1. Introduction
In recent years, environmental concerns and regulatory measures to manufacture sustainable products have increased, and recyclability already stands out as one of the most promising ways to achieve sustainable circularity[1], [2].
Among polymeric materials, polyurethanes (PUs) are one of the most widely used polymers [3], [4]. PUs can be produced as thermoplastics and thermosets. Their excellent performance and versatility allow them to be used in a wide range of applications, which include foams, coatings, adhesives, sealants and elastomers (CASE). Cross-linked thermosets generally have outstanding properties compared to linear PUs, but with the drawback that they cannot be recycled or reprocessed [5], [6].
As an alternative to the recycling of cross-linked PUs and polymers in general, the introduction of thermally reversible bonds into the polymer chains has been studied in recent years, allowing the polymers to be recycled or reprocessed, since the bonds are broken when heat is applied and reformed when heat is removed [7]–[9].
The furan-maleimide Diels-Alder adduct (DA) is one of the most studied thermally reversible covalent bond. At low temperatures, the DA bonds are closed, allowing cross-linking in properly designed structures and resulting in materials with improved mechanical properties. At high temperatures, however, the retro-DA reaction takes place, uncoupling the DA reactants, facilitating the reprocessing of cross-linked materials, thus allowing to cross the boundary between thermoplastics and thermosets [9], [10].
In this work, a trifunctional alcohol with three Diels-Alder adducts in the structure (DA-triol) was synthesized from a trismaleimide (TMI) and furfuryl alcohol, component that can be obtained from polysaccharides or sugars from vegetable renewable sources [7], [11]. Then, the DA-triol was used along with a fully biobased diol (Velvetol® 500 from Weylchem) and an aromatic isocyanate to synthesize a cross-linked and partially bio-based PU. The obtained PU behaves like a thermoset in terms of mechanical properties, but thanks to the Diels-Alder adduct, it can be reprocessed and is suitable for recycling.
2. DA-triol synthesis and characterization
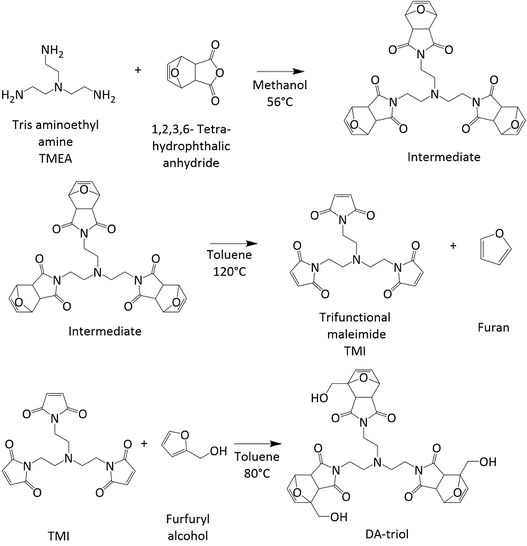
The DA-triol synthesis was carried out in three main reactions (Figure 1). In the first one, tris (2-aminoethyl) amine and exo-3,6-epoxy-1,2,3,6-tetrahydrophthalic anhydride reacted to produce the intermediate. Reactants were added at (1:3) stoichiometric ratio to HPLC grade methanol and left to react at 56 °C for three days under constant stirring. The final precipitate was collected and dried as the intermediate [11].
Next, the intermediate was diluted in toluene and left on continuous stirring for 24 hours at 120 °C. The resulting mixture was rapidly cooled and then filtered. The collected solids were discarded and the filtrate was dried obtaining solid TMI, which was washed with hexane and dried again.
Finally, TMI and furfuryl alcohol were mixed to synthesize the DA-triol. Reactants were added at a stoichiometric ratio along with toluene. The mixture was heated to 80 °C and let under constant stirring for 16 hours. At the end of the reaction time, a precipitate was obtained which was collected, washed with fresh toluene and dried.
