Abstract
Background
The chemokine receptor, CC-chemokine receptor 3 (CCR3), and its major ligands, eotaxin, RANTES, and MCP-4, are involved in eosinophil chemotaxis. It is thought that CCR3 plays an important role in the recruitment and activation of eosinophils in nasal polyposis. We examined nasal polyp extract-induced eosinophil chemotaxis and the effect of a CCR3 antagonist using EZ-TAXIScan, a novel real-time chemotaxis assay device.
Methods
Nasal polyps were obtained from chronic rhinosinusitis (CRS) patients during surgery. The polyps were homogenized and eotaxin levels in the extracts were measured. Eosinophils were purified from human peripheral blood by the CD16 negative selection method. Nasal polyp extract-induced eosinophil chemotaxis, with or without CCR3 antagonist, was assessed by EZ-TAXIScan.
Results
There was a significant positive correlation between the eosinophil counts in nasal polyp and eotaxin levels in the nasal polyp extracts. Using EZ-TAXIScan, eosinophil chemotactic responses were observed following stimulation with nasal polyp extracts. There was a significant positive correlation between the chemotactic index toward the nasal polyp extracts and their eotaxin levels. Nasal polyp extract-induced chemotaxis was completely inhibited by CCR3 antagonist but not by chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonist which inhibited PGD2-induced eosinophil chemotaxis.
Conclusions
The CCR3 pathway may play an important role in the pathogenesis of eosinophil recruitment in nasal polyps through selective eosinophil chemotaxis.
Keywords
Asthma; Chronic sinusitis; Eosinophils; Eotaxin; Nasal polyps
Abbreviations
CCR3, CC-chemokine receptor 3; CRS, chronic rhinosinusitis; CRTH2, chemoattractant receptor-homologous molecule expressed on Th2 cells
Introduction
Nasal polyps occur more commonly in asthmatic patients, and there are greater densities of activated eosinophils in nasal polyps from asthmatic patients compared to non-asthmatics.1, 2 and 3 Eosinophils play an important role in the pathogenesis of allergic airway disease by secreting a wide variety of cationic proteins, lipid mediators, and cytokines/chemokines that mediate terminal effector functions and the innate immune response.4 CC-chemokine receptor 3 (CCR3) belongs to a family of seven transmembrane-spanning G protein-coupled receptors and binds three eotaxin family proteins, eotaxin-1/CCL11, eotaxin-2/CCL24 and eotaxin-3/CCL26, that are potent chemoattractants for eosinophils.5, 6 and 7 The eotaxin concentrations of nasal polyp extracts are significantly increased in eosinophilic chronic rhinosinusitis (CRS) patients compared to the non-eosinophilic CRS group.8 and 9 CCR3 plays an essential role in eosinophil recruitment to the skin and the lung and in the development of airway hyperresponsiveness in a CCR3-deficient mouse model.10 Because CCR3 expression is associated with Th2 lymphocytes, eosinophils, and mast cells, CCR3 may be central to the induction of the inflammation associated with allergic disease.11 and 12 However, the role of CCR3 in eosinophil recruitment in nasal polyps is not clear. To address this issue, we employed a nasal polyp extract-induced eosinophil chemotaxis assay with a novel real-time chemotaxis assay device, EZ-TAXIScan, which can monitor and record horizontal cell migration.13 We found that nasal polyp extract-induced eosinophil chemotaxis was correlated with the eotaxin levels of nasal polyps, and addition of a CCR3 antagonist completely inhibited chemotaxis. On the other hand chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonist which inhibited PGD2-induced eosinophil chemotaxis did not inhibit nasal polyp extract-induced eosinophil chemotaxis. These findings suggest that the CCR3 pathway may play an important role in the pathogenesis of eosinophil recruitment in nasal polyps through selective eosinophil chemotaxis, raising the possibility that CCR3 antagonists may be effective for the treatment of eosinophilic nasal polyps.
Methods
Tissue collection
Nasal polyps were obtained from 12 patients with CRS (five male and seven female) during endoscopic sinus surgery. There were six asthmatic and six non-asthmatic patients. Systemic corticosteroids were discontinued at least one month prior to surgery. The nasal polyp samples were divided in half, and one specimen was immediately snap-frozen in liquid nitrogen and stored at −80 °C until processed. The other was fixed in a 10% neutral formaldehyde solution and embedded in paraffin. Counting of the number of eosinophils in the nasal polyps was performed at high magnification (400×). In each patient, 5 visual fields were randomly selected and the mean eosinophil count per field was calculated. The study was approved by the ethics committee of Akita University School of Medicine. Informed consent was obtained from each patient before collecting samples.
