Abstract
Perfluorooctanoic acid (PFOA) is a man-made surfactant with a number of industrial applications. It has a long half-life environmentally and biologically. Past studies suggest a direct relationship between plasma cholesterol and PFOA serum concentrations in humans and an inverse one in rodents fed standard rodent chow, making it difficult to examine mechanisms responsible for the potential PFOA-induced hypercholesterolemia and altered sterol metabolism. To examine dietary modification of PFOA-induced effects, C57BL/6 and BALB/c mice were fed PFOA in a fat- and cholesterol-containing diet. When fed these high fat diets, PFOA ingestion resulted in marked hypercholesterolemia in male and female C57BL/6 mice and less robust hypercholesterolemia in male BALB/c mice. The PFOA-induced hypercholesterolemia appeared to be the result of increased liver masses and altered expression of genes associated with hepatic sterol output, specifically bile acid production. mRNA levels of genes associated with sterol input were reduced only in C57BL/6 females, the mice with the greatest increase in plasma cholesterol levels. Strain-specific PFOA-induced changes in cholesterol concentrations in mammary tissues and ovaries paralleled changes in plasma cholesterol levels. mRNA levels of sterol-related genes were reduced in ovaries of C57BL/6 but not in BALB/c mice and not in mammary tissues. Our data suggest that PFOA ingestion leads to hypercholesterolemia in mice fed fat and cholesterol and effects are dependent upon the genetic background and gender of the mice with C57BL/6 female mice being most responsive to PFOA.
Keywords
Perfluorooctanoic acid ; C8 ; PFC ; PFAS ; Cholesterol ; Dietary fat
1. Introduction
Perfluorooctanoic acid (PFOA) is a saturated eight carbon chain organic acid, perfluoroalkyl substance (PFAS) with all hydrogen replaced by fluorine. PFOA is primarily used to produce other PFASs for stain- and stick-resistant coatings (Teflon), water-resistant coatings (Gor-Tex), and food contact paper [29] , [4] and [42] . Because of their widespread use over the past several decades and relatively long half-lives [45] some of these PFASs, including PFOA, are prevalent throughout the world from populated, industrialized locations to remote locations [3] and [11] . Consequently, PFOA exposure is widespread throughout the US general population [7] and [39] . Though essentially everyone has measurable levels of PFOA due to their detection in almost every ecosystem worldwide, serum levels are somewhat greater in areas near facilities that use PFOA [13] and [51] and considerably higher in perfluoroalkyl production workers [9] , [43] , [44] , [46] , [47] , [56] , [57] and [66] . Though PFOA production in the US has decreased markedly in the past several years, along with serum concentrations in people in the US (NHANES), and should cease in 2015 (EPA Stewardship Plan), PFOA remains a human health concern due to continuing production and use worldwide, relatively long half-lives in humans [45] , persistence in the environment, and degradation of other PFAAs into PFOA [4] .
Controversy exists over whether PFOA is hypo- or hypercholesteremic. Human gene expression data from different studies suggest a hypercholesteremic hypothesis. PFOA upregulated cholesterol biosynthesis genes in human hepatocytes [5] and [50] . In a study of population co-exposed to PFOA and perfluorooctanoate sulfonate, expression of genes involved in reverse cholesterol transport expression, a cause of hypercholesterolemia, were reduced with PFOA exposure [17] . In addition, PFOA exposure has been significantly associated with an increased risk of clinical hypercholesterolemia [60] and [18] and increased plasma cholesterol concentrations in national population studies [39] , [14] , [20] and [75] , in communities near perfluoroalkyl production facilities [60] , [18] , [16] and [19] and in perfluoroalkyl workers [9] , [26] , [56] and [57] . While most studies have been cross-sectional, significant associations between PFOA and cholesterol have been found in exposed communities [16] and [71] and occupational cohorts as well [9] , [56] and [57] though increases attributable to PFOA are small compared to other hypercholesteremic factors [44] . In many studies, effect estimates, and resultant cholesterol increases, are strongest at serum PFOA concentrations below 10 ng/mL (corresponding to 95% of US population exposure) although they remain significant for all concentrations, with the inflection point ≈25–40 ng/mL [60] and [18] . Regardless, though direction, magnitude and significance of the PFOA-cholesterol association vary across studies, especially for HDL and LDL, human experimental and epidemiological data suggest PFOA is indeed hypercholesterolemic.
A change in plasma cholesterol levels is often an indication of a change in hepatic sterol balance [59] and can lead to altered sterol metabolism in a variety of extra-hepatic tissues, including steroidogenic tissues. Hepatic sterol balance plays a key role in maintaining sterol balance in the whole body [59] . A positive sterol balance would occur with increased dietary cholesterol and will often lead to a reduction in LDL receptor (LDLR) expression levels which will in turn lead to fewer LDL particles being taken up by the liver and an increase in plasma LDL-cholesterol levels [12] . As most LDL is taken up by the liver [72] , a reduction in uptake would lead to an increase in plasma levels. A change in sterol balance can also occur if sterol synthesis or bile acid production rates are altered. As the peripheral tissues take up a majority of LDL-cholesterol via receptor-independent processes or via receptors that are independent of tissue sterol balance, an increase in circulating lipoprotein-cholesterol would lead to an increase in uptake by peripheral tissues. An increase in tissue cholesterol could directly affect tissues, such as ovaries that synthesize estradiol and indirectly affect tissues that rely on the steroid products produced by the other tissues, including mammary tissues that rely on estradiol.