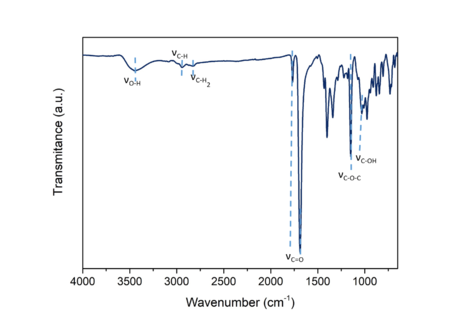
The DA-triol was characterized by Fourier transformed infrared spectroscopy (FTIR) in a Perkin Elmer Two N FT-NIR spectrometer equipped with an ATR golden gate accessory. The spectrum was recorded between 4000 and 650 cm-1, with 64 scans and a resolution of 4 cm-1. The FTIR spectra of the synthesized DA-triol is shown in Figure 2. The characteristic broad band around 3400 cm-1 corresponds to the stretching vibration of the hydroxyl groups and the bands around 2940 and 2826 cm-1 are related to the stretching vibration of the CH- and –CH2- groups, respectively. The presence of the bands at 1770 cm-1 and 1688 cm-1 represent the carbonyl characteristic doublet of DA adducts, indicating the successful DA bond formation between furfuryl alcohol and maleimide [12], [13]. The band located at 1147 cm-1 is related to the stretching of C-O-C bonds inside the maleimide rings; and the band located at 1030 cm-1 is associated with the C-O stretching vibration in the primary alcohol, confirming the presence of the primary alcohol in the DA-triol.
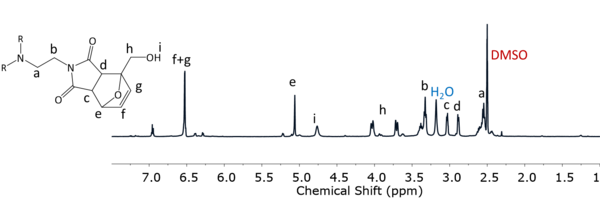
The DA-triol was also characterized by proton nuclear magnetic resonance spectroscopy (1H-NMR). The measurement was carried out in a Bruker Avance 500 spectrometer at 55 °C using deuterated DMSO as solvent. The spectrum shows that the expected DA-triol was obtained (Figure 3). 1H NMR (500 MHz, DMSO-d6) δ 6.53 (m, 2H, -CH=CH-, Hf and Hg), 5.06 (m, 1H, CH-COH-CH, He), 4.76 (s, 1H, C-OH, Hi), 4.03 (dd, 1H, COH-CH2-CO, Hh, J = 12.7), 3.71 (dd, 2H, COH-CH2-CO, Hh, J = 12.4), 3.33 (t, CH2-CH2-N, Hb), 3.03 (m, 1H, CO-CH(CH)-CO, Hc), 2.88 (d, 1H, CO-CH(CH)-CO, Hd), 2.55 ( t, 2H, N-CH2-CH2, Ha) [12], [14], [15].
3. Thermoset polyurethane synthesis and characterization
To synthesize the thermoset polyurethane, the fully biobased polyether diol (Velvetol® 500) and the synthesized DA-triol were first mixed at 50 °C for 12 hours under nitrogen atmosphere at an 1:1 OH molar ratio. Then, polymeric methylene diphenyl diisocyanate (pMDI) was added to the mixture, so the final formulation was 0.5 OHDA-triol:0.5 OHdiol:1 NCOpMDI in molar ratio. The mixture was magnetically stirred for 5 minutes under nitrogen atmosphere, and afterwards, the sample was cured on a hot plate press at 110 °C for 24 hours at a pressure of 50 KPa to obtain a brownish 1.8 mm thick plate as can be observed in Figure 4.
Figure 4. Thermoset PU plate synthesized using the DA-triol.