Eotaxin assay
Nasal polyp samples were homogenized in 500 μl of buffer containing 1% NP-40, 150 mM NaCl, 50 mM Hepes, and centrifuged at 13,000 rpm for 10 min. The concentration of eotaxin in the nasal polyp extracts was measured by an ELISA kit (R & D Systems, Minneapolis, USA), according to the manufacturers instructions.
Eosinophil preparation
Eosinophils were purified from normal donor blood by negative selection, as previously described.14 Briefly, eosinophils were isolated by sedimentation with 6% dextran saline solution followed by centrifugation on 1.088 Percoll (Pharmacia, St. Louis, USA) density gradients. The cells were further purified by negative selection using anti-CD16 immunomagnetic beads and a MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). Eosinophil purity of >98% was routinely obtained as determined by microscopic analyses.
Chemotaxis assay
Eosinophil chemotactic responses were measured using the real-time chemotaxis assay device, EZ-TAXIScan (Effector Cell Institute, Tokyo, Japan). The EZ-TAXIScan chamber was assembled with a 260 μm wide × 4 μm thick silicon chip on a 2 mm untreated glass base, as described by the manufacturer, and filled with RPMI medium containing 1% FBS. Eosinophil suspension (1 μl containing 2 × 106 cells/ml) was injected into one side of the EZ-TAXIScan chamber. Chemoattractant solution (1 μl eotaxin, PGD2 or nasal polyp extract) was injected into the opposite side of the chamber to initiate chemotaxis. Migration of eosinophils over the glass surface was recorded with a CCD camera located beneath the glass every minute for 60 min at 37 °C. At the end of the assays, we calculated the chemotactic index by dividing the number of eosinophils that had migrated over the halfway line towards the chemoattractants by the number of eosinophils remaining at baseline.
CCR3 or CRTH2 antagonist TREATMENT
Eosinophils (2 × 106 cells/ml in RPMI medium containing 1% FBS) were incubated for 30 min with or without CCR3 antagonist, SB328437 (1000 nM) (R & D Systems, Minneapolis, USA) and CRTH2 antagonist, CAY10471 (1000 nM) (Cayman Biochemical, Michigan, USA).
Statistical analyses
Data are presented as mean ± SE. Comparisons of two groups of data were performed using the Wilcoxon single rank test, and simple regression analysis was performed. Significance was established at the p < 0.05 level.
Results
Eosiniphil count and eotaxin level in nasal polyp
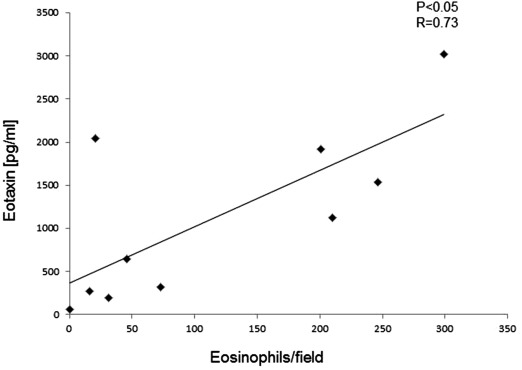
There was a significant positive correlation between the eosinophil counts in nasal polyp and eotaxin levels in the nasal polyp extracts (Fig. 1).
|
|
|
Fig. 1. There was a significant positive correlation between the eosinophil counts in nasal polyp and eotaxin levels in the nasal polyp extracts. |
Eosinophil chemotaxis induced by eotaxin
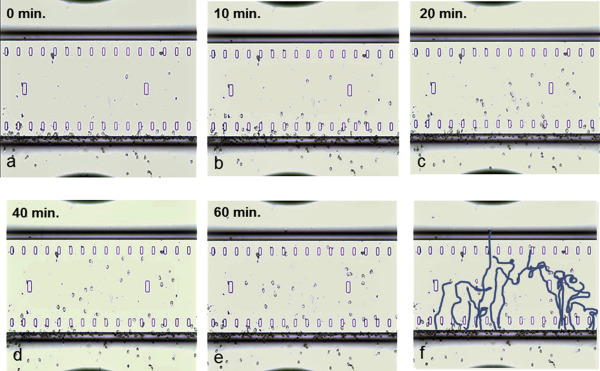
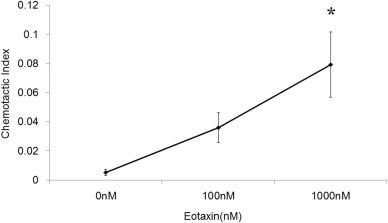
Migration of human eosinophils from normal donor blood toward a concentration gradient of eotaxin (1000 nM) was recorded using EZ-TAXIScan. Images were recorded every minute for 60 min; images at 0, 10, 20, 40, 60 min tracing the trajectory of eosinophil chemotaxis are presented (Fig. 2). The chemotactic index with 1000 nM eotaxin was significantly higher than that of the control (Fig. 3).
