Highlights
- The most pronounced changes in gene expression were detected at 4 h exposure compared to the 24 h exposure.
- At 4 h exposure the larger (50 nm) nanosilver altered more genes by more different pathways than the smaller (20 nm) one.
- Metallothioneins and heat shock proteins were highly up-regulated as a result of exposure to both the nanosilvers.
- The toxicogenomic characterization of Caco2 cells is a valuable in vitro model for assessing toxicity of nanosilver.
Abstract
Extensive consumer exposure to food- and cosmetics-related consumer products containing nanosilver is of public safety concern. Therefore, there is a need for suitable in vitro models and sensitive predictive rapid screening methods to assess their toxicity. Toxicogenomic profile showing subtle changes in gene expressions following nanosilver exposure is a sensitive toxicological endpoint for this purpose. We evaluated the Caco2 cells and global gene expression profiles as tools for predictive rapid toxicity screening of nanosilver. We evaluated and compared the gene expression profiles of Caco-2 cells exposed to 20 nm and 50 nm nanosilver at a concentration 2.5 μg/ml. The global gene expression analysis of Caco2 cells exposed to 20 nm nanosilver showed that a total of 93 genes were altered at 4 h exposure, out of which 90 genes were up-regulated and 3 genes were down-regulated. The 24 h exposure of 20 nm silver altered 15 genes in Caco2 cells, out of which 14 were up-regulated and one was down-regulated. The most pronounced changes in gene expression were detected at 4 h. The greater size (50 nm) nanosilver at 4 h exposure altered more genes by more different pathways than the smaller (20 nm) one. Metallothioneins and heat shock proteins were highly up-regulated as a result of exposure to both the nanosilvers. The cellular pathways affected by the nanosilver exposure is likely to lead to increased toxicity. The results of our study presented here suggest that the toxicogenomic characterization of Caco2 cells is a valuable in vitro tool for assessing toxicity of nanomaterials such as nanosilver.
Keywords
Nanosilver ; Silver nanoparticles ; Nanoparticles ; Toxicogenomics ; DNA microarray ; Global gene expression profiles ; Caco2 cells
1. Introduction
An exponential increase in the use of nanomaterials in consumer products has been reported in recent years. The human exposure to these products is rapidly expanding and, therefore, their potential for adverse health effects is of concern. The silver nanoparticles show a wide spectrum of antibacterial and antifungal properties [20] , [42] and [52] . Therefore, nanosilver is one of the most commonly used nanomaterials in consumer products. However, in spite of their widespread use, very limited information is available on their potential toxicity. Use of nanosilver in food, food contact materials, and dietary supplements has significantly increased in recent years [53] . Thus, the use of food-related nanosilver is highly relevant for human exposure [53] , and its toxicity screening is necessary to better ensure the consumer safety.
Ingestion is a major route of human exposure of food-related nanoparticles. It has been reported that the nanoparticles present in gastrointestinal tract play an important role in the development of colon disease [54] . The epithelium of the small intestine and colon provide protection against toxicants in the blood stream [55] . The epithelial cells separate the gastrointestinal tract from the systemic circulation and prevent the uptake of toxicants from the bloodstream. Injury to these epithelial cells impairs their protective function.
Animal studies required for toxicity screening are costly and time consuming. Therefore, the search for suitable in vitro models to accurately predict toxicity in vivo is of interest. One of the important requirements of safety evaluation of a potential toxicant is its reliable and reproducible toxicity information. The costly and time consuming animal studies and the lack of human data led to the evaluation of alternative in vitro models for reliable and reproducible mechanistic information that can be used for risk assessment. Human cell lines in culture are sensitive tools for high-throughput toxicity screening and they have the potential to reduce the use of animals for toxicological testing in the 21st century [27] .
The human gastrointestinal epithelial Caco2 cells are widely used as an in vitro model for traditional chemical toxicity testing, representing the oral route of exposure. They are well characterized and show many of the morphological and biochemical characteristics of small intestine enterocytes [56] and [57] . Recently, [61] have demonstrated that the differentiated Caco2 cells closely resemble the physiological intestinal epithelium. [58] have used the Caco2 cells as an in vitro model for the prediction of intestinal drug absorption in vivo. Therefore, the Caco2 cells have been successfully used as a useful in vitro screening tool for toxicity evaluation of compounds [59] and [60] . Therefore, in this study we evaluated the Caco2 cells to determine if they can be used as an in vitro model for predictive rapid screening of food-related nanomaterials.
Multicellular organisms react to environmental changes primarily at the cellular level. Genomic responses to toxic exposures offer valuable tools for toxicity evaluation of potential toxicants [62] . The gene expression profiles provide molecular information on the cell-toxicant interactions. The cells respond to environmental stress through adaptive stress response pathways. These pathways are activated at significantly lower toxicant concentrations than those causing cellular injury detected by conventional biochemical methods [62] . The toxicogenomic effects of nanoparticles at low levels especially below the detection limits of traditional biochemical endpoints of toxicity are unknown. Therefore, molecular biomarkers such as gene expression changes which can detect cellular injury at low levels of toxic exposure are of importance. Toxicogenomic endpoints are very useful for detection of early stages of toxicity that cannot be detected by conventional endpoints. Toxicogenomics shows subtle changes in gene expression. Identification of differentially expressed genes reveals the molecular mechanisms of toxic exposures. This technology uses DNA microarrays to evaluate the effects of potential toxicants at the molecular level. It allows the analysis of expression of thousands of genes simultaneously. Toxicogenomic techniques and human cells in vitro have the potential to reduce the use of animals and to eliminate the need for interspecies extrapolation [27] .
