1. INTRODUCTION
The development of reshapable, recyclable and repairable polymer materials or resins is a key research focus currently due to their environmental issues, and non-renewability concerns. In this context, several alternatives are being developed in order to reach new resins that exhibit strength, durability and chemical resistance approaching that of traditional thermosets, while exhibiting end of life recyclability, re-processability and reparability capabilities, that is why they are also called 3R resins. Due to current environmental challenges that the aeronautical industry is facing, resins based on vitrimer chemistry become a potential and attractive alternative to be considered in order to combine main advantages of current thermoset resin (processing conditions / temperature, means, mechanical properties...) and thermoplastic resin (recycling, re-processability, repairability, welding), and, then, overcome main current constraints of widely used thermoset epoxy resin in airframe.
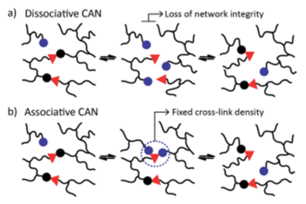
The chemical strategy of vitrimers is based on the introduction of plasticity in the crosslinked polymer network through the introduction of exchangeable chemical bonds, leading to dynamic cross-links. The internal bond exchanged between different positions of the organic polymer chain can lead to a macroscopic flow without risking structural damage or permanent loss of resin properties. This kind of resins are called Covalent Adaptable Networks or CAN [1]. CANs may be further classified in two groups according to the exchange mechanism. In CANs the network dynamism is achieved by applying an external stimulus such as heat, pH or light via a dissociative or associative pathway (see Figure 1).
The first group of CANS is based on a dissociative mechanism that consists of a preliminary step where the covalent bonds are first broken and then, in a second step, formed again in a different place (Figure 1a). This mechanism brings a viscosity drop due to the polymer density decrease during the first step. The second group of CANs involves an associative mechanism that is characterized by fixed cross-link density. Thus, covalent bonds are broken only when the new ones are formed (Figure 1b), making this network both permanent and dynamic.
Vitrimers, which are a new class of plastics / resins introduced by Leibler in 2011 [2], belong to associative CAN . They behave as cross-linked materials at temperatures below Tg, and as viscoelastic liquids at higher temperatures. The presence of dynamic bonds facilitates the reprocessing of network and recycling despite having permanent crosslinks. The rearrangement process of vitrimers can be controlled by two different transition temperatures: glass transition temperature (Tg) and topology freezing temperature (Tv), which is theoretically defined as the temperature at which the viscosity reaches 1012 Pa.s. Above Tv, exchangeable reaction happens fast and vitrimer is able to be reprocessed and recycled and below Tv, exchangeable reaction is slow and vitrimer is similar to the traditional thermoset. Both Tg and Tv are key for the application of such a kind of vitrimer resins in aeronautics due to aircraft operation and production conditions. Vitrimers are categorized based on the nature of the dynamic associative exchange reaction. Thus, following chemical exchange mechanisms as transesterification, transamination of Vinylogous Acyls, transcarbamoylation, transalkylation, Si-O exchange in Siloxanes and Silyl Ethers, Olefin metathesis, Boroxine exchange, Thiol-disulfide vitrimer, Disulfide exchange, which are described in bibliography [3], can be considered to obtain vitrimers.
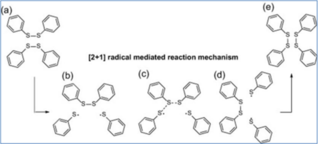
Among all the chemical exchange mechanisms mentioned above, the disulfide exchange (see figure 2) seems to be the most promising for the aircraft industry, since resulting polymers show operational Tg ranges almost in line with those reached in thermoset materials currently used.
Thus, the resin referenced as AIR-RES-13B, a vitrimer epoxy resin based on disulfide exchange dynamic mechanism, was firstly developed within the framework of European Project AIRPOXY to be used for Resin Infusion (RI) manufacturing technology. Now, within the framework of the national funding PTA (Plan Tecnológico Aeronáutico), an overall mechanical, phys-chemical and dynamic characterization has been carried out in order to develop a thorough understanding of such materials and the potential application in the Aircraft industry. Moreover, some laminates have been manufactured by Resin Transfer Moulding (RTM) in order to study the material thermoforming capability by using an omega mould geometry.
2. MATERIALS AND METHODS
2.1. Raw materials and manufacturing procedures