|
|
|
Fig. 2. Chemotaxis of human eosinophils from normal donor blood toward a concentration gradient of eotaxin (1000 nM) was recorded using a real-time chemotaxis assay device, EZ-TAXIScan. Images of eosinophil chemotaxis were recorded every minute for 60 min. Images at 0, 10, 20, 40, 60 min (a–e) and tracing the trajectory (f) of eosinophil chemotaxis are presented. |
|
|
|
Fig. 3. The chemotactic index was calculated by dividing the number of cells that migrated past the halfway line by the number of cells remaining at baseline. The chemotactic index of eotaxin (1000 nM) was significantly higher than that of the control. *p < 0.05 vs control. |
Effect of CCR3 antagonist on eosinophil chemotaxis
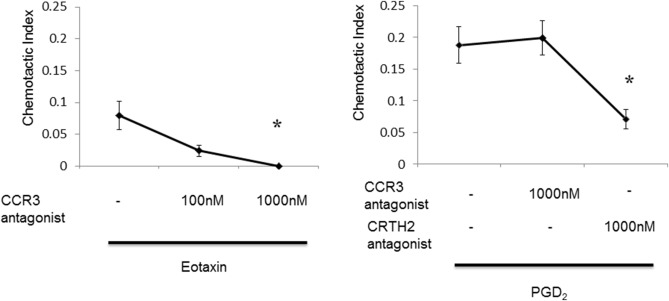
We investigated the effect of the CCR3 antagonist, SB328437 or CRTH2 antagonist, CAY10471 on eotaxin (1000 nM) or PGD2 (1000 nM)-induced eosinophil chemotaxis to establish whether the CCR3 antagonist directly modulated the eosinophil chemotactic response. Dose-dependent inhibition of eotaxin-induced chemotaxis of eosinophils by CCR3 antagonist and inhibition of PGD2-induced chemotaxis of eosinophils by CRTH2 antagonist were observed. CCR3 antagonist did not inhibit PGD2 -induced chemotaxis of eosinophils (Fig. 4). Our results indicated that the CCR3 antagonist inhibited the eotaxin/CCR3 chemotactic response directly.
|
|
|
Fig. 4. Dose-dependent inhibition of eotaxin (1000 nM)-induced chemotaxis of eosinophils by CCR3 antagonist and inhibition of PGD2 (1000 nM) -induced chemotaxis of eosinophils by CRTH2 antagonist were observed. CCR3 antagonist did not inhibit PGD2 -induced chemotaxis of eosinophils. *p < 0.05 vs control. |
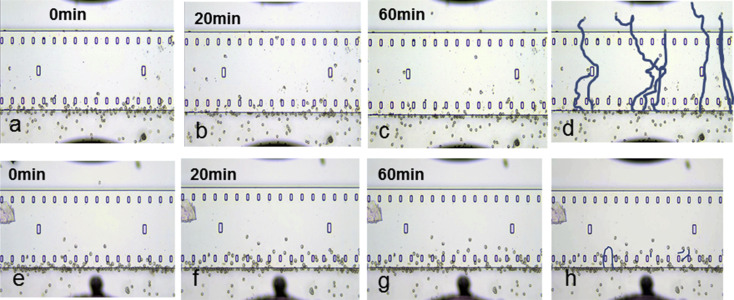
Eosinophil chemotaxis induced by nasal polyp extracts
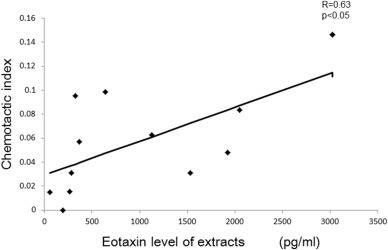
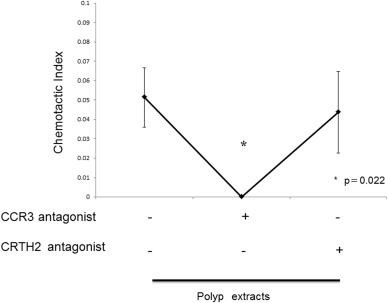
The numbers of eosinophils in nasal polyps were significantly higher in asthmatic patients than in non-asthmatic patients (data not shown). Migration of human eosinophils toward nasal polyp extracts was recorded and images at 0, 10, 20, 40, 60 min tracing the trajectory of asthmatic patients' nasal polyp extract-induced chemotaxis are presented (Fig. 5a–d). Inhibition of nasal polyp extract-induced chemotaxis of eosinophils by CCR3 antagonist was observed (Fig. 5e–h). There was a significant positive correlation between the chemotactic index toward the nasal polyp extracts and their eotaxin concentration (Fig. 6). In addition, CCR3 antagonist (1000 nM) inhibited nasal polyp extract-induced eosinophil chemotaxis completely. On the other hand CRTH2 antagonist (1000 nM) did not inhibit nasal polyp extract-induced chemotaxis of eosinophils (Fig. 7). These results indicated that the CCR3 pathway has an important role in eosinophil recruitment in nasal polyps.
|
|
|