Recently we have reported the cytotoxic and genotoxic effects of 20 nm and 50 nm nanosilver in HepG2 and Caco2 cells [34] , [35] , [36] and [38] and toxicogenomic effects of these nanosilvers in HepG2 cells [37] . The purpose of the current study presented here was to determine if the Caco2 cells and the global gene expression profiles can be used as useful tools for predictive rapid toxicity screening of food-related nanomaterials such as 20 nm and 50 nm nanosilver.
2. Materials and methods
2.1. Materials
The 20 nm and 50 nm BioPure® silver nanoparticle citrate solution was purchased from nanoComposix (San Diego, CA). The human colon carcinoma Caco2 cells (ATCC HTB-37), were obtained from the American Type Culture Collection (ATCC), Manassas, VA. Deep-frozen vials of stock cells were routinely stored in liquid nitrogen freezer. Dulbecco’s modified Eagle’s medium (DMEM) GlutaMax, Hanks’ balanced salt solution (HBSS), HEPES, phosphate buffered saline (PBS), trypsin-EDTA solution and 0.4% trypan blue solution were purchased from Invitrogen Corporation (Grand Island, NY). Fetal bovine serum (FBS) was purchased from the Hyclone Labs (Logan, UT). The sterile nonpyrogenic polystyrene cell culture flasks and plates were purchased from Corning (Corning, NY) and Becton-Dickinson (Franklin Lakes, NJ), respectively. All other chemicals were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO). Buffer RLT, QIAshredder spin column and EZ1 RNA Cell Mini Kit were purchased form Qiagen (Valencia, CA). The RNA 6000 Nano Reagent Kit was purchased from Agilent (Santa Clara, CA). The Affymetrix GeneChip 3′ IVT Express Kit was purchased from Affymetrix (Santa Clara, CA).
2.2. Methods
2.2.1. Characterization of nanoparticles
The silver nanoparticles were characterized by the dynamic light scattering (DLS), transmission electron microscopy (TEM) and inductively coupled plasma-mass spectrometry (ICP-MS) analysis as described previously [34] and [37] . The stock solution of the nanosilver in aqueous 2 mM citrate was stored at 4 °C in small aliquots. The desired concentrations of silver nanoparticles for cell exposures were prepared fresh by diluting the stock solution with the cell culture medium just before the experiment [63] , [64] , [65] , [34] and [35] ].
2.2.2. Cell culture
Human colon carcinoma Caco2 cells were stored routinely in small aliquots in liquid nitrogen and the experimental cultures were prepared from the frozen stock cells and always kept in a sub-confluent state [66] , [67] , [34] , [35] and [37] ]. The cells were cultured in 75 cm2 culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) with GlutaMax containing 1.5% glucose and supplemented with 10% fetal bovine serum, 1% MEM non-essential amino acids and 10 mM HEPES buffer [67] , [68] , [69] , [70] , [9] , [34] , [35] and [37] in a saturating humidified atmosphere of 5% CO2 in air at 37 °C. The culture medium was changed every 3–4 days. The cultures were used for testing within 10 passages after the cells were received from the ATCC.
2.2.3. Cytotoxicity assay
The cytotoxicity of 20 nm and 50 nm nanosilver was determined fluorometrically by the resazurin (Alamar Blue) reduction assay as described previously [34] and [37] . Briefly, cells were seeded in 96-well plates and treated with varying concentrations (0.5–25.0 μg/ml) of the 20 nm or 50 nm nanosilver, or with the vehicle control (cell culture medium), for 4 h or 24 h at 37 °C, washed with sterile HBSS, and then incubated with resazurin for 30 min at 37 °C in a plate reader using the Sigma Resazurin Assay kit. The rate of increase of resorufin fluorescence was measured at 545 nm excitation and 590 nm emission. Each concentration was tested with ten replicates. Statistical analysis of the results was conducted using one-way ANOVA.
2.2.4. Treatment of cells with nanosilver
When the cells were grown to approximately 70–80% confluence, they were prepared for the experimental procedures. The cells were washed with Ca- and Mg-free HBSS and harvested from the 75 cm2 culture flasks by 0.05% trypsin-EDTA. A single cell suspension in the culture medium was obtained by repeated trituration. Cell counts and cell viability were determined by trypan blue dye exclusion using a hemocytometer. A single cell suspension in the culture medium at an approximate density 1 × 105 cells/ml was prepared by serial dilution and this stock cell suspension was used for seeding cells for the study. The cells were seeded in 6-well plates (3 ml/well) for 24 h before treatment.
On the day of cell exposure the dosing solutions of 20 nm silver were prepared by serial dilutions of the stock solution in the cell culture medium immediately before use. The logarithmically growing cells were treated with the test agents. The cells were washed once with HBSS and the dosing solutions were added to the cells. The concentrations of the dosing solution and the time of exposure were selected to induce minimum toxicity. The cells were exposed to the nanosilver at a concentration of 2.5 μg/ml for 4 h or 24 h at 37 °C in a saturating humidified atmosphere of 5% CO2 in air. The control cells received an equal volume of the vehicle (cell culture medium). After exposure the cells were washed with HBSS and prepared for the global gene expression profile analysis by DNA microarray.
2.2.5. Global gene expression profile analysis by DNA microarray
DNA microarray analysis was performed as described previously [37] using GeneChip® PrimeView™ human gene expression array (Affymetrix, Santa Clara, CA) following the standard Affymetrix protocol.
