Summary
This is a report of seven cases of infected thoracic wounds treated with an adapted low-cost vacuum therapy in the Thoracic Surgery Unit of Santa Marcelina Hospital. The vacuum system used was designed and adapted to our hospital due to financial limitations on the acquisition of commercial kits. The vacuum-assisted closure kit used in this study consisted of chlorhexidine sponges (which are usually used for antisepsis of the surgical team), a 16F nasogastric tube, and two sterile adhesive films (OPSITE) for surgical field reinforcement. The mean duration of vacuum therapy was 13.4 days (range, 10–20 days), with an average of three dressing changes (range, 1–5). After treatment with vacuum-assisted closure, three wounds (3/7) were closed with simple primary sutures, one of the lesions (1/7) was closed by muscle flap rotation, and three wounds (3/7) healed by second intention. This adapted vacuum therapy was safe and easy to apply in our institution, including its use in patients with thoracostomies.
Keywords
infections;thoracic wounds;vacuum therapy
1. Introduction
Destruction of chest wall secondary to trauma or local infection might limit closure options, especially when muscle flaps have been destroyed. Since vacuum-assisted closure (VAC) therapy (KCI International, San Antonio, TX, USA) was introduced,1 it has been increasingly used in wound management.2.; 3.; 4.; 5. ; 6. Successful treatment of chest wall wounds with vacuum therapy was described in 1997 in a limited number of patients.7 Since then, small series have reported on the use of this technique, including for the closure of open window thoracostomy. The commercial value of routinely used materials is high, hindering the use of this technology in Brazils public health services. We describe our initial experience with an adapted vacuum therapy used on chest wall wounds.
2. Case report
We report seven cases of infected thoracic wounds treated using adapted vacuum therapy in the Thoracic Surgery Unit and approved by the Ethics Committee Review Board of our institution. Chest wall wounds that did not show a reduction in pus discharge within 48 hours, despite the use of antibiotics, were considered complex wounds and selected for treatment with vacuum therapy. This treatment was not used in patients with active bleeding or wounds with exposed blood vessels. (There was one patient who did not tolerate the treatment because of intractable chest pain due to the negative pressure, so is not included in this report.)
Vacuum dressing technique was used in six patients, with a total of seven thoracic wounds treated (Table 1) as Patient 5 had two different complex wounds. Among the seven wounds, two were from pleurostomies (Patients 5 and 6), four were infected surgical wounds with dehiscence (Patients 1, 2, 4 and 5), and one was an extensive abscess of the chest wall associated with cellulitis (Patient 3).
| Patient | Sex | Age (y) | Initial disease | Initial procedure | Comorbidities | Complex wound |
|---|---|---|---|---|---|---|
| 1 | M | 21 | Spinal column osteosarcoma | Rib biopsy | Malnutrition | Infected surgical wound |
| 2 | M | 28 | Right hemithorax GSW with spinal cord injury | Thoracotomy to treat retained hemothorax | Paraplegia | Infected surgical wound |
| 3 | F | 35 | Left subscapular abscess | Abscess drainage | DM and morbid obesity | Extensive chest wall abscess |
| 4 | M | 30 | Prolonged aerial fistula after spontaneous pneumothorax | Thoracotomy & bullectomy | Malnutrition | Infected surgical wound |
| 5 | F | 44 | Chronic sternal osteomyelitis & parapneumonic pleural empyema | Resection of manubrium & pleurostomy | DM, morbid obesity and CHF NYHA functional class 3 | Infected surgical wound & pleurostomy |
| 6 | M | 52 | Tuberculous empyema | Pulmonary decortication | Malnutrition | Pleurostomy |
GSW = gunshot wound; DM = diabetes mellitus; CHF = congestive heart failure; NYHA = New York Heart Association.
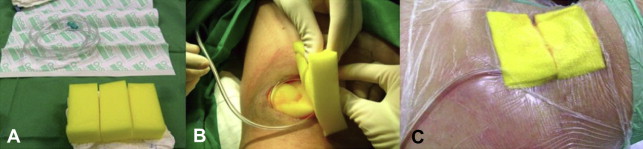
The VAC kit used in this study consisted of 5–10 sterile chlorhexidine sponges (which are usually used for antisepsis by the surgical team), a 16F nasogastric tube, and two sterile adhesive films (OPSITE) for surgical field reinforcement (Fig. 1). The sponges were sampled and all cultures were negative for bacterial growth.
|
|
|
Figure 1. (A) Materials used for the dressing. (B) Placing the dressing on the infected wound. (C) Final aspect of the dressing. |
Wound treatment was performed using a standard technique as follows.
- Before the dressing was applied, the infected wound was measured at its largest diameter.
- The surface of the wound was thoroughly cleansed with saline solution to remove secretions.
- Chlorhexidine sponges were applied to cover the entire wound.
- The nasogastric tube was placed between the sponges for suction in the closed system.
- The surface was covered with OPSITE plastic dressings, creating a sealed surface and isolating the wound from the external environment.
- The nasogastric tube was connected to the vacuum suction system of the hospital.