The vitrimer epoxy resin AIR-RES-13B, based on dynamic disulfide covalent bonds, was formulated by mixing different components. Part A was based on a mixture of Tetraglycidyl-4,4´ methylene dianiline (TGMDA), Bisphenol F diglycidyl ether (BFDGE) and Poly Bisphenol A-co-epichlorohydrin resins from Huntsman. Part B was a 4-Aminophenyl disulfide (4-AFD) dynamic hardener from Carbosynth. HexForce G0926 5 HS fabric with 375 g/m2 from Hexcel was used to produce the composite panels. To prepare neat epoxy resin sheets, Part A and Part B were mixed at 80ºC and degassed under vacuum. Afterwards the resulting viscous homogeneous liquid was poured into a metallic mould and cured in an oven at 130ºC for 1 h and post-cured at 180ºC for 0,5 h. The reaction was followed by FTIR, where a complete curing was confirmed by the disappearance of the epoxide bands at 3056 and 915 cm-1. Differential scanning calorimetry (DSC) was used to confirm the complete curing. No residual curing exothermic peak was observed by DSC, confirming the complete cure of the epoxy network.
Carbon laminates were manufactured using the low-pressure RTM process, using Walther Pilot MDG2 pressurized tank, an aluminium mould with a 500x500 mm cavity and 6 mm thickness, and a hot plate press to heat and close the mould. The vitrimer epoxy resin mixture was heated at 80ºC and injected at a pressure of 3 bars into the mould previously heated at 130ºC. Once completed the injection, the compaction was performed at 4 bars and the total curing cycle was 1 hour @ 130ºC followed by a post-curing of 0,5h @ 180ºC. 16 plies with a lay-up of (45/0/-45/90)2S was used to produce laminates with a fibre volume content of 58%.
2.2. Characterization tests
The uncured vitrimer epoxy resin was characterized according to Airbus internal standards to measure several properties related to its processability: glass transition temperature (Tg), gel time, dynamic viscosity and isothermal viscosity. DSC measurements were performed using a differential scanning calorimeter (DSC) from TA Instruments (Discovery DSC25 Auto) over a temperature range from -80°C to 210°C under nitrogen. The Tg was obtained as the inflection point of the heat flow step recorded at a scan rate of 10°C min-1. Rheological and gelation time tests were carried out in a TA instrument AR2000ex rheometer using a 40 mm plate–peltier geometry. The viscosities were determined at 10 rad/s and 10% of strain, being the heating rate of 2ºC/min to measure the dynamic viscosity. The gelation time was determined as the crossover point of the elastic modulus (G´) and loss modulus (G´´).
The cured vitrimer resin was characterized to measure its mechanical properties: tensile, flexural and fracture toughness. Tensile and flexural tests were performed using an INSTRON 5985 universal testing machine and 2650-563 static axial clip-on extensometer controlled by Bluehill 3 software. Tensile properties were measured according to ISO 527-2 standard at an elongation rate of 1 mm min-1using 1BA type test specimens. Flexural properties were carried out according to ISO 178 standard using 80x10x4 mm test specimens at deformation rate of 2 mm min-1. Fracture toughness tests were performed according to ISO 13586 using a Galdabini SUN2500 equipment. The pre-notch was machined with a depth of 0.45 times the width of the specimen using a CEAST notching machine and the notch was sharpened with a femtosecond laser to a depth of approximately 0,5 times the width of the specimen. Due to the dynamic hardener, the resin was expected to show vitrimer behaviour and thus, some interesting features derived from it such as thermoformability. The time and temperature dependent relaxation modulus of the vitrimer was tested by DMA to characterize its heat induced malleability. Stress relaxation tests were carried out in DMA TA Q800 equipment. Specimens of 38x6x1 mm were tested at different temperatures, applying a preload of 10-3 N to assure straightness. After reaching the required temperature, 1% of strain was applied and the relaxation modulus was monitored as a function of time.
Finally, the thermoformability of composite laminates was studied using a steel mould with omega geometry and an INSTRON 5985 universal testing machine equipped with a temperature chamber. The mould was designed to obtain a profile with omega geometry of approximate dimensions of 120x140x30 mm and internal radius of 4 mm. Specimens extracted from the thermoformed omega were analysed by microscopy using a Nikon Epiphot 200 Inverted Microscope.
3. RESULTS AND DISCUSSION
3.1. Characterization of the vitrimer epoxy resin
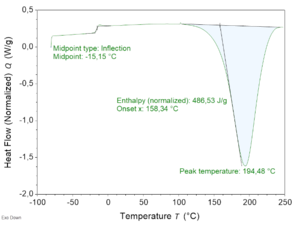
To determine reactivity of the vitrimer resin, DSC analysis at 10 ºC/min was performed first (Figure 3). The exothermic onset and peak temperatures were found to be 159°C and 194°C respectively, Therefore, the resin curing temperature for the composite panels produced afterwards by RTM was set at 130°C and the post-curing temperature was set at 180°C.