2.2.6. Total RNA isolation and quality assurance
Total RNA was isolated as described previously [66] and [37] . After treatment cells were immediately lysed in Buffer RLT (Qiagen; Valencia, CA) supplemented with β-mercaptoethanol, homogenized by QIAshredder spin column (Qiagen), and kept in a −80 °C freezer until further processing. Total RNA was isolated on the EZ1 Advanced XL (Qiagen) automated RNA purification instrument using the EZ1 RNA Cell Mini Kit (Qiagen) following the manufacturer’s protocol, including an on-column DNase digestion. RNA concentration and purity (260/280 ratio) were measured with the NanoDrop 2000 UV–vis spectrophotometer (NanoDrop Products, Wilmington, DE). Integrity of RNA samples was assessed by the Agilent 2100 Bioanalyzer (Santa Clara, CA) with the RNA 6000 Nano Reagent Kit from the same manufacturer. The threshold of acceptability for Bioanalyzer RIN values was ≥8.5 for the RNA samples.
2.2.7. DNA microarray analysis
DNA microarray analysis was performed as described previously [37] . Total RNA samples were preprocessed for hybridization to GeneChip PrimeView Human Gene Expression Array (Affymetrix, Santa Clara, CA) using the Affymetrix GeneChip 3′ IVT Express Kit following the manufacturer’s protocol. In brief, 0.1 μg of total RNA was used to generate first strand cDNA using reverse transcriptase and a T7-linked oligo(dT) primer. After second strand synthesis, the double stranded cDNA was then used for in vitro transcription with biotinylated UTP and CTP to amplify the product, referred to as cRNA amplification. Subsequent hybridization, wash, and staining were carried out using the Affymetrix GeneChip Hybridization, Wash, and Stain Kit and the manufacturer’s protocols were followed. Briefly, biotinylated target cRNA was fragmented using heat and Mg2+ to sizes of 35–200 bp. Each fragmented cRNA target sample (approximately 10 μg) was individually hybridized to a GeneChip PrimeView Human Gene Expression Array at 45 °C for 16 h in Affymetrix GeneChip Hybridization Oven 645. After hybridization, the array chips were stained and washed using an Affymetrix Fluidics Station 450. The chips were then scanned on Affymetrix GeneChip Scanner 3000 7G and the image (.DAT) files were preprocessed using the Affymetrix GeneChip Command Console (AGCC) software v.4.0 to generate cell intensity (.CEL) files. Prior to data analysis, all arrays referred to in this study were assessed for data quality using the Affymetrix Expression Console software v.1.3 and all quality assessment metrics (including spike-in controls during target preparation and hybridization) were found within boundaries.
2.2.8. Data processing and statistical analysis
Data analysis was carried out as described previously [37] primarily using the U.S. FDA’s ArrayTrack software system [46] and [47] . The values of individual probes belonging to one probe set in .CEL files were summarized using the robust multi-array average (RMA) algorithm [16] embedded in ArrayTrack, which comprises of convolution background correction, quantile normalization, and median polish summarization. Differentially expressed genes (DEGs) were selected using one-way analysis of variance (ANOVA) based on Welch t -test. To improve moderated t -statistics a gene filtering procedure, namely I/NI-calls [12] , was applied before the Welch t -test to exclude non-informative genes. For the global gene expression analysis, the control and treated cells were compared by using the fold change (FC) of every annotated gene, together with their false discovery rate (FDR). Genes were considered differentially altered and of potential biological significance if FC ≥1.5 and FDR ≤0.05 compared to vehicle controls. The genes were considered up-regulated if FC ≥1.5 and FDR ≤0.05, and down-regulated if FC ≤1.0.
2.2.9. Function and pathway analysis of differentially expressed genes
The DEGs were subjected to gene ontology (GO) and pathway analysis as described previously [37] using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) [7] and [14] to find overrepresentations of GO terms in the biological process (BP) category at level 5 (GOTERM_BP_5) and KEGG pathways. As background, the Homo sapiens (human) whole genome was used. Statistical enrichment was determined using Fisher exact test p < 0.05 and count threshold ≥5. The statistically enriched GO terms were grouped and counted after classification according to GO Slim using the freely available web tool CateGOrizer [13] . Functional and pathway analysis were also conducted using the online Ingenuity Pathway Analysis (IPA) software (http://www.ingenuity.com/products/ipa ) with default settings to identify biological functions, canonical pathways, and networks associated with the significantly regulated genes.
3. Results
3.1. Nanosilver characterization
The 20 nm and 50 nm nanosilver used in this study were characterized by DLS, TEM and ICP-MS analysis as described previously [34] and [37] . Both the nanosilvers used in our study were stable in the cell culture medium [34] and [37] in agreement with the stability report from another independent laboratory [30] for the citrate-coated nanosilver. The average size of the silver nanoparticles and the concentration of the nanosilver solutions were close to the manufacturer provided values [34] and [37] . The TEM images of nanosilver particles showed no aggregation or agglomeration [34] and [37] . The dosing solutions in this study were prepared based on the nanosilver concentration determined by our analysis.
3.2. Cytotoxicity of nanosilver in Caco2 cells
We have reported previously the cytotoxic effects of 20 nm and 50 nm nanosilver in Caco2 cells [34] , [37] and [38] . The concentrations of 20 nm and 50 nm nanosilver for the global gene expression analysis by DNA microarray was selected from the results of these studies [34] , [37] and [38] . In this study we selected 2.5 μg/ml as exposure concentration at which no significant cytotoxicity was observed in Caco2 cells after 4 h exposure of either nanosilver [37] .
3.3. Global gene expression profiles following nanosilver exposure
Global gene expression of Caco2 cells following nanosilver exposure was evaluated by Affymetrix microarrays [37] . The cells were treated with 2.5 μg/ml 20 nm or 50 nm nanosilver for 4 h or 24 h.