In studies with rodents, PFOA-treated animals often become hypocholesterolemic, opposite of that which occurs in humans [2] , [22] , [28] , [35] , [36] , [37] , [52] and [73] . In most of these previous studies, rodents consumed a standard chow diet containing 4% fat and very little cholesterol. It has been proposed that the effect occurs because PFOA will activate peroxisome proliferator-activated receptor α (PPARα) more readily in rodents than in humans, and PPARs can be hypolipidemic [24] , [2] , [38] , [54] and [68] . However, other studies suggest that some PFOA metabolic effects are PPARα-independent [55] , [27] and [54] . How animals respond to PFOA when sterol balance is altered with dietary factors and cholesterol, as occurs in humans, is unknown, though it appears that dietary fat can modify the effect of PFOA on other parameters [61] .
Thus, the purpose of the current study was to determine the impact of dietary PFOA when fed in combination with a diet containing fat and cholesterol, more similar to that consumed by humans, using strains of mice that respond differently to PFOA with respect to sexual maturity [74] and to dietary factors [30] and [48] , and have been shown previously to become hypocholeserolemic when exposed to PFOA [52] and [73] . The use of mice that become hypercholesteremic in response to PFOA and a high-fat diet would be very useful in assessing human PFOA response and addressing the contradictions between human and rodent data.
2. Materials and methods
2.1. Animals and diets
Male and female C57BL/6 and BALB/c mice were purchased from The Jackson Laboratories (Bar Harbor, ME) at weaning, randomly separated into 8 groups, and fed pelleted chow upon arrival (Harlan, Madison, WI); 8 groups consisted of 2 different sexes, two different strains of mice, and two different PFOA treatments. Mice were housed 3 per cage in a temperature and humidity controlled room and subjected to 12 h of light and 12 h of darkness. Four to five days after arrival, mice were weighed and distributed between cages to ensure similar initial body weights per treatment, and began consuming one of two diets (Research Diets, Inc.); we chose to study younger mice just prior to sexual maturity as PFOA has been described as an endocrine disruptor and could impact metabolism in endocrine tissues as they mature [1] and [74] . Both diets contained 0.25% cholesterol and 32% fat (kcal) with soybean oil as the primary oil. One diet was the control diet and contained no added PFOA. The other diet contained 3.5 mg PFOA (Sigma–Aldrich)/kg diet. We chose this amount of PFOA to add because we wanted mice to consume 0.5 mg PFOA/kg body weight and adult mice consume ≈3.5 g high fat diet [33] . Thus, a 22 g mouse would consume ≈12.3 μg PFOA per whole body per day or ≈0.56 mg PFOA per kg body weight per day. PFOA was added to the diet and not gavaged daily so that mice would be exposed to PFOA throughout the day and mimic human exposure to PFOA. Weekly individual body weights and food consumption of each cage were obtained to ensure adequate growth and to determine the dose of PFOA consumed each day per kg body weight, respectively; each group consisted of 2 cages of 3 mice each (6 male and 6 female mice of each strain per treatment). Animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
After 5 weeks, body fat mass was measured by MRI (Echo). The following week the mice were fasted 6 h, anesthetized, exsanguinated and tissues collected rapidly and stored as needed. Diethyl ether was used as an anesthesia (prior to the AVMA report in 2013) to ensure accurate PFOA measurements in plasma not confounded by interfering anesthetics. Additional care was taken so blood and plasma were collected and stored using syringes and tubes made of polypropylene. Plasma from each animal per group was pooled, stored frozen, and shipped to the Centers for Disease Control and Prevention (CDC) where it was analyzed for PFOA using online solid phase extraction coupled with reversed-phase high-performance liquid chromatography isotope-dilution tandem mass spectrometry as described [27] ; samples were pooled in this initial study to ensure adequate volumes for all plasma measurements proposed. To ensure that the measured PFOA concentrations fell within the calibration range, necessary dilution of the plasma was performed. Blanks and quality control materials were analyzed along with the study samples to ensure the accuracy and reliability of the data [27] .
2.2. Tissue and plasma steroid levels
Plasma cholesterol levels in each sample were measured enzymatically in duplicate (Thermo Electron). Plasma from each animal per group was pooled and separated into lipoproteins by fast-protein liquid chromatography (FPLC) using two Sephadex columns in tandem [31] . Pieces of liver (males and females) and mammary tissue and one ovary (females) were collected after exsanguination, saponfied in alcoholic KOH, sterol extracted, and mass of sterol measured by gas liquid chromatography using stigmastanol as an internal standard. Estradiol levels were measured in duplicate in plasma of some of the females (2–5 mice per group) by EIA (Cayman Chemical).
2.3. Real time PCR
Livers, ovaries, and pieces of mammary tissues were collected rapidly after exsanguination, snap frozen in liquid nitrogen, and stored at −80 °C until use. Tissue RNA was isolated using TRIzol® and stored in FORMAzol® at −80 °C. RNA was treated with RNase-free DNase I and reverse transcribed to cDNA by SuperScript II reverse transcriptase using random hexamers. PCR assays were performed on a Bio-Rad iCycler iQ real-time PCR Detection System using SYBR green as our fluorophore; primers will be given upon request. A serial dilution of a randomly-picked sample was used to generate a standard curve for each gene examined, using Ct values and log of dilutions as axes. Relative mRNA levels in each sample were obtained from dilution curves for each gene, and numbers converted back from log transformations. The relative levels of mRNA for the genes of interest were determined by generating a ratio of the gene of interest vs cyclophilin as the reference/housekeeping gene.