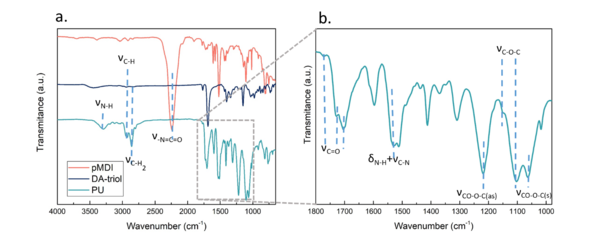
Figure 5 shows the FTIR spectra of the synthesized thermoset PU, the DA-triol and the pMDI. A band centered at 3317 cm-1 can be observed in the spectrum of PU, ascribed to the stretching vibration of urethane N-H bond. The characteristic band associated with OH groups in the DA-triol (Figure 2) disappears in the PU spectrum, indicating full conversion of hydroxyl groups. The bands between 2800 and 3000 cm-1 attributed to the -C-H stretching vibration that appear in the DA-triol, are preserved in the PU as well. The typical band of isocyanate group around 2270 cm-1 observed in the pMDI, disappears in the PU sample suggesting that there is no residual isocyanate in the PU, and thus ratifying the complete conversion of the reaction. The PU still presents the characteristic bands at 1770 and 1688 cm-1 specific to DA adducts [13], [16], as well as a new band around 1702 cm-1 related to the carbonyl of the urethane functional group of PU. Furthermore, the PU shows a new absorption band in the amide II region (1600-1500 cm-1) at 1529 cm-1 , related to the bending vibration of N-H combined with the stretching vibration of C-N, characteristic of the urethane group [17], [18]. Finally, the bands located in the interval 1000- 1250 cm-1 are related to the asymmetric and symmetric stretching vibrations of CO-O-C, characteristic of urethane, at 1218 and 1062 cm-1, respectively, and to the C-O-C bonds at 1147 and 1100 cm-1, inside the maleimide rings in the DA-triol and of the diol, respectively [19], [20]. Moreover, the typical maleimide absorption bands at 829 and 696 cm-1 are negligible indicating that the adduct still remains in the material [21].
4. Thermoset polyurethane recyclability
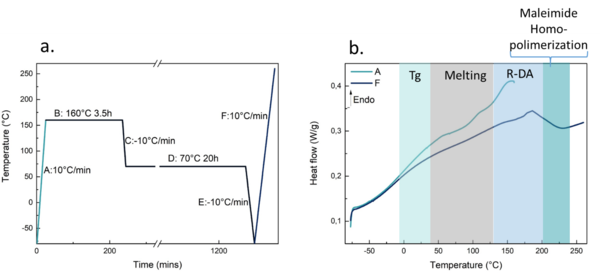
In order to recycle or reprocess the synthesized thermoset PU, the retro Diels-Alder reaction must be completed to uncrosslink the polymer, and then the Diels-Alder reaction must take place again [9], [22]. The recycling or reprocessing conditions were set by differential scanning calorimetry (DSC), and tests were performed in a Mettler Toledo DSC3+ (Columbus, Ohio, USA) equipment, equipped with a robotic arm and an electric intercooler as a cooling unit. A sample of 5 to 10 mg was encapsulated in aluminum pans and as shown in Figure 6a, it was subjected to different dynamic heating and cooling scans and isothermal steps under nitrogen atmosphere. The thermograms of the heating scans obtained are shown in Figure 6b.