Table 1 shows the differentially expressed genes (DEGs) in Caco2 cells induced by exposure to the 20 nm and 50 nm nanosilvers. The 20 nm nanosilver exposure of Caco2 cells for 4 h resulted in a significantly changed expression of a total of 93 genes (Table 1 and Table S1), out of which 90 genes were up-regulated (FC ≥ 1.5) and 3 genes down-regulated (FC ≤ 1.0). However, the 24 h exposure altered only 15 genes, out of which 14 genes were up-regulated and one gene down-regulated (Tables 1 and S2). In comparison, the 50 nm nanosilver exposure of Caco2 cells for 4 h resulted in a significantly changed expression of a total of 189 genes, out of which 184 genes were up-regulated and 5 genes down-regulated (Tables 1 and S3). The 24 h exposure altered only 43 genes, out of which 41 genes were up-regulated and 2 genes down-regulated (Tables 1 and S4).
| Nanosilver exposure period | Nanosilver-induced differentially altered genes | 20 nm nanosilver | 50 nm nanosilver |
|---|---|---|---|
| 4h | Total number of altered genes | 93 | 189 |
| Up-regulated genes | 90 | 184 | |
| Down-regulated genes | 3 | 5 | |
| 24h | Total number of altered genes | 15 | 43 |
| Up-regulated genes | 14 | 41 | |
| Down-regulated genes | 1 | 2 | |
The gene expression profiles of the treated cells were compared with those of the control cells. The fold change (FC) of the annotated genes and their false discovery rate (FDR) were used for selection of differentially expressed genes. Genes were considered differentially altered and of potential biological significance if FC ≥1.5 and FDR ≤0.05 compared to vehicle controls. The genes were considered up-regulated if FC ≥1.5 and FDR ≤0.05, and down- regulated if FC ≤1.0.
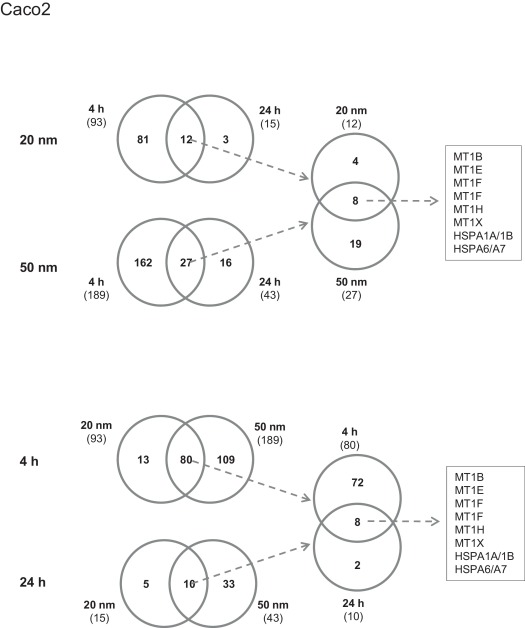
Fig. 1 shows the Venn diagram of overlapping of the DEGs between different treated groups in Caco2 cells. For the 20 nm nanosilver, 12 genes regulated after 24 h exposure were also found in the genes regulated after 4 h exposure. These genes were members of the metallothionein (MT) and heat shock (HSPA) family. For the 50 nm nanosilver, 27 genes were shared by the two time points. These genes were also members of the MT and HSPA family. The MTs and HSPAs were the most affected genes in Caco2 cells exposed to both 20 nm and 50 nm nanosilver.
|
|
|
Fig. 1. Venn diagrams showing overlap of differentially expressed genes between different experiment groups. The total number of differentially expressed genes in each group is included in the parentheses under the group name. The fold change (FC) of the annotated genes and their false discovery rate (FDR) were used for selection of differentially expressed genes. Genes were considered differentially altered and of potential biological significance if FC ≥ 1.5 and FDR ≤ 0.05 compared to vehicle controls. The genes were considered up-regulated if FC ≥ 1.5 and FDR ≤ 0.05, and down- regulated if FC ≤ 1.0. |
3.4. Identification of biological functions and pathways affected by nanosilver exposure
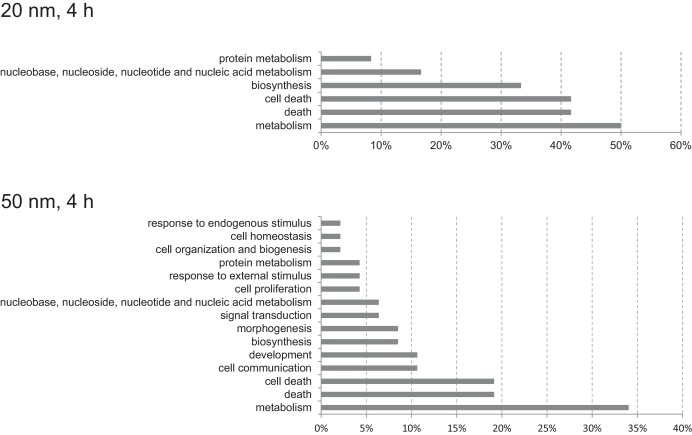
Potential mechanisms of nanosilver toxicity in Caco2 cells were evaluated by the gene ontology (GO) and pathway analysis of DEGs using DAVID. Fig. 2 shows the GO function classification by evaluating the distribution of GO terms for the genes identified in Caco2 cells exposed to 20 nm and 50 nm nanosilver for 4 h. Only the data of 4 h exposure with the two nanosilvers were analyzed because other conditions did not yield adequate number of DEGs for analysis. Using the CateGOrizer tool, these GO terms for the 20 nm nanosilver were grouped into 6 categories of the pre-defined set of parent/ancestor GO terms (Fig. 2 ): metabolism (50%), cell death (42%), biosynthesis (33%), nucleic acid metabolism (17%) and protein metabolism (9%). For the 4 h exposure to the 50 nm nanosilver the GO terms were grouped into 15 categories (Fig. 2 ): metabolism (34%), cell death (19%), communication (11%), development (11%), biosynthesis (8%), morphogenesis (8%), signal transduction (7%), nucleic acid metabolism (7%), cell proliferation (4%) and cell homeostatis (2%).