2.4. Statistics
Sex-specific means ± SEM of body weight, body fat%, liver (% body weight) and plasma and liver cholesterol concentrations were calculated for PFOA exposed and non-exposed within each strain. Strain specific mammary and ovary cholesterol concentrations were calculated for the female animals. For the sake of presentation, significant differences between each dietary group (±PFOA) of each strain and sex were determined by two-tailed student’s t -tests (P < 0.05). Significant differences between each dietary group (±PFOA) of each strain and sex were determined by two-tailed students t-tests for mRNA analyses (P < 0.05).
Linear regression analyses were performed with PFOA exposure as a binary variable (±PFOA) to test for the effect of the PFOA exposure on various outcomes with simultaneous adjustment for sex and strain. When comparing plasma and tissue cholesterol concentrations between mice of different groups, plasma cholesterol was used as a covariate for tissue cholesterol concentrations, because uptake of cholesterol can be directly related to plasma cholesterol in extrahepatic tissues, and liver weight, as liver is a source of plasma cholesterol, was used as a covariate for plasma cholesterol levels [59] .
3. Results
These studies are unique compared to other studies in rodents in that mice were fed diets with fat and cholesterol to simulate human dietary composition and results. Dietary PFOA did not affect body weight after treatment in males or females of either strain (Table 1 , Supplemental Fig. 1). Percent body fat was also similar in each group of animals (Table 1 ). Not surprisingly, liver weight (expressed as a percent of body weight) was increased ≈60% in mice fed PFOA compared to control mice (Table 1 ). When liver weight was subtracted from body weight, the only group that had significantly lower body weights with dietary PFOA was C57BL/6 males (P = 0.003; 25.0 ± 0.3 vs 23.6 ± 0.2 g); body weights minus liver weights were not different for female C57BL/6 mice (19.3 ± 0.4 vs 18.5 ± 0.5) or male or females BALB/c mice respectively (23.5 ± 0.8 vs 22.8 ± 0.4 and 19.5 ± 0.2 vs 19.0 ± 0.3 g). It was not surprising that body weights were slightly less in PFOA-fed mice as mice consuming diets laced with PFOA appeared to eat slightly less food (Supplemental Table 1). Plasma estradiol levels were not significantly affected by dietary PFOA in this study of sexually mature mice fed fat and cholesterol (Table 1 ).
| C57BL/6 | BALB/c | |||
|---|---|---|---|---|
| Control | PFOA | Control | PFOA | |
| Male | ||||
| Body weight (g) | 26.3±0.4 | 25.6±0.2 | 24.8±1.0 | 24.8±0.4 |
| Body fat (%) | 9.4±1.1 | 8.4±1.1 | 10.6±0.8 | 11.0±1.1 |
| Liver (% body) | 5.2±0.1 | 8.7±0.3* | 5.5±0.3 | 8.5±0.4* |
| PFOA levels (ng/ml) | 2 | 26,900 | 5 | 28,200 |
| Female | ||||
| Body weight (g) | 20.4±0.6 | 20.3±0.5 | 20.7±0.2 | 21.0±0.4 |
| Body fat (%) | 10.5±0.8 | 10.6±0.9 | 10.1±0.6 | 10.1±0.5 |
| Liver (% body) | 5.6±0.3 | 9.3±0.7* | 5.7±0.2 | 9.4±0.3* |
| PFOA levels (ng/ml)a | 28 | 44,300 | 86 | 35,600 |
| Estradiol (ng/ml)b | 11.7±1.8 | 8.6±2.0 | 7.5±1.5 | 10.8±1.7 |
Note : Data are presented as means ± SEM (n = 6).
- . Significant differences from control (P < 0.05).
a. Plasma of mice within each group are pooled for PFOA levels only.
b. n = 5, 2, 4, and 4 for control C57, PFOA C57, control BALB/c, and PFOA BALB/c, respectively.
The amount of food consumed by each cage of PFOA-fed mice was measured each week with an average of 2.6 ± 0.1 and 2.5 ± 0.1 g/day for each female and male mouse, respectively. As the size of the mice differed from week 1 to week 6 of the study, mice consumed ≈0.55 mg PFOA per day per kg body weight at 4 weeks of age in both males and females as they have similar body masses. At 10 weeks of age, male mice consumed ≈0.33 and female mice consumed ≈0.44 mg PFOA per day per kg body weight due to the gender differences in body weights of the older mice. This amount of PFOA resulted in average plasma levels of circulating PFOA in mice of ≈27,600 ng PFOA/ml plasma in males and ≈39,000 ng PFOA/ml plasma in females. Even though the plasma PFOA concentrations in our study are many fold greater than the means of a number of human cohorts, they are within the range of human occupational exposures, though at the higher end [9] , [44] and [57] .