In the first thermogram, related to the dynamic heating scan and named as A (from -80 °C to 160 °C at a heating rate of 2 °C/min), a glass transition temperature (Tg) associated with the polyol is detected around 0 °C, followed by two endothermic peaks in the 50-100 °C temperature range, related to the melting observed in the DSC of pure DA-triol (the thermogram of this scan is deliberately omitted). Some authors report two melting peaks due to the possible formation of two stereoisomers in the DA-triol synthesis [10], [14]. Subsequently, the PU shows the retro-DA reaction in the temperature range of 150-160 °C, selecting the higher one for the recycling cycle. The PU is then subjected to two consecutive isothermal steps. In the first one, the PU is held at 160 °C for 3.5 hours to allow the retro-DA reaction to occur and the chains to become uncrosslinked. In the second, after cooling rapidly to 70 °C, it is held for 20 hours to allow the DA reaction to take place and the polymer chains to crosslink again. In the last dynamic heating scan, labeled as F, the temperature ramp goes from -80 °C to 250 °C to check how the polymer behaves after the recycling cycle. The thermogram shows only one peak related with the DA-triol melting, probably due to the formation of only one of the isomers (Figure 6b). Furthermore, around 160 °C the signal related to the retro-DA is still present, corroborating that after 20 hours at 70 °C the DA-reactions takes place, and the thermoset polyurethane is recyclable and/or reprocessable. Finally, above 200 °C maleimide homopolymerization takes place, which prevents subsequent recycling cycles, so this temperature should not be reached.
In order to assess the efficiency of bond reformation, tensile tests were carried out on the original and the recycled samples using an Instron 5967 testing machine equipped with a 500 N load cell. Rectangular samples ( 3 mm x 3 mm x 1.5 mm) were cut from the original thermoset polyurethane sheet and according to the DSC results, the samples were first heated to 160 °C for 3.5 hours, then rapidly cooled to 70 °C and kept at this temperature for 20 hours.
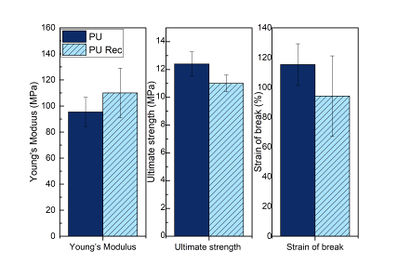
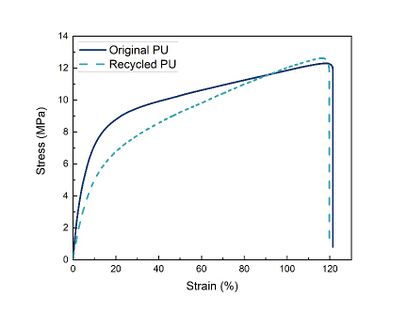
The measured mechanical properties are summarized in Figure 7, while stress-strain curves of the original and the recycled thermoset PU are shown in Figure 8. As can be seen, the mechanical properties remain similar after the recycling process, as the mechanical properties largely restore after recycling. When comparing the mechanical properties values, the recycled PU presents a good bond reforming efficiency, 97.7% for the Young’s modulus, 88.7% for ultimate strength, and 73.5% for strain at break; however, these values could be improved by increasing the DA adduct reformation time at 70 °C, but this would require more energy.
5. Conclusions
A new trifunctional polyol with dynamic covalent bonds has been successfully synthesized and characterized by 1H NMR and FTIR within this work, then the trifunctional polyol was used as a crosslinker in the construction of a bio-based polyurethane. The thermal properties of the final crosslinked polyurethane were studied by DSC finding an endothermic peak between 150 and 160°C, due to the thermal reversibility given by the decoupling of furan and maleimide adduct, which was inserted thought the DA-triol in the crosslinked structure. Thus, this thermal reversibility gives the polyurethane the property of recyclability and reprocessability, which has been demonstrated in mechanical tests of samples before and after reprocessing , recovering up to 97.7% of Young’s modulus, 88.7% of ultimate strength, and 73.5% of strain at break compared to unrecycled material. Thereby, with this work, authors present a novel strategy to add recyclability to thermoset polymeric materials, and thus overcome the environmental issues associated with this type of materials.