|
|
|
Fig. 2. Distribution of GO terms according to GO slim for the genes identified in Caco2 cells exposed to 20 nm and 50 nm nanosilver for 4 h. The percentage indicates the number of GO terms in each class as a percentage of the total number of unique GO terms enriched by the differentially expressed genes. |
The pathways, enriched in DEGs in Caco2 cells exposed for 4 h to 20 nm and 50 nm nanosilver, were analyzed by DAVID. The DEGs associated with the 20 nm nanosilver showed the MAPK signaling pathway (Table S5). The DEGs associated with the 50 nm nanosilver showed three pathways: the MAPK signaling, p53 signaling and focal adhesion pathways (Table S6). The MAPK signaling (Fig. S1) was the common pathways associated with both 20 nm and 50 nm nanosilvers. The p53 Signaling (Fig. S2) and Focal adhesion (Fig. S3) pathways were associated with the 50 nm nanosilver only.
4. Discussion
Recently we have reported the toxicogenomic response of the human liver HepG2 cells to 20 nm and 50 nm nanosilver using global gene expression profiling determined by DNA microarray [37] . In the present study we evaluated genomic changes, induced by 20 nm and 50 nm nanosilver in human gastrointestinal Caco2 cells, using the same experimental conditions and toxicogenomic endpoint to determine if these gastrointestinal epithelial cells can be used as an in vitro model representing the oral exposure to nanosilver for rapid predictive toxicity screening. The cells were exposed to the nanosilver at the same dose, 2.5 μg/ml, a concentration that showed no cell viability loss after 4 h exposure [37] . Our results presented here (Table 1 ) show that exposure for 4 h to the 50 nm nanosilver induced higher number of DEGs (189 genes) than to the 20 nm nanosilver (93 genes), suggesting that the larger size nanosilver was more toxic than the smaller one. This observation is opposite of what we observed in HepG2 cells under the same experimental conditions and genotoxic endpoint, showing that the smaller 20 nm nanosilver was more toxic than the larger 50 nm one [37] . These results suggest that cell types influence the toxicity of nanosilver. After 24 h only 15 DEGs remained after exposure to 20 nm nanosilver and 43 genes following the exposure to 50 nm nanosive (Table 1 ), suggesting the cellular repair mechanism that had taken effect in response to the toxic insult exerted by the nanosilvers. Only a small portion of genes was shared by the two time points (Fig. 1 ) compared with the total number of genes suggesting a dynamic nature of the gene expression changes. The MAPK signaling without transcript evidence for alteration of cellular repair genes may also indicate the adjustment of cell signaling after exposure to nanosilver.
Members of the MT and HSAP family genes were upregulated at all the conditions studied (Tables S1–S4). MTs are a family of low molecular weight, cysteine-rich proteins existing in several isoforms [15] . The MT genes have the capacity to bind both physiological and xenobiotic heavy metals through the thiol group of its cysteine residues [5] . It has been suggested that MTs may provide protection against metal toxicity by being involved in regulation of physiological metals, that results in protection against oxidative stress [45] . MT expression has been implicated as a transient response to many forms of stress or injury providing cytoprotective action [45] . HSPA genes are a group of proteins induced by heat shock. Their expression is increased when cells are exposed to elevated temperatures or other stress [6] . The upregulation of MTs and HSAPs found in our study suggests that nanosilver exposure induced cellular stresses and elicited cellular protective responses in Caco2 cells as they did in HepG2 cells [37] . Several independent studies [10] , [19] , [51] and [50] reported up regulation of MTs and HSPAs in cultured cells after exposure to nanosilver similar to our findings.
The transient nature of the gene expression changes was also reflected by the fold change of MTs and HSPAs relative to the control. At 4 h, the average fold change was higher than that at 24 h (Tables S2–S5).
The mechanism(s) of nanosilver toxicity in Caco2 cells could be proposed from the cellular pathways (Tables S5 and S6). The nanosilver exposure regulates the MAPK signaling pathway (Fig. S1). Several HSPA genes involved in the pathway were significantly upregulated by the exposure to both 20 nm and 50 nm nanosilvers. The p53 pathway (Fig. S2) is an important signaling pathway controlling the molecular response to genotoxic stress that may ultimately lead to cell cycle arrest, apoptosis, and cancer [28] . Under normal conditions, p53 is constitutively expressed, but is inactivated by its negative regulator, Mdm2. Upon DNA damage or other cellular stresses, various pathways will lead to the dissociation of the p53 and Mdm2 complex. It has been demonstrated that genotoxic stress activates the p53 gene [2] and the p53 pathway plays an important role in genotoxicant-induced cell apoptosis [71] . Therefore, the p53 signaling pathway and apoptosis may be associated with the genotoxicity of nanosilver in Caco2 and HepG2 cells [34] and [35] . Once activated, p53 will induce a cell cycle arrest to allow either repair of DNA damage or apoptosis to discard the damaged cell. Failure to do this may result in cell survival with damaged DNA. This may cause further tumerigenesis leading to cancer. When this study was in progress another toxicogenomic study on the effects of nanogold in Caco2 cells, similar to our nanosilver study, has been published [3] with similar results as ours.