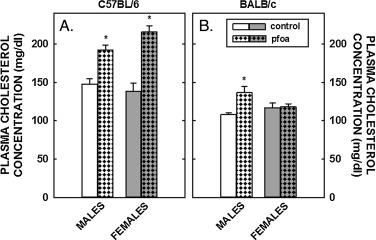
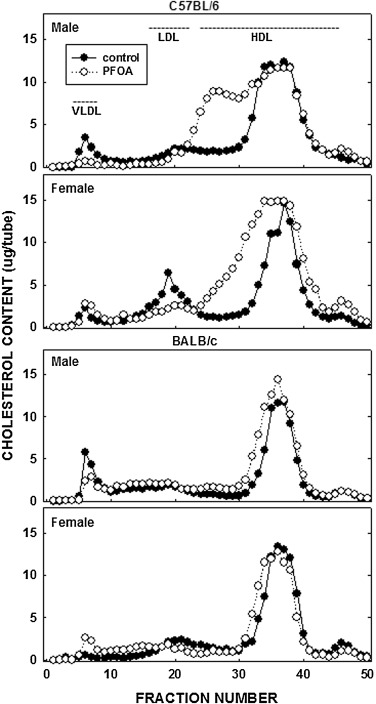
Unlike the lack of effect of PFOA on body fat, PFOA resulted in a 70% increase in plasma cholesterol levels in female C57BL/6 mice and a more modest increase of 35% in male C57BL/6 mice (Fig. 1 ); dietary fat and cholesterol resulted in plasma cholesterol levels in control mice of ≈150 mg/dl in C57BL/6 mice and ≈100 mg/dl in BALB/c mice. In BALB/c mice, plasma cholesterol levels increased somewhat (≈20%) in male mice fed PFOA whereas PFOA had no effect on plasma cholesterol levels in female mice. In regression analyses, the effect of PFOA on plasma cholesterol levels was significant in both C57BL/6 and BALB/c mice in models without liver weight, but not significant with liver weight in the model. These PFOA-induced increases in plasma cholesterol levels in C57BL/6 animals were associated with a “large HDL” as determined by FPLC (Fig. 2 ); additional studies would need to determine what type of particle these are, i.e. do they contain apoB, apoE, or apoAI.
|
|
|
Fig. 1. Plasma cholesterol concentrations in C57BL/6 (A) and BALB/c (B) male and female mice. Mice were weaned and fed diets containing fat plus cholesterol. Half of the mice received no dietary PFOA and half received 3.5 mg/kg diet so that mice would consume ≈1 mg/kg body weight. After 6 weeks of dietary treatment, mice were anesthetized, exsanguinated and plasma collected. Data represent averages ± SEM (n = 5–6). Differences from mice fed control diets are depicted by * (P < 0.05). |
|
|
|
Fig. 2. Lipoprotein-cholesterol in C57BL/6 and BALB/c male and female mice. Plasma collected in animals described in Fig. 1 was pooled by group and lipoproteins separated by FPLC. Cholesterol content in each fraction was measured enzymatically. |
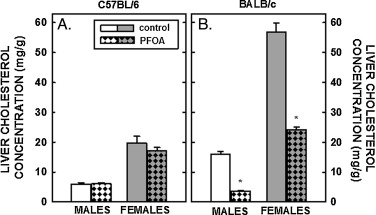
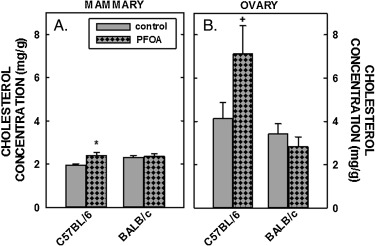
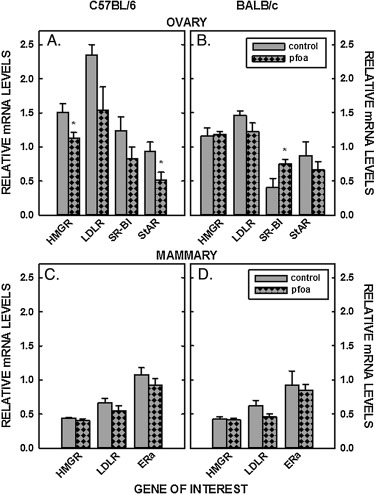
Tissue cholesterol concentrations were determined next. Hepatic cholesterol concentrations in male and female C57BL/6 mice fed fat and cholesterol were ≈5 and ≈20 mg/g, respectively (Fig. 3 A), higher than liver cholesterol concentrations in chow-fed mice of ≈2 mg/g [49] . Dietary fat and cholesterol resulted in even greater elevated liver cholesterol concentrations in BALB/c mice (Fig. 3 B). Whereas dietary PFOA had no impact on hepatic cholesterol levels in C57BL/6 mice, hepatic cholesterol concentrations were reduced significantly in male and female BALB/c mice fed PFOA (Fig. 3 B and Table 2 ). In mammary tissues, there was a modest increase (≈25%) in cholesterol concentrations in C57BL/6 mice fed PFOA, whereas there was no statistical effect of PFOA in cholesterol concentrations in mammary tissues of BALB/c mice (Fig. 4 A). In regression analyses, mammary cholesterol was increased only when plasma cholesterol was included as the covariate. In ovaries, cholesterol concentration in C57BL/6 mice tended to be increased (P=0.069) with PFOA whereas there was no little change in ovarian cholesterol in BALB/c mice (Fig. 4 B). In regression analyses of both strains combined, there was no effect of ovarian cholesterol when plasma cholesterol was included as a covariate but the strain effect was significant or marginally significant (Table 2 ). The limited number of animals in each group prevented us from doing strain-specific regression analyses of the mammary and ovary cholesterol outcomes, which were measured only in females. Model fit was much better for the models examining the effects of PFOA on liver weight and liver cholesterol with R-Square >0.80 for all models (explaining more than 80% of the total variance) except for liver weight% in C57BL/6 where the R-Square was a very respectable 0.73. Model fit for plasma cholesterol was not as good (C57BL/6, R-Square = 0.59 and BALB/c R-Square = 0.49) and <0.24 all for models examining mammary and ovary cholesterol concentration as outcomes.