Acknowledgments
Financial support from the Basque Country Government in the frame of Grupos Consolidados (IT-1690-22) and ELKARTEK 2021 (Project NEOMAT KK-2021/00059) is gratefully acknowledged. The authors also acknowledge the Macobehavior-Mesostructure-Nanotechnology SGIker unit from the University of the Basque Country (UPV/EHU). A R-M. would like to thank the 'Materials + Technologies' group for the PhD grant (PIFG21/34).
Bibliography
[1] J. Banik, D. Chakraborty, M. Rizwan, A. H. Shaik, and M. R. Chandan, “Review on disposal, recycling and management of waste polyurethane foams: A way ahead,” Waste Manag. Res., 2023, doi: 10.1177/0734242X221146082.
[2] S. Burattini, B. W. Greenland, D. Chappell, H. M. Colquhoun, and W. Hayes, “Healable polymeric materials: A tutorial review,” Chem. Soc. Rev., vol. 39, no. 6, pp. 1973–1985, 2010, doi: 10.1039/b904502n.
[3] J. O. Akindoyo, M. D. H. Beg, S. Ghazali, M. R. Islam, N. Jeyaratnam, and A. R. Yuvaraj, “Polyurethane types, synthesis and applications-a review,” RSC Adv., vol. 6, no. 115, pp. 114453–114482, 2016, doi: 10.1039/c6ra14525f.
[4] R. Kaur, P. Singh, S. Tanwar, G. Varshney, and S. Yadav, “Assessment of Bio-Based Polyurethanes: Perspective on Applications and Bio-Degradation,” Macromol, vol. 2, no. 3, pp. 284–314, 2022, doi: 10.3390/macromol2030019.
[5] B. Zhao, H. Mei, G. Hang, L. Li, and S. Zheng, “Shape recovery and reprocessable polyurethanes crosslinked with double decker silsesquioxane via Diels-Alder reaction,” Polymer (Guildf)., vol. 230, Sep. 2021, doi: 10.1016/j.polymer.2021.124042.
[6] A. A. Caraculacu and S. Coseri, "Isocyanates in polyaddition processes. Structure and reaction mechanisms", Process in polymer science, vol. 26, no. 5. 2001. doi: 10.1016/S0079-6700(00)00033-2.
[7] Z. Karami, M. Zolghadr, and M. J. Zohuriaan-Mehr, "Self-healing Diels–Alder engineered thermosets", Self-Healing Polymer-Based Systems. INC, 2020. doi: 10.1016/b978-0-12-818450-9.00008-8.
[8] B. Willocq, F. Khelifa, J. Brancart, G. Van Assche, P. Dubois, and J. M. Raquez, “One-component Diels-Alder based polyurethanes: A unique way to self-heal,” RSC Adv., vol. 7, no. 76, pp. 48047–48053, 2017, doi: 10.1039/c7ra09898g.
[9] C. J. Kloxin and C. N. Bowman, “Covalent adaptable networks: Smart, reconfigurable and responsive network systems,” Chem. Soc. Rev., vol. 42, no. 17, pp. 7161–7173, 2013, doi: 10.1039/c3cs60046g.
[10] Q. Zhou, Z. Sang, K. K. Rajagopalan, Y. Sliozberg, F. Gardea, and S. A. Sukhishvili, “Thermodynamics and Stereochemistry of Diels − Alder Polymer Networks: Role of Crosslinker Flexibility and Crosslinking Density,” Macromolecules, 2021, doi: 10.1021/acs.macromol.1c01662.
[11] A. Gandini, D. Coelho, M. Gomes, B. Reis, and A. Silvestre, “Materials from renewable resources based on furan monomers and furan chemistry: Work in progress,” J. Mater. Chem., vol. 19, no. 45, pp. 8656–8664, 2009, doi: 10.1039/b909377j.
[12] Z. Xu, Y. Zhao, X. Wang, and T. Lin, “A thermally healable polyhedral oligomeric silsesquioxane (POSS) nanocomposite based on Diels–Alder chemistry,” Macromolecules Chem. Commun., vol. 49, no. 60, pp. 6755–6757, 2013, doi: 10.1039/c3cc43432j.