An alternate interpretation of our results could be that the nanosilver might induce a generalized cellular metal response by primarily upregulating metallothionein (MT) and heat shock protein (HSP) transcripts, mediated by MAPK signaling [25] and [18] . Lim et al. [25] evaluated by DNA microarray the effects of nanosilver of varying sizes from 5 to 100 nm at sub-lethal concentrations in a macrophage cell line. They screened a total of 28,000 cDNA profiles. They observed a significant increase in levels of hemeoxygenase-1 (HO-1), heat shock protein-70 (HSP-70) and interleukin-8 (IL-8). Kaur and Tikoo [18] examined toxicity of silver nanoparticles in different cell types (lung epithelial A549, skin epithelial A431, and murine macrophages RAW264.7) over a range of concentrations (5–100 μg/ml). They reported increased expression of stress markers HSP-70, pp38MAPK, and TNF-α.
We also recognize a “data gap” as a need to provide confirmation and further support of our results by undertaking additional RT-PCR studies to validate the expression of some of the highly differentially regulated genes observed in our studies.
The results of the current study suggest that nanosilver exposure has the potential to cause carcinogenicity. Numerous in vitro studies have revealed that nanosilver induces genotoxicity in various cell types [24] . Recently, in vivo genotoxicity and carcinogenicity of nanosilver were also reported. One study by Xu et al. [49] reported that nanosilver-hydrogel induced micronuclei, nuclei disruption, chromatin concentration and cell apoptosis in rabbit reproductive organ tissues. On the other hand, study by Kim et al. [23] reported that there was no genotoxic effect in rats after 28 days oral exposure to nanosilver. Kim et al. [21] also reported that no genotoxic effect in rats after 90 days inhalation of nanosilver. The reasons for the different findings in potential genotoxic and carcinogenic risks of nanoparticles are not known, but are possibly due to differences in experimental design, use of different nanomaterials and doses, use of different animal models, and different targeting organs investigated [4] . In addition, all these in vivo studies were relatively short considering the time needed to observe tumor development in animals. In this regard, it has to be noted that it took over 10 years to confirm the carcinogenicity of asbestos nanofibers [39] . Therefore long-term studies on nanoparticle in vivo toxicity are warranted in order to gain a better understanding of their genotoxicity and carcinogenicity.
5. Conclusions
This study demonstrates that 20 nm and 50 nm nanosilver interact with Caco2 cells to induce cellular toxicogenomic responses that could be further exploited as biomarkers of nanosilver exposure. The Caco2 cells exposed to nanosilvers responded to the toxic insult by upregulating stress response genes such as metallothioneins and heat shock proteins. The cellular pathways affected by the nanosilver exposure may lead to increased toxicity. The results of our study presented here suggest that toxicogenomic characterization of human Caco2 cells is a valuable in vitro tool for assessing toxicities of nanoparticles.
Conflict of interest
The author declares that there is no conflict of interest.
Transparency document
Transparency Document.
Acknowledgments
The views presented in this article are those of the author and do not necessarily reflect those of the US Food and Drug Administration. The author thanks Dr. Xiugong Gao for his help in microarray analysis, Dr. Jeffrey Yourick and Dr. Robert Sprando for consultations and support for this study.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [2] E. Appella, C.W. Anderson; Post-translational modifications and activation of p53 by genotoxic stresses; Eur. J. Biochem., 268 (2001), pp. 2764–2772
- [3] E. Bajaka, M. Fabbria, J. Pontia, I. Ojea-Jiméneza, A. Collottac, V. Mariania, D. Gillilanda, F. Rossia, L. Gribaldod; Changes in Caco-2 cells transcriptome profiles upon exposure to gold nanoparticles; Toxicol. Lett., 233 (2015), pp. 187–199
- [4] H. Becker, F. Herzberg, A. Schulte, M. Kolossa-Gehring; The carcinogenic potential of nanomaterials, their release from products and options for regulating them; Int. J. Hyg. Environ. Health, 214 (2011), pp. 231–238
- [5] S.R. Davis, R.J. Cousins; Metallothionein expression in animals: a physiological perspective on function; J. Nutr., 130 (2000), pp. 1085–1088
- [6] A. De Maio; Heat shock proteins: facts thoughts, and dreams; Shock, 11 (1999), pp. 1–12
- [7] G. Dennis Jr, B.T. Sherman, D.A. Hosack, J. Yang, W. Gao, H.C. Lane, R.A. Lempicki; DAVID: database for annotation, visualization, and integrated discovery; Genome Biol., 4 (2003), p. 3
- [9] J.L. Fang, F.A. Beland; Long-term exposure to zidovudine delays cell cycle progression, induces apoptosis, and decreases telomerase activity in human hepatocytes; Toxicol. Sci., 111 (2009), pp. 120–130