|
|
|
Fig. 3. Cholesterol concentration in the livers of C57BL/6 (A) and BALB/c (B) male and female mice. Livers were collected from mice described in Fig. 1 and cholesterol measured by gas chromatography. Data represent averages ± SEM (n = 5–6). Differences from mice fed control diets are depicted by * (P < 0.05). |
| Dependent variable | PFOA | Sex | Liver weight % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C57BL/6 | Beta | SE | P value | Beta | SE | P value | Beta | SE | P value |
| Liver weight% | 3.594 | 0.479 | <0.0001 | 0.438 | 0.479 | 0.371 | |||
| Liver cholesterol | −1.195 | 1.182 | 0.324 | 12.275 | 1.183 | <0.0001 | |||
| Plasma cholesterol | 52.319 | 11.992 | 0.0003 | −1.829 | 11.992 | 0.88 | |||
| Plasma cholesterol | 13.921 | 20.687 | 0.509 | −6.692 | 11.214 | 0.558 | 10.730 | 4.897 | 0.041 |
| BALB/c | Beta | SE | P value | Beta | SE | P value | Beta | SE | P value |
|---|---|---|---|---|---|---|---|---|---|
| Liver weight% | 3.350 | 0.365 | <0.0001 | 0.567 | 0.365 | 0.136 | |||
| Liver cholesterol | −22.343 | 2.793 | <0.0001 | 30.781 | 2.793 | <0.0001 | |||
| Plasma cholesterol | 14.700 | 6.068 | 0.025 | −4.850 | 6.068 | 0.433 | |||
| Plasma cholesterol | 12.456 | 13.899 | 0.381 | −5.230 | 6.669 | 0.435 | 0.670 | 3.711 | 0.859 |
| PFOA | Strain (BALB/c) | Plasma cholesterol | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Both strains | Beta | SE | P value | Beta | SE | P value | Beta | SE | P value |
| Ovarya | 0.789 | 1.185 | 0.514 | −2.625 | 1.185 | 0.039 | |||
| Ovary | 0.947 | 1.354 | 0.493 | −2.895 | 1.584 | 0.084 | −0.005 | 0.020 | 0.794 |
| Mammarya | 0.180 | 0.129 | 0.177 | 0.080 | 0.129 | 0.541 | |||
| Mammary | 0.299 | 0.137 | 0.041 | −0.118 | 0.161 | 0.47 | −0.004 | 0.002 | 0.074 |
Estimates (p values) for PFOA and cofactors. Bold represents significant differences.
a. Line one for each tissue represents analyses without using plasma cholesterol as a covariate whereas line two uses plasma cholesterol.
|
|
|
Fig. 4. Cholesterol concentration in the mammary tissues and ovaries of C57BL/6 (A) and BALB/c (B) female mice. Tissues were collected from mice described in Fig. 1 and cholesterol measured by gas chromatography. Data represent averages ± SEM (n = 5–6). Differences from mice fed control diets are depicted by * (P < 0.05) and by # (P = 0.069). |
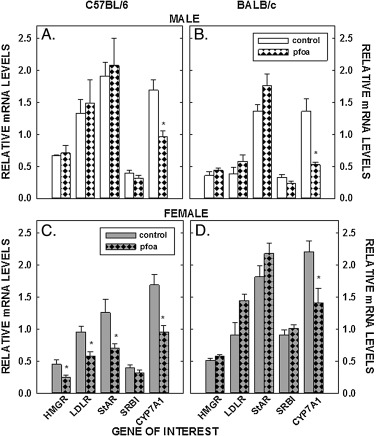
In addition to concentrations of sterol in tissues, mRNA levels of genes involved in sterol input (LDLR and HMGR) and output (cyp7a1), transport of sterol into the mitochondria (StAR), and uptake of estradiol (ERα) were measured in livers, ovaries, and/or mammary tissues. PFOA did not affect the relative levels of mRNA of LDLR and HMGR in the liver in male C57BL/6 mice or male or female BALB/c mice (Fig. 5 A&B&D). However, mRNA levels were reduced in the C57BL/6 female mice fed PFOA (P < 0.05) (Fig. 5 C). As with LDLR and HMGR, dietary PFOA led to a decrease of StAR mRNA only in the C57BL/6 female (Fig. 5 ). SR-BI was not affected by PFOA in any group of mice (Fig. 5 ). The biggest change in liver mRNA levels was a reduction in cyp7a1 mRNA as all mice fed PFOA had reduced levels of cyp7a1 mRNA. The effect could be direct because mRNA levels of FXR, a major regulator of cyp7a1, were not affected by PFOA in male and female C57BL/6 (0.97 ± 0.07 vs 0.95 ± 0.05 and 1.15 ± 0.08 vs 0.99 ± 0.08, respectively) or BALB/c mice (0.77 ± 0.06 vs 0.73 ± 0.08 and 1.19 ± 0.07 vs 1.11 ± 0.07, respectively). In ovaries, LDLR and HMGR mRNA levels were reduced or tended to be reduced in C57BL/6 females, respectively, and were unaffected in BALB/c mice (Fig. 6 A&B). StAR was also reduced significantly in ovaries of the C57BL/6 females but remained unaffected in the BALB/c mice. PFOA did not affect gene expression in mammary tissue (Fig. 6 C&D).