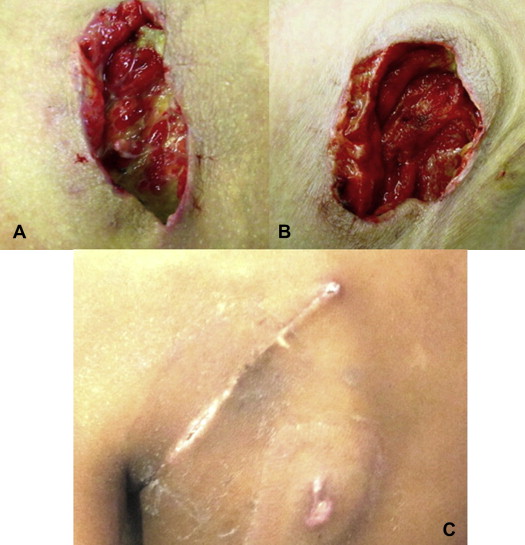
- The dressing was changed according to the appearance and volume of the aspirated secretions, at an average of 2.4 days (range, 1–5 days) until the wounds showed granulation tissue and absence of purulent secretion (Fig. 2).
|
|
|
Figure 2. (A) Infected wound areas with deposits of fibrin and pus. (B) Same wound after first dressing change showing reduced secretion and formation of granulation tissue in the wound bed. (C) Completely healed wound after treatment with primary suture after vacuum therapy. |
The demographic and preoperative characteristics of the patients are shown in Table 1. The mean duration of vacuum therapy was 12 days (range, 5–19 days), with an average of three dressing changes (range, 1–5 changes). Patients remained hospitalized for a mean of 41 days (range, 3–77 days).
After treatment with VAC, three wounds (3/7) were closed with simple primary sutures (wounds 2, 3 and 4). One of the lesions (1/7) was closed by rotation of the serratus muscle flap (wound 1). Three wounds (3/7), including both patients who underwent pleurostomies, showed healing by second intention (wounds 5, 6 and 7) (Table 2). Patients 5 and 6 who underwent pleurostomy and vacuum treatment developed granulation tissue formation and cessation of purulent secretion after 15 days. The closing of their pleurostomy wounds occurred within 2 months by second intention.
| Wound | Patient | Size of infected wound (cm) | Duration of vacuum therapy (d) | Complication | Infectious agent | No. of dressing changes | Closure type |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 25 | 17 | — | Not isolated | 3 | Muscle flap (serratus) |
| 2 | 2 | 15 | 7 | — | Staphylococcus aureus | 1 | Primary closure |
| 3 | 3 | 7 | 10 | Contact dermatitis | Not isolated | 1 | Primary closure |
| 4 | 4 | 10 | 20 | Contact dermatitis | Acinetobacter baumannii | 5 | Primary closure |
| 5 | 5 | 20 | 10 | — | Staphylococcus aureus | 2 | Secondary intention |
| 6 | 5 | 10 | 15 | — | Pseudomonas aeruginosa | 3 | Secondary intention |
| 7 | 6 | 13 | 15 | Contact dermatitis | Providencia stuartii | 3 | Secondary intention |
With regard to complications, contact dermatitis was noted on the areas where the dressings were applied in three cases. There were no deaths related to this procedure.
3. Discussion
The benefit of vacuum therapy in complex wounds is established in the medical literature.5 The mechanisms underlying the effectiveness of this procedure include obliteration of dead space between the tissue layers and stimulation of angiogenesis caused by negative pressure in the tissue adjacent to the dressing to a depth of 5 mm,4 favoring healing. Few studies have evaluated vacuum therapy in the treatment of complex wounds of the chest wall. We report the same characteristics described in the literature4.; 5. ; 6. with a simple, low-cost and easily reproducible technique: preventing unnecessary dressing changes every day, without the need for patient transportation to the surgical center with less handling of the wound. Additionally, decreased healing time seems to be a favorable factor.
As for the duration of treatment, O'Connor et al6 reported a mean of 9 days (range, 3–21 days). However, we experienced a longer treatment time of 12 days (range, 5–19 days). The difference in these data may be influenced by differences in wound severity.
Palmen et al7 reported on the use of VAC in 11 patients who underwent pleurostomies, with no complications or need for surgical closure of the pleural cavity. This series reported VAC therapy time of 31 days (range, 12–50 days). In our study, we used VAC in two thoracostomy patients for 15 days, in whom there was no need for subsequent surgical closure. The use of this type of therapy benefits patients in the long term as there is no need for daily dressing changes (often up to three times daily for weeks). However, the burden is hospitalization until cavity obliteration is attained, which, in our experience, occurred within 15 days. This need for hospitalization is due to the use of the vacuum suction system of the hospital.
To sum up, patients with complex wounds of the chest wall can be treated with the proposed technique, in addition to antibiotic therapy, with low complication rates.
References
- 1. T.F. Molnar; Current surgical treatment of thoracic empyema in adults; Eur J Cardiothorac Surg, 32 (2007), pp. 422–430
- 2. J.I. Miller, K.A. Mansour, F. Nahai, et al.; Single stage complete muscle flap closure of the post-pneumonectomy empyema space: a new method and possible solution to a disturbing complication; Ann Thorac Surg., 38 (1984), pp. 227–231
- 3. M.K. Widmer, T. Krueger, D. Lardinois, et al.; A comparative evaluation of intrathoracic latissimus dorsi and serratus anterior muscle transposition; Eur J Cardiothorac Surg, 4 (2000), pp. 435–439
- 4. M.J. Morykwas, L.C. Argenta, E.I. Shelton-Brown, et al.; Vacuum assisted closure. A new method for wound control and treatment: animal studies and basic foundation; Ann Plast Surg, 38 (1997), pp. 553–562
- 5. P. Segers, A.P. de Jong, J.J. Kloek, et al.; TNP in wounds after cardiothoracic surgery: successful experience supported by literature; Thorac Cardiovasc Surg, 54 (2006), pp. 289–294
- 6. J. O'Connor, A. Kells, S. Henry, et al.; Vacuum-assisted closure for the treatment of complex chest wounds; Ann Thorac Surg, 79 (2005), pp. 1196–1200
- 7. M. Palmen, H.N.A.M. van Breugel, G.G. Geskes, et al.; Open window thoracostomy treatment of empyema is accelerated by vacuum-assisted closure; Ann Thorac Surg, 88 (2009), p. 1131
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?