- [10] R. Foldbjerg, E.S. Irving, Y. Hayashi, D.S. Sutherland, K. Thorsen, H. Autrup, C. Beer; Global gene expression profiling of human lung epithelial cells after exposure to nanosilver; Toxicol. Sci., 130 (2012), pp. 145–157
- [12] S. Hochreiter, D.A. Clevert, K. Obermayer; A new summarization method for affymetrix probe level data; Bioinformatics, 22 (2006), pp. 943–949
- [13] Z.L. Hu, J. Bao, J.M. Reecy; CateGOrizer: a web-based program to batch analyze gene ontology classification categories; Online J. Bioinform., 9 (2008), pp. 108–112
- [14] W. Huang da, B.T. Sherman, R.A. Lempicki; Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources; Nat. Protoc., 4 (2009), pp. 44–57
- [15] P.E. Hunziker, J.H. Kägi; Isolation and characterization of six human hepatic isometallothioneins; Biochem. J., 231 (1985), pp. 375–382
- [16] R.A. Irizarry, B. Hobbs, F. Collin, Y.D. Beazer-Barclay, K.J. Antonellis, U. Scherf, T.P. Speed; Exploration normalization, and summaries of high density oligonucleotide array probe level data; Biostatistics, 4 (2003), pp. 249–264
- [18] J. Kaur, K. Tikoo; Evaluating cell specific cytotoxicity of differentially charged silver nanoparticles; Food Chem. Toxicol., 51 (2013), pp. 1–14
- [19] K. Kawata, M. Osawa, S. Okabe; In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells; Environ. Sci. Technol., 43 (2009), pp. 6046–6051
- [20] J.S. Kim, E. Kuk, K.N. Yu, J.H. Kim, S.J. Park, H.J. Lee, S.H. Kim, Y.K. Park, Y.H. Park, C.Y. Hwang, Y.K. Kim, Y.S. Lee, D.H. Jeong, M.H. Cho; Antimicrobial effects of silver nanoparticles; Nanomedicine, 3 (2007), pp. 95–101
- [21] J.S. Kim, J.H. Sung, J.H. Ji, K.S. Song, J.H. Lee, C.S. Kang, I.J. Yu; In vivo genotoxicity of silver nanoparticles after 90-day silver nanoparticle inhalation exposure; Saf. Health Work, 2 (2011), pp. 34–38
- [23] Y.S. Kim, J.S. Kim, H.S. Cho, D.S. Rha, J.M. Kim, J.D. Park, B.S. Choi, R. Lim, H.K. Chang, Y.H. Chung, I.H. Kwon, J. Jeong, B.S. Han, I.J. Yu; Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats; Inhal. Toxicol., 20 (2008), pp. 575–583
- [24] A. Kumar, A. Dhawan; Genotoxic and carcinogenic potential of engineered nanoparticles: an update; Arch. Toxicol., 87 (2013), pp. 1883–1900
- [25] D.H. Lim, J. Jang, S. Kim, T. Kang, K. Lee, I.H. Choi; The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis; Biomaterials, 33 (2012), pp. 4690–4699
- [27] B. Meek, J. Doull; Pragmatic challenges for the vision of toxicity testing in the 21st century in a regulatory context: another Ames test? …or a new edition of the red book?; Toxicol. Sci., 108 (2009), pp. 19–21
- [28] S.E. Morgan, M.B. Kastan; p53 and ATM: cell cycle, cell death, and cancer; Adv. Cancer Res., 71 (1997), pp. 1–25
- [30] C.M. Powers, A.R. Badireddy, I.T. Ryde, F.J. Seidler, T.A. Slotkin; Silver nanoparticles compromise neurodevelopment in PC12 cells: critical contributions of silver ion, particle size, coating, and composition; Environ. Health Perspect., 119 (2011), pp. 37–44
- [34] S.C. Sahu, J. Njoroge, S.M. Bryce, J.J. Yourick, R.L. Sprando; Comparative genotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells evaluated by a flow cytometric in vitro micronucleus assay; J. Appl. Toxicol., 34 (2014), pp. 1226–1234
- [35] S.C. Sahu, S. Roy, J. Zheng, J.J. Yourick, R.L. Sprando; Comparative genotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells evaluated by fluorescent microscopy of cytochalasin B-blocked micronucleus formation; J. Appl. Toxicol., 34 (2014), pp. 1200–1208
- [36] S.C. Sahu, J. Zheng, L. Graham, L. Chen, J. Ihrie, J.J. Yourick, R.L. Sprando; Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture; J. Appl. Toxicol., 34 (2014), pp. 1155–1166
- [37] S.C. Sahu, J. Zheng, J.J. Yourick, S.L. Sprando, X. Gao; Toxicogenomic responses of human liver HepG2 cells to silver nanoparticles; J. Appl. Toxicol., 35 (2015), pp. 1160–1168
- [38] S.C. Sahu, J. Njoroge, S.M. Bryce, J. Zheng, J. Ihrie; Flow cytometric evaluation of the contribution of ionic silver to genotoxic potential of nanosilver in human liver HepG2 and colon Caco2 cells; J. Appl. Toxicol. (2016) http://dx.doi.org/10.1002/jat.3276
- [39] V.C. Sanchez, J.R. Pietruska, N.R. Miselis, R.H. Hurt, A.B. Kane; Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos?; Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 1 (2009), pp. 511–529
- [42] I. Sondi, B. Salopek-Sondi; Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria ; J. Colloid Interface Sci., 275 (2004), pp. 177–182
- [45] S.E. Theocharis, A.P. Margeli, A. Koutselinis; Metallothionein: a multifunctional protein from toxicity to cancer; Int. J. Biol. Markers, 18 (2003), pp. 162–169
- [46] W. Tong, X. Cao, S. Harris, H. Sun, H. Fang, J. Fuscoe, A. Harris, H. Hong, Q. Xie, R. Perkins, L. Shi, D. Casciano; ArrayTrack—supporting toxicogenomic research at the U.S. Food and Drug Administration National Center for Toxicological Research; Environ. Health Perspect., 111 (2003), pp. 1819–1826
- [47] W. Tong, S. Harris, X. Cao, H. Fang, L. Shi, H. Sun, J. Fuscoe, A. Harris, H. Hong, Q. Xie, R. Perkins, D. Casciano; Development of public toxicogenomics software for microarray data management and analysis; Mutat. Res., 549 (2004), pp. 241–253