|
|
|
Fig. 5. Relative mRNA levels of genes involved in sterol metabolism of livers of C57BL/6 (A, C) and BALB/c (B, D) male and female mice. Livers were collected from mice described in Fig. 1 and mRNA levels measured by real time PCR using cyclophilin as our housekeeping gene. Data represent averages ± SEM (n = 5–6). Differences from mice fed control diets are depicted by * (P < 0.05). |
|
|
|
Fig. 6. Relative mRNA levels of genes involved in sterol metabolism of mammary tissues and ovaries of C57BL/6 (A, C) and BALB/c (B, D) female mice. Tissues were collected from mice described in Fig. 1 and mRNA levels measured by real time PCR using cyclophilin as our housekeeping gene. Data represent averages ± SEM (n = 5–6). Differences from mice fed control diets are depicted by * (P < 0.05) and by # (P = 0.056). |
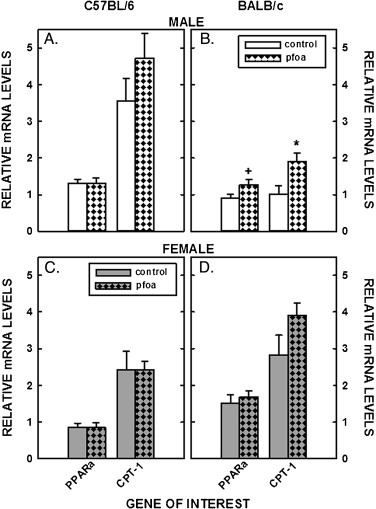
Finally, PPARα activation was examined as a possible cause of metabolic changes by measuring the mRNA levels of PPARα and of a downstream target of PPARα (CPT-1). There was little effect of PFOA on PPARα or CPT-1 mRNA levels in livers of C57BL/6 mice or female BALB/c mice (Fig. 7 A,C,&D). However, there was a trend for an increase in PPARα (P = 0.08) and a significant increase in CPT-1 levels in male BALB/c mice fed PFOA-laced diets (Fig. 7 B).
|
|
|
Fig. 7. Relative mRNA levels of genes involved PPARα signaling in livers of C57BL/6 (A, C) and BALB/c (B, D) male and female mice. Livers were collected from mice described in Fig. 1 and mRNA levels measured by real time PCR using cyclophilin as our housekeeping gene. Data represent averages ± SEM (n = 5–6). Differences from mice fed control diets are depicted by * (P < 0.05) and # (P = 0.08). |
4. Discussion
The major findings of these studies were two-fold. First and possibly most important, mice exposed to PFOA became hypercholesterolemic (defined as a significant increase in plasma cholesterol levels compared to control mice) when consuming diets containing cholesterol and fat. The effect was modified by genetics (C57BL/6 vs BALB/c) as well as gender (male vs female). Both C57BL/6 male and female mice became hypercholesterolemic when PFOA was fed in combination with cholesterol and fat. In contrast, only male BALB/c mice became hypercholesterolemic when fed PFOA-laced diets in combination with cholesterol and fat. A strain difference was not unexpected as previous studies have shown that C57BL/6 and BALB/c mice respond differently to dietary factors with respect to lipid metabolism [30] and mammary tissue metabolism [48] and [74] . Importantly, though diets resulted in marked increases in PFOA levels, there was not a reduction in body weight, as can happen with exposure to increased levels of PFOA.
The difference in the response to PFOA with respect to plasma cholesterol levels in these studies versus past studies was likely because PFOA was fed in combination with components of a typical Westernized diet, with increased levels of fat (≈33% calories) and cholesterol (≈300 mg/day), more like diets consumed by humans in resource-rich countries [8] and [40] , compared to typical rodent chow. By adding cholesterol to the diet, whole body sterol balance was changed to mimic that found in humans consuming similar diets. This is important as adaptive changes in an individual’s plasma cholesterol level appear to depend, at least partly, on their hepatic sterol synthesis rates, LDLR levels, and bile acid synthesis rates [59] and [63] . When exogenous cholesterol enters hepatocytes, intracellular cholesterol concentrations increase. Regulation of intracellular cholesterol occurs through a balance of input versus output of cholesterol. To account for the exogenous cholesterol, input from other sources, including de novo synthesis and lipoprotein uptake, is reduced and output of cholesterol, including bile acid synthesis, is increased. On a mechanistic level, the levels of HMGR and LDLR are regulated by SREBP-2 such that decreases in the mature form of SREBP result in reductions in HMGR and LDLR levels [23] . Because most LDL is cleared by the liver [72] , a reduction in hepatic LDLR levels will lead to more cholesterol being retained in the plasma [12] . Likewise, cholesterol is retained in the body when bile acid synthesis rates are decreased [12] and [62] . In the current study, the control groups (without PFOA) already have elevated plasma and/or liver cholesterol levels compared to previous studies in which mice were fed standard rodent chow [49] and [65] ; standard rodent chows have on average 4% fat (wt/wt) and 0.002% cholesterol (wt/wt).
In our mice, the increase in plasma cholesterol occurred in the HDL fraction. In humans, significant increases in LDL with increasing PFOA have been seen in national adolescent studies [20] and [75] , exposed communities [60] , [16] , [18] and [19] and occupational cohorts [56] . Human HDL cholesterol and PFOA studies are less consistent, though all show a decrease in HDL as PFOA increases [20] , [21] , [47] and [66] . As such, PFOA-induced hypercholesteremia in humans appears to be the result of elevated LDL and reduced HDL cholesterol. The difference in where the excess cholesterol is carried in the plasma in humans and mice is likely due to one of two metabolic differences between species. It is possible that HDL-cholesterol increases in humans but the cholesterol is transferred to VLDL, via cholesteryl ester transport protein (CETP), and subsequently converted to LDL, whereas mice cannot transfer cholesterol between lipoproteins as they lack this enzyme. Alternatively, excess cholesterol is secreted as VLDL in humans and as HDL in mice. Additional studies would need to be completed in mice to determine if HDL production was increased followed by a transfer of HDL-cholesterol to VLDL using CETP transgenic mice.