[13] K. Adachi, A. K. Achimuthu, and Y. Chujo, “Synthesis of organic-inorganic polymer hybrids controlled by diels-alder reaction,” Macromolecules, vol. 37, no. 26, pp. 9793–9797, 2004, doi: 10.1021/ma0400618.
[14] E. Dolci, G. Michaud, F. Simon, B. Boutevin, S. Fouquay, and S. Caillol, “Remendable thermosetting polymers for isocyanate-free adhesives: A preliminary study,” Polym. Chem., vol. 6, no. 45, pp. 7851–7861, 2015, doi: 10.1039/c5py01213a.
[15] V. Froidevaux, M. Borne, E. Laborbe, R. Auvergne, A. Gandini, and B. Boutevin, “Study of the diels-alder and retro-diels-alder reaction between furan derivatives and maleimide for the creation of new materials,” RSC Adv., vol. 5, no. 47, pp. 37742–37754, 2015, doi: 10.1039/c5ra01185j.
[16] P. Du, X. Liu, Z. Zheng, X. Wang, T. Joncheray, and Y. Zhang, “Synthesis and characterization of linear self-healing polyurethane based on thermally reversible Diels-Alder reaction,” RSC Adv., vol. 3, no. 35, pp. 15475–15482, Sep. 2013, doi: 10.1039/c3ra42278j.
[17] T. Calvo-Correas et al., “Thermoplastic polyurethanes with glycolysate intermediates from polyurethane waste recycling,” Polym. Degrad. Stab., vol. 144, pp. 411–419, 2017, doi: 10.1016/j.polymdegradstab.2017.09.001.
[18] D. K. Chattopadhyay, A. K. Mishra, B. Sreedhar, and K. V. S. N. Raju, “Thermal and viscoelastic properties of polyurethane-imide/clay hybrid coatings,” Polym. Degrad. Stab., vol. 91, no. 8, pp. 1837–1849, 2006, doi: 10.1016/j.polymdegradstab.2005.11.004.
[19] L. Irusta and M. J. Fernandez-Berridi, “Aromatic poly(ether-urethanes): Effect of the polyol molecular weight on the photochemical behaviour,” Polymer (Guildf)., vol. 40, no. 17, pp. 4821–4831, 1999, doi: 10.1016/S0032-3861(98)00708-3.
[20] K. Sugane, R. Takagi, and M. Shibata, “Thermally healable/heat-resistant properties of thermosets bearing dynamic and thermally stable bonds formed by the Diels-Alder and thiol-maleimide ‘click’ reactions,” React. Funct. Polym., vol. 131, no. May, pp. 211–218, 2018, doi: 10.1016/j.reactfunctpolym.2018.08.001.
[21] T. T. Truong et al., “Tailoring the Hard-Soft Interface with Dynamic Diels-Alder Linkages in Polyurethanes: Toward Superior Mechanical Properties and Healability at Mild Temperature,” Chem. Mater., vol. 31, no. 7, pp. 2347–2357, Apr. 2019, doi: 10.1021/acs.chemmater.8b04624.
[22] H. Tu, M. Zhou, Y. Gu, and Y. Gu, “Conductive, self-healing, and repeatable graphene/carbon nanotube/polyurethane flexible sensor based on Diels-Alder chemothermal drive,” Compos. Sci. Technol., vol. 225, no. March, p. 109476, 2022, doi: 10.1016/j.compscitech.2022.109476.
Document information
Published on 12/04/24
Accepted on 04/12/23
Submitted on 17/05/23
Volume 08 - COMUNICACIONES MATCOMP21 (2022) Y MATCOMP23 (2023), Issue Núm. 4 - Sostenibilidad y Reciclaje, 2024
DOI: 10.23967/r.matcomp.2024.04.02
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?