Fig. 5. Migration of human eosinophils toward a nasal polyp extract was recorded using EZ-TAXIScan. Images at 0, 20, 60 min and tracing the trajectory of asthmatic patients' nasal polyp extract-induced chemotaxis are presented (a–d). Inhibition of nasal polyp extract-induced chemotaxis of eosinophils by CCR3 antagonist was observed (e–h). |
|
|
|
Fig. 6. There was a significant positive correlation between the chemotactic index toward the nasal polyp extracts and the eotaxin levels in nasal polyps. R = 0.63, p < 0.05. |
|
|
|
Fig. 7. CCR3 antagonist inhibited polyp extract-induced chemotaxis of eosinophils completely. On the other hand CRTH2 antagonist did not inhibit nasal polyp extract-induced chemotaxis of eosinophils. *p < 0.05 vs CCR3 antagonist (−). |
Discussion
This study demonstrated that nasal polyp extract-induced eosinophil chemotaxis is correlated with the eotaxin level of nasal polyps, and chemotaxis was completely blocked by CCR3 antagonist. These findings suggest that the CCR3 pathway plays an important role in the pathogenesis of eosinophilic nasal polyps, and CCR3 antagonists may be effective for the treatment of this condition.
Nasal polyps occur more commonly in asthmatic patients, and asthma is associated with a higher rate of recurrence of nasal polyps after endoscopic sinus surgery.2 and 15 Eosinophilic inflammation is the major histologic hallmark of both asthma and CRS.16 The histopathologic characteristics of asthma, namely, an intense eosinophilic inflammation, basement membrane thickening, erosion of the epithelium and features of airway remodeling, are also present in CRS. CCR3 is highly expressed not only on eosinophils12 and 17 but also on basophils, mast cells and highly activated T helper type 2 (Th2) cells.11, 18 and 19 CCR3 antagonist prevents not only the infiltration of eosinophils into the airways, but also the development of allergen-induced subepithelial and peribronchial fibrosis in a mouse model of allergic asthma.20 However, there is no appropriate in vitro model of eosinophilic accumulation in nasal polyps, and the role of CCR3 in eosinophil accumulation in nasal polyps is not clear. To measure eosinophilic chemotaxis in vitro, the trans-well or Boyden chamber method has most commonly been used. However, while this method can provide endpoint data, temporal analysis of cellular migration is not assessed, and comparatively large numbers of eosinophils are needed for this experiment (50,000 to 100,000 cells). EZ-TAXIScan is a novel technique in which microchannels formed between a silicon substrate and a flat glass plate are used to observe cell migration, including cellular chemotaxis. 13 and 21 Cells moving horizontally via microchannels can be observed with a microscope, and the morphological changes cells undergo during chemotaxis, as well as the directions they travel, can be observed in real time using a small sample of cells (as few as 100 cells). Furthermore, computer control of multiple channels mounted on a chip permits time-series recordings of cell migration.