We also examined possible causes of the hypercholesterolemia. Initially we examined expression of genes involved in sterol balance in the liver. Importantly, PFOA treatment led to a reduction in the mRNA levels cyp7a1 in every group treated; PFOA-induced cyp7a1 downregulation was previously observed in rats fed a normal chow diet [36] and human hepatocytes [53] . All groups with ≥50% reduction in cyp7a1 mRNA levels (C57BL/6 male and female and BALB/c male mice) had increased plasma cholesterol levels. The increase was greatest in C57BL/6 females, possibly due to the suppression of LDLR in those mice as well. A reduction in LDLR mRNA levels that are accompanied by reductions of HMGR mRNA levels, as detected in the C57BL/6 females, suggests an increase in the subcellular pool of cholesterol regulating transcription of both genes [10] . The decrease in liver cholesterol levels in BALB/c mice initially seemed inconsistent in mice with reduced bile acid production. However, results could be confounded by the fact that PFOA can activate PPARα and BALB/c mice have more PPARα than C57BL/6 mice [76] ; increased mRNA levels of PPARα and CPT-1 is an indication of activated PPARα and was found in BALB/c male mice. As PPARα is hypolipidemic and can lead to a reduction in liver cholesterol levels [64] , the hypolipidemic effect of PFOA in BALB/c might override any other metabolic effect. At present, no human in vivo studies have examined reduced bile acid production. However, human in vivo studies found PFOA downregulated gene expression associated with cholesterol mobilization and reverse cholesterol transport [17] and upregulated circulating microRNAs that impair reverse cholesterol transport [66] , both consistent with reduced bile acid production and cholesterol excretion. Though the current studies focused on the liver, the intestine can also play a role in sterol balance if cholesterol absorption were changed by PFOA as well as other extrahepatic tissues and should be the focus of future studies.
The effect on plasma cholesterol levels was not due solely to changes in genes involved in sterol balance. In linear regression analyses, we also found that the larger liver size, containing a significant pool of cholesterol, also played a role in the PFOA-induced hypercholesterolemia. Based on previous studies, the increased liver size is most likely the result of hypertrophy [61] . The effect was also not mediated by an increase in food consumption (Supplemental Table I), and in fact mice may have consumed less food when 3.5 mg PFOA was added to each kg of diet. Thus, a change in sterol balance as the result of regulation of genes involved with sterol balance combined with larger livers appeared to be responsible for the hypercholesterolemia in the C57BL/6 mice.
Our second major finding was that dietary PFOA affected sterol metabolism in ovaries and mammary tissues. In the current study, cholesterol concentrations were increased in mammary tissues and trended to be increased in ovaries in the C57BL/6 females but not BALB/c females. When plasma cholesterol was also included as a covariate, mammary cholesterol was significantly increased by PFOA exposure suggesting that the effect on the mammary was the result of a change in plasma cholesterol levels. The β standard error estimates for these outcomes were quite large relative to the effect estimates, probably reflecting some scatter in the small number of mice studied. No effects were found for ovarian cholesterol, most likely as the differences were not <0.05. Clearly larger sample sizes are needed to better contrast the relationships between PFOA and mammary and ovarian cholesterol concentrations and metabolism in these two mouse strains when sterol balance is altered.
Even if tissue cholesterol concentrations are not affected, it is possible to detect differences in metabolism if the putative regulatory pool of cholesterol is changed [10] . For example, though ovarian total cholesterol was not statistically different in groups with or without dietary PFOA, sterol balance across this tissue of C57BL/6 females was likely affected as there were reductions in the genes regulated by sterol balance in these tissues (LDLR and HMGR), suggesting altered sterol balance. The C57BL/6 females also had reduced StAR mRNA levels which could be the reason for the lack of increase in estradiol levels in the tissues with greater sterol levels; StAR mRNA levels have been shown to be reduced by other perfluronated compounds [58] and [32] . Though a decrease in StAR mRNA levels might suggest a reduction in transport of precursor for steroidogenesis, substrate supply could be greater in the ovaries of PFOA-treated mice, leading to relatively similar estradiol levels between both strains of mice. There was no change in mRNA levels of any of the proteins measured in the mammary tissues of mice fed PFOA, possibly because there was only a 20% increase in tissue cholesterol levels.
The lack of effect of PFOA on mRNA levels in tissues of BALB/c female mice could help delineate the process by which PFOA is affecting peripheral tissues. As tissue cholesterol levels and mRNA levels of sterol-related proteins did not change in BALB/c female mice fed PFOA, one might assume that the effects of PFOA on sterol metabolism in mammary tissues and ovaries are mediated by uptake of plasma cholesterol and not a direct effect of PFOA; BALB/c mice were normocholesterolemic with dietary PFOA whereas C57BL/6 female mice were hypercholesterolemic. There has been much speculation of the impact of PFOA on peripheral tissue metabolism, especially in females, due to its potential function as an endocrine disruptor [68] . As cholesterol is needed for tissue proliferation, i.e. mammary tissue development, a change in sterol content or metabolism could affect mammary tissue development. Indeed, PFOA does affect mammary tissue development in a strain dependent manner [74] and [76] . In mice that become hypocholesterolemic with PFOA, i.e. previous studies in which mice were fed chow, PFOA can delay mammary tissue development in BALB/c mice at all doses of PFOA (0.1–10 mg/kg BW) and in C57BL/6 mice at the high dose only [74] . It will be interesting to know how PFOA affects mammary tissue development in mice that become hypercholesterolemic with consumption of PFOA. In fact, the combination of diet and PFOA could be quite important as it relates to the human population in that a high fat diet alone can affect mammary tissue development [48] . Though a full study on breast maturation and PFOA has not been completed in humans, PFOA does appear to delay menarche in young girls [34] .