Next rheology profiles were obtained by measure of dynamic viscosity at 2ºC/min and isothermal viscosities at different temperatures. From isothermal viscosity measurements gel time was also determined. To do this, G' and G" versus time were plotted, corresponding the time correlating to the crossover point of G' and G" to the gel point. The dynamic curves showed a minimum viscosity of the resin of 50 mPa.s at 122°C, while isothermal curves showed a relatively fast curing, being the time to gelation between 50 min @ 120°C and 13 min @ 150°C. These results, summarized in Table 1, show that the vitrimer resin has a higher viscosity and reactivity than aero grade commercial resins currently used in aeronautics, e.g. RTM6 [4]. The values were considered to adjust RTM process parameters to produce composite panels.
| Test | Value | Test method | Units | Value |
| DSC | Tg | AITM 3-0002
(heating rate 10°C/min) |
°C | -15 |
| Tos | °C | 159 | ||
| Tp | °C | 194 | ||
| -AH100 | J/g | 477 | ||
| Gel time | AITM 3-0004 | min | 13 @ 150°C | |
| 30 @ 130°C | ||||
| 50 @ 120°C | ||||
| Dynamic viscosity | Viscosity min | AITM 3-0004
(heating rate of 2°C/min) |
mPa.s | 58 |
| T viscosity min | °C | 122,5 | ||
| Tgel | °C | 154 | ||
| tr | min | 16 | ||
| Isothermal viscosity | Initial | AITM 3-0004 | mPa.s | 38 @130°C |
| 40 @120°C | ||||
| 200 @ 80°C | ||||
| 513 @ 70°C | ||||
| 2 hours | 670 @ 80°C | |||
| 800 @ 70°C | ||||
Next, the mechanical properties of the cured vitrimer resin were measured. Table 2 summarizes the results obtained. Comparing again with the properties of commercial resins currently used in aeronautics, e.g. RTM6 resin, the vitrimer resin has similar flexural properties and better tensile and fracture properties, which may be because the vitrimer resin has lower Tg than RTM6 (160°C against 230°C).
| Test | Value | Test method | Units | Value |
| DSC | Tg | °C | 160 | |
| Tensile | Strength | ASTM D638 | MPa | 101 |
| Modulus | MPa | 3371 | ||
| Flexure | Strength | ASTM D790 | MPa | 143 |
| Modulus | MPa | 3296 | ||
| Fracture toughness | K1c | ISO13586 | MPa.m1/2 | 1 |
| G1c | J/m2 | 430 |
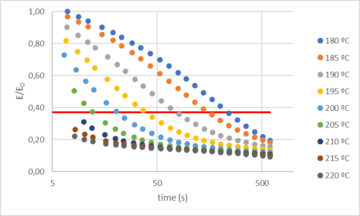
Based on these results, vitrimer resin could be used to obtain thermal and mechanical properties comparable to classical epoxy resins. Additionally, due to the dynamic hardener, the resin was expected to show vitrimer behavior and therefore some interesting features derived from it, such as thermoformability. To verify this, the time and temperature dependent relaxation modulus of the dynamic network was tested by DMA. Figure 4 shows the normalized stress relaxation curves at different temperatures. The results showed that at temperatures above Tg the epoxy network was able to relax stress and flow. Based on the Maxwell’s model for viscoelastic fluids, relaxation times were determined as the time required to relax 63% of the initial stress. The obtained relaxation times (τ*) ranged from 243 seconds at 180 ºC to less than 10 seconds at temperatures equal to or greater than 210 ºC. Due to the presence of dynamic bonds, this material relaxes stresses when applying the strain, contrary to traditional thermosets where no significant relaxation occurs [5].
|
|
It can be also observed that full relaxation is not achieved, being the maximum around 90%. This is because a small part of the network is not dynamic. Nevertheless, our previous experience had already shown that 100% relaxation is not necessary to thermoform the resin, being relaxation times below 20 s suitable for thermoforming of the vitrimer resin.
3.2. Thermoforming of composite panels
6 mm thick panels produced by RTM were cut into pieces of 180x120mm for the thermoforming trials. To verify if a ply gliding happened during thermoforming, holes of 3 mm diameter were drilled perpendicular to the surface in the laminates at different positions. Additionally, 0,5 mm thick aluminium sheets were used to fill the mould cavity and help to homogenize pressure distribution and consolidation during thermoforming of the part.
The procedure followed during the thermoforming process was as follows (see Figure 5): Firstly the mould was heated to 210°C inside the chamber. Next the laminate was placed with the aluminium sheet on top of the lower mould and heated to 210°C. Once the whole was tempered, the laminate was thermoformed. For that, after closing the mould at a speed of 10 mm/min, a load of 500 N/s was applied until reaching the final consolidation force of 90 KN, which corresponded to 50 bar. Finally, keeping the mould closed, the chamber was cooled by forced ventilation until the temperature was below 130°C.At that time de-moulding was carried out.
.