- [49] L. Xu, L. Chen, Z. Dong, J. Wang, Z. Wang, A. Shao; In vivo toxicity in reproductive organs of rabbit and in vitro cytotoxicity of silver nanoparticle based-hydrogel; Chin. J. Pharm. Anal., 2 (2012), pp. 194–201
- [50] L. Xu, X. Li, T. Takemura, N. Hanagata, G. Wu, L.L. Chou; Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel; J. Nanobiotechnol., 10 (2012), p. 16 http://dx.doi.org/10.1186/1477-3155-10-16
- [51] L. Xu, T. Takemura, M. Xu, N. Hanagata; Toxicity of silver nanoparticles as assessed by global gene expression analysis; Mater. Express, 1 (2011), pp. 74–79
- [52] C.N. Lok, C.M. Ho, R. Chen, H. Sun, P.K. Tam, J.F. Chiu, C.M. Che; Proteomic analysis of the mode of antibacterial action of silver nanoparticles; J. Proteome Res., 5 (2006), pp. 916–924
- [53] B.K. Gaiser, S. Hirn, A. Kermanizadeh, N. Kanase, K. Fytianos, A. Wenk, N. Haberl, A. Brunelli, W.G. Kreyling, V. Stone; Effects of silver nanoparticles on the liver and hepatocytes in vitro; Toxicol. Sci., 131 (2013), pp. 537–547
- [54] A.M. Gatti; Biocompatibility of micro- and nano-particles in the colon; Biomaterials, 25 (2004), pp. 385–392
- [55] P.R. Gibson, R.P. Anderson, J.M. Mariadason, A.J. Wilson; Protective role of the epithelium of the small intestine and colon; Inflamm. Bowel Dis., 2 (1996), pp. 279–302
- [56] I. Chantret, A. Barbat, E. Dussaulx, M.G. Brattain, A. Zweibaum; Epithelial polarity, villin expression and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines; Cancer Res., 48 (1988), pp. 1936–1942
- [57] D.P. Chopra, A.A. Dombkowski, P.M. Stemmer, G.C. Parker; Intestinal epithelial cells in vitro; Stem Cells Dev., 19 (2010), pp. 131–142
- [58] S. Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, H. Tokuda; Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells; Eur. J. Pharm. Sci., 10 (2000), pp. 195–204
- [59] E.F.A. Brandon, T.M. Bosch, M.J. Deenen, R. Levink, E. van der Wal, J.B.M. van Meerveld, M. Bijl, J.H. Beijnen, J.H.M. Schellen, I. Meijerman; Validation of in vitro cell models used in drug metabolism and transport studies: genotypning of cytochrome P450 and drug transporter polymorphism in the human hepatoma (HepG2), ovarian carcinoma (IGROV-1) and colon carcinoma (Caco2, LS180) cell lines ; Toxicol. Appl. Pharmacol., 211 (2005), pp. 1–10
- [60] K. Gerloff, C. Albrecht, A.W. Boots, I. Forster, R.P.F. Schins; Cytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco2 cells; Nanotoxicology, 3 (2009), pp. 355–364
- [61] K. Gerloff, D. Pereira, N. Faria, A.W. Boots, J. Kolling, I. Forster, C. Albrecht, J.J. Powell, R.P.F. Schins; Influence of simulated gastrointestinal conditions on particle-induced cytotoxicity and interleukin-8 regulation in differentiated and undifferentiated Caco2 cells; Nanotoxicology, 7 (2013), pp. 353–366
- [62] S.O. Simmons, C. Fan, R. Ramabhadran; Cellular stress response pathway system as a sentinel ensemble in toxicological screening; Toxicol. Sci., 111 (2009), pp. 202–225
- [63] P.V. AshaRani, G.L.K. Mun, M.P. Hande, Valiyaveettil; Cytotoxicity and genotoxicity of silver nanoparticles in human cells; ACS Nano, 3 (2009), pp. 279–290
- [64] A. Haase, S. Rott, A. Mantion, P. Graf, J. Plendl, A. Thunemann, W. Meier, A. Taubert, A. Luch, G. Reiser; Effects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responses; Toxicol. Sci., 126 (2012), pp. 457–468
- [65] Y. Kao, Y. Chen, T. Cheng, Chiung, P. Liu; Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity; Toxicol. Sci., 125 (2012), pp. 462–472
- [66] L. Horev-Azaria, C.J. Kirkpatrick, R. Korenstein, P.N. Marche, O. Maimon, J. Ponti, R. Romano, F. Rossi, U. Golla-Schindler, D. Sommer, C. Uboldi, R.E. Unger, C. Villiers; Predictive toxicology of cobalt nanoparticles and ions: comparative in vitro study of different cellular models using methods of knowledge discovery from data ; Toxicol. Sci., 122 (2011), pp. 489–501
- [67] S.C. Sahu, M.W. O’Donnell, R.L. Sprando; Interactive toxicity of usnic acid and lipopolysaccharides in human liver HepG2 cells; J. Appl. Toxicol., 32 (2012), pp. 739–748
- [68] C.L. Merrill, H. Ni, L.W. Yoon, M.A. Tirmenstein, P. Narayanan, G.R. Benavides, M. Easton, D. Creech, C. Hu, D. McFarland, L. Hahn, H.C. Thomas, K.T. Morgan; Etomoxir-induced oxidative stress in HepG2 cells detected by differential gene expression is confirmed biochemically; Toxicol. Sci., 68 (2002), pp. 93–101
- [69] A.J. Harris, S.L.D.A. Dial Casciano; Comparision of basal gene expression profiles and effects of hepatocarcinogens on gene expression in cultured primary human hepatocytes and HepG2 cells; Mutat. Res., 549 (2004), pp. 79–99
- [70] J. Dudas, T. Mansuroglu, F. Moriconi, F. Haller, J. Wilting, T. Lorf, L. Fuzes, G. Ramadori; Altered regulation of Prox1-gene-expression in liver tumors; BMC Cancer, 8 (2008), pp. 92–106
- [71] X. Shi, B. Zhou; The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebra fish embryo; Toxicol. Sci., 115 (2010), pp. 391–400
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?