An interesting observation that is currently unexplained was that the plasma PFOA levels were much greater than expected with the amount of PFOA added to the diet. For example, PFOA levels measured in previous studies in mice that received 5 mg/kg body weight by oral gavage [67] and [69] were comparable to values obtained here when mice received lower PFOA doses (≈0.5 mg/kg body weight). There are several possibilities for this result. First, PFOA was fed with dietary fat and cholesterol. It is possible that absorption rates of PFOA differ when fed in the presence of these dietary factors. However, a recent study in hamsters fed fat and a small amount of cholesterol and given daily doses of PFOA suggests that this is not true as PFOA levels were similar in hamsters fed fat or chow [15] . Second, it is possible that a greater proportion of PFOA entered the circulation due to it being consumed throughout the day, similar to what occurs in humans, and not in one bolus. Third, though the PFOA does not appear to be carried in lipoproteins [6] , it is unknown if changes in metabolism with high fat diets are such that clearance from the plasma could be delayed compared to chow-fed rodents. Delayed clearance could result in accumulation of PFOA over time. Though an analysis of NHANES data has found a direct but non-significant association between total fat intake and serum PFOA [25] , which was apparently not due to the presence of PFOA in fatty foods [41] , human dietary studies have not considered dietary fat a moderator of PFOA uptake by the gut. The very high serum concentrations found in our animals do limit the interpretation of findings and generalization to humans. Future studies will need to repeat these studies with lower amounts of PFOA in the same diet, individual PFOA plasma measurements, and with different modes of administration, as well as address the potential interaction of PFOA and dietary lipid.
Another unexplained finding was that female control mice had plasma PFOA concentrations one order of magnitude higher than the males (regardless of strain), assuming the pooled sample is representative of an average of all 6 mice per group. While PFOA in the control male mice was in the range of the median concentrations in NHANES, the concentrations were much higher in the females. As the control mice were not administered PFOA, they must have been exposed to PFOA prior to reaching the university or be present in our environment at low doses, i.e. in the chow. This is not unique in that PFOA has been detected in control mice in previous studies as well [69] and [70] . It should be noted that PFOA levels also were greater in the treated females versus males, though this was likely due to consumption of similar amounts of food while having less body mass leading to increased PFOA consumption per kg body weight. The causes of the sex differences are unknown, though in humans males have higher serum concentrations of PFOA compared to women [25] . Future studies with more animals should help to further address the sex differences in response to PFOA administration.
5. Conclusions
To summarize, we have shown that C57BL/6 and male BALB/c mice exposed to PFOA have significantly increased plasma cholesterol levels when fed Westernized diets, consistent with human observational findings at the national [39] and [14] and community levels [16] , [19] and [60] and in adolescent [18] and [20] and occupational [44] , [56] , [57] and [9] cohorts. As the majority of these human studies are cross-sectional and unable to assess causality, it has been difficult to study causality because of the directionality difference between epidemiologic human and experimental rodent studies [46] . Our findings suggest mouse PFOA responses are diet dependent, as suggested previously with other metabolic parameters [61] , and this dependency may explain some of the differences in human and rodent response observed in the literature. Though additional studies with lower levels of circulating PFOA are needed to model non-occupational human PFOA exposure and with mice genetically altered such that cholesterol can be transferred in plasma by CETP, the fat-fed rodents can be used to delineate the mechanism responsible for altered sterol metabolism in humans exposed to PFOA. Future studies will need to take caution with the gender and strain used as these variables affect how mice respond to dietary factors with respect to the development of hypercholesterolemia.
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Acknowledgements
These studies were supported by a pilot project from the Center for Environmental Genetics from the University of Cincinnati (NIEHS P30-ES006096), and also NIEHS U01ES019453, R21ES017176, T32ES010957, and EPA-RD-834788. We thank Kayoko Kato, Brian Basden, and Tao (Lily) Jia for the PFOA measurements.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [1] B.D. Abbott, C.R. Wood, A.M. Watkins, K. Tatum-Gibbs, D.P. Das, C. Lau; Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator-activated receptors (PPAR) and nuclear receptor-regulated genes in fetal and postnatal CD-1 mouse tissues; Reprod. Toxicol., 33 (2012), pp. 491–505
- [2] J. Berthiaume, K.B. Wallace; Perfluorooctanoate, perflourooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis; Toxicol. Lett., 129 (2002), pp. 23–32
- [3] K.S. Betts; What is the evidence telling us?; Env. Health Persp., 115 (2007), pp. A250–A256
- [4] R.C. Buck, J. Franklin, U. Berger, J.M. Conder, I.T. Cousins, P. de Voogt, A.A. Jensen, K. Kannan, S.A. Mabury, S.P. Van Leeuwen; Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins; Intger. Environ. Assess Manag., 7 (2011), pp. 513–541
- [5] T. Buhrke, E. Kruger, S. Pevny, M. Robler, K. Bitter, A. Lampen; Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes; Toxicology, 333 (2015), pp. 53–62
- [6] J.L. Butenhoff, E. Pieterman, D.J. Ehresman, G.S. Gorman, G.W. Olsen, S.C. Chang, H.M. Princen; Distribution of perfluorooctanesulfonate and perfluorooctanoate into human plasma lipoprotein fractions; Toxicol. Lett., 210 (2012), pp. 360–365
- [7] A.M. Calafat, Z. Kuklenyik, J.A. Reidy, S.P. Caudill, J.S. Tully, L.L. Needham; Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the national health and nutrition examination survey (NHANES); Environ. Sci. Technol., 41 (2007), pp. 2237–2242
- [8] CDC fastsats. www.cdc.gov/nchs/fastats/diet.htm .
- [9] G. Costa, S. Sartori, D. Consonni; Thirty years of medical surveillance in perfluooctanoic acid production workers; J. Occup. Environ. Med., 51