(1) laminate with holes |

(2) laminate in the mold with the aluminium sheet on top |

(3) laminate after thermoforming |
Figure 6 shows the omega profile obtained. Some roughness was observed by visual inspection in the outer face of the webs in the area near the transition zone to the head. Also some blisters were detected in the inner face of the head. Finally, inspection of the holes confirmed that the ply gliding happened in the webs, which is necessary to avoid internal breakage in the laminate.

(1) Thermoformed omega |

(2) Roughness in the web |

(c) Blisters in the head (inner face) |

(3) Ply gliding in the web |
Microscopic analysis performed on the transition zone from web to head showed only some small imperfections in the web and the radius (see Figure 7).

|

|

|

(1) Web |
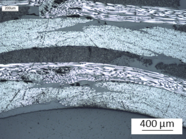
(2) Radius |

(3) Head |
4. CONCLUSIONS
The detailed characterization of the AIR-RES-13B resin as well as the thermoforming trials carried out in this project led to the following conclusions:
- AIR-RES-13B has shown a higher viscosity and reactivity than conventional RI thermoset resins, however the RTM process has been adjusted to produce panels successfully.
- The final Tg of the resin (158°C onset) is lower than the thermoset resin reference (typical RTM resin used in aeronautics). Although the resins could be reformulated stoichiometrically to have a higher Tg, this would lead to a loss of 3R properties due to the thermal degradation of the hardener during the reprocessing phase. Therefore, the formulation of the AIR-RES-13B should be adjusted in order to maximise the dynamic properties to obtain a Tg as high as possible to be a potential alternative for thermoset resin implied in the aeronautical field.
- A maximum of 90% of relaxation of the resin has been achieved with the current AIR-RES-13B formulation. Although it is not clear to what extent it affects thermoforming, it can hinder the resin chemical recycling capability. Therefore, new formulation strategies need to be addressed in order to improve the relaxation of the resin and therefore the recycling capability of the system.
- It has been demonstrated the thermoforming capability of the system. The internal quality of the thermoformed parts carried out using symmetric mould geometry has shown that there has been ply gliding, showing only a few small imperfections.
Then, although a high potential has been seen in this type of chemistry, it is necessary to work on readjusting the formulations to achieve a higher Tg, lower reactivity, higher percentage of relaxation of the resin to improve the internal quality of the thermoformed parts, achieve a better performance and 100% chemical recyclability of the resin.
Regarding next steps for this project, different chemical strategies and solutions will be explored to increase the vitrimer resin Tg values as well as to increase the maximum % of resin relaxation, trying to keep the good properties observed in this technology up to now. Additionally, it is time to start to focus on the development and characterization of a prepreg based on vitrimer resin and carbon fibre, since this is the type of composite materials most widely used in the aeronautical structure sector for time being
ACKNOWLEDGEMENTS
The technical support of Edurne Elorza from CIDETEC is acknowledged. All the work done on the AIRPOXY project (H2020 No 769274) is also acknowledged, especially the thermoforming studies carried out by Stefan Weidmann from the Leibniz IVW and Conor Kelly from ÉireComposites.
CDTI funding PTA (Plan Tecnológico Aeronáutico) - project PEGASO led by Airbus, where this Airbus - CIDETEC collaboration first phase is included. Next phase of the collaboration project will be placed in the CTDI funding MISIONES - project OPTIMUS led by Airbus.
REFERENCES
[1] Recyclable and reformable epoxy resins based on dynamic covalent bonds – Present, past, and future. Volume 105, 107420 2022 Polymer Testing.
[2] Silica-like malleable materials from permanent organic networks. Volume 334, 6058 (965-968) 2011 L. Science
[3] Vitrimers: permanent organic networks with glass-like fluidity. Volume 7, 30-38, 2016 Chemical Science
[4] https://www.hexcel.com/user_area/content_media/raw/RTM6_DataSheetPDF.pdf
[5] Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Materials Horizons 2016, 3 (3), 241-247
Document information
Published on 10/01/23
Accepted on 16/06/22
Submitted on 29/04/22
Volume 07 - COMUNICACIONES MATCOMP21 (2022), Issue Núm. 3 - Materiales y Estructuras - Modelos Numéricos, 2023
DOI: 10.23967/r.matcomp.2023.01.01
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?