In this study, the PGD2-induced eosinophil chemotaxis index was higher than eotaxin-induced chemotaxis according to EZ-TAXIScan. The speed of eosinophil chemotaxis toward PGD2 was also higher than towards eotaxin (data not shown). Nitta et al. reported that using EZ-TAXIScan, rapid eosinophil chemotactic responses were observed by stimulation with PGD2 and fMLP compared to RANTES, although the migration lifetime of eosinophils was longer when they were stimulated with RANTES.21 The mechanisms underlying the differences in response to chemoattractants was not clear. RANTES and eotaxin augment the functional adherence of eosinophils. 22 and 23 The establishment of a gradient of the molecules (RANTES: M.W. 8720, PGD2: M.W. 352), or the induced functional adherence of eosinophils by chemoattractant may affect the eosinophil chemotactic response. There is a discrepancy in eotaxin levels between in polyp extract-induced chemotaxis assay and eotaxin-induced chemotaxis assay. It was reported that the levels of MCP-4, eotaxin, eotaxin-2 and eotaxin-3 in nasal polyp tissue were increased compared with sinonasal tissue from control subjects.24 Not only eotaxin but also these chemokines may play an important role in the eosinophil migration in the nasal polyp through CCR3 pathway in eosinophils. PGD2 regulates various immunological responses via two distinct PGD2 receptors, prostaglandin D receptor (DP), and CRTH2 on eosinophils and induces the migration of eosinophils through CRTH2.25 In this study, eotaxin-induced chemotaxis was completely inhibited by the CCR3 antagonist, while CRTH2 antagonist did not inhibit eotaxin-induced chemotaxis. These results suggest that the CCR3 antagonist may inhibit the CCR3 pathway directly. Moreover, nasal polyp extract-induced chemotaxis of eosinophils was completely inhibited by CCR3 antagonist but not by CRTH2 antagonist. Taken together, our data indicate the CCR3 pathway may play an important role in the pathogenesis of eosinophilic nasal polyps through a selective effect on eosinophil chemotaxis. Importantly, the quantitative methods described in the study could be useful for drug screening to identify more effective options for the treatment of nasal polyps.
Conflict of interest
The authors have no conflict of interest to declare.
Authors' contributions
HS and KH designed the study and wrote the paper. CA performed pathological study. SU supervised the study. KI contributed to revision of the manuscript.
References
- 1 M.M. Ardehali, A. Amali, M. Bakhshaee, Z. Madani, M. Amiri; The comparison of histopathological characteristics of polyps in asthmatic and nonasthmatic patients; Otolaryngol Head Neck Surg, 140 (2009), pp. 748–751
- 2 N.D. Bateman, A. Shahi, K.M. Feeley, T.J. Woolford; Activated eosinophils in nasal polyps: a comparison of asthmatic and non-asthmatic patients; Clin Otolaryngol, 30 (2005), pp. 221–225
- 3 C. Asaka, K. Honda, E. Ito, N. Fukui, J. Chihara, K. Ishikawa; Peroxisome proliferator-activated receptor-gamma is expressed in eosinophils in nasal polyps; Int Arch Allergy Immunol, 155 (2011), pp. 57–63
- 4 S.P. Hogan, H.F. Rosenberg, R. Moqbel, S. Phipps, P.S. Foster, P. Lacy, et al.; Eosinophils: biological properties and role in health and disease; Clin Exp Allergy, 38 (2008), pp. 709–750
- 5 K. Honda, J. Chihara; Eosinophil activation by eotaxin–eotaxin primes the production of reactive oxygen species from eosinophils; Allergy, 54 (1999), pp. 1262–1269
- 6 T.G. Uhm, B.S. Kim, I.Y. Chung; Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma; Ann Allergy Asthma Immunol, 4 (2012), pp. 68–79
- 7 M. Kitaura, N. Suzuki, T. Imai, S. Takagi, R. Suzuki, T. Nakajima, et al.; Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3; J Biol Chem, 274 (1999), pp. 27975–27980
