin, J. Peng; Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo; Toxicol. Lett., 214 (2012), pp. 69–80 http://dx.doi.org/10.1016/j.toxlet.2012.08.005
- [34] A.B.C.R. Alves, R.S. Santos, S.S. Calil, R. Niero, J.S. Lopes, F.F. Perazzo, P.C.P. Rosa, S.F. Andrade, V. Cechinel-Filho, E.L. Maistro; Genotoxic assessment of Rubus imperialis (Rosaceae) extract in vivo and its potencial chemoprevention against cyclophosphamide-induced DNA damage; J. Ethnopharmacol., 153 (2014), pp. 694–700
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?