- 8 T. Yao, Y. Kojima, A. Koyanagi, H. Yokoi, T. Saito, K. Kawano, et al.; Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients; Laryngoscope, 119 (2009), pp. 1053–1059
- 9 H. Olze, U. Forster, T. Zuberbier, L. Morawietz, E.O. Luger; Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3; Rhinology, 44 (2006), pp. 145–150
- 10 W. Ma, P.J. Bryce, A.A. Humbles, D. Laouini, A. Yalcindag, H. Alenius, et al.; CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation; J Clin Invest, 109 (2002), pp. 621–628
- 11 F. Sallusto, C.R. Mackay, A. Lanzavecchia; Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells; Science, 277 (1997), pp. 2005–2007
- 12 P.D. Ponath, S. Qin, D.J. Ringler, I. Clark-Lewis, J. Wang, N. Kassam, et al.; Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils; J Clin Invest, 97 (1996), pp. 604–612
- 13 S. Kanegasaki, Y. Nomura, N. Nitta, S. Akiyama, T. Tamatani, Y. Goshoh, et al.; A novel optical assay system for the quantitative measurement of chemotaxis; J Immunol Methods, 282 (2003), pp. 1–11
- 14 S. Ueki, T. Adachi, J. Bourdeaux, H. Oyamada, Y. Yamada, K. Hamada, et al.; Expression of PPARgamma in eosinophils and its functional role in survival and chemotaxis; Immunol Lett, 86 (2003), pp. 183–189
- 15 K. Dejima, T. Hama, M. Miyazaki, S. Yasuda, K. Fukushima, A. Oshima, et al.; A clinical study of endoscopic sinus surgery for sinusitis in patients with bronchial asthma; Int Arch Allergy Immunol, 138 (2005), pp. 97–104
- 16 J.U. Ponikau, D.A. Sherris, G.M. Kephart, E.B. Kern, T.A. Gaffey, J.E. Tarara, et al.; Features of airway remodeling and eosinophilic inflammation in chronic rhinosinusitis: is the histopathology similar to asthma?; J Allergy Clin Immunol, 112 (2003), pp. 877–882
- 17 H. Oyamada, Y. Kamada, T. Kuwasaki, Y. Yamada, Y. Kobayashi, C. Cui, et al.; CCR3 mRNA expression in bronchial epithelial cells and various cells in allergic inflammation; Int Arch Allergy Immunol, 120 (1999), pp. 45–47
- 18 M. Uguccioni, C.R. Mackay, B. Ochensberger, P. Loetscher, S. Rhis, G.J. LaRosa, et al.; High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines; J Clin Invest, 100 (1997), pp. 1137–1143
- 19 A. de Paulis, F. Annunziato, L. Di Gioia, S. Romagnani, M. Carfora, C. Beltrame, et al.; Expression of the chemokine receptor CCR3 on human mast cells; Int Arch Allergy Immunol, 124 (2001), pp. 146–150
- 20 M. Komai, H. Tanaka, K. Nagao, M. Ishizaki, D. Kajiwara, T. Miura, et al.; A novel CC-chemokine receptor 3 antagonist, Ki19003, inhibits airway eosinophilia and subepithelial/peribronchial fibrosis induced by repeated antigen challenge in mice; J Pharmacol Sci, 112 (2010), pp. 203–213
- 21 N. Nitta, T. Tsuchiya, A. Yamauchi, T. Tamatani, S. Kanegasaki; Quantitative analysis of eosinophil chemotaxis tracked using a novel optical device – TAXIScan; J Immunol Methods, 320 (2007), pp. 155–163
- 22 N. Saito, Y. Yamada, S. Sannohe, K. Honda, T. Adachi, H. Kayaba, et al.; Possible involvement of C-C chemokines in functional augmentation of adhesion molecules in asthmatic patients; Lung, 180 (2002), pp. 251–263
- 23 T. Chiba, Y. Kamada, N. Saito, H. Oyamada, S. Ueki, Y. Kobayashi, et al.; RANTES and eotaxin enhance CD11b and CD18 expression on eosinophils from allergic patients with eosinophilia in the application of whole blood flow cytometry analysis; Int Arch Allergy Immunol, 137 (2005), pp. 12–16
- 24 W.W. Stevens, C.J. Ocampo, S. Berdnikovs, M. Sakashita, M. Mahdavinia, L. Suh, et al.; Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease; Am J Respir Crit Care Med, 192 (2015), pp. 682–694
- 25 T. Chiba, S. Ueki, W. Ito, H. Kato, R. Kamada, M. Takeda, et al.; The opposing role of two prostaglandin D2 receptors, DP and CRTH2, in human eosinophil migration; Ann Allergy Asthma Immunol, 106 (2011), pp. 511–517
Document information
Published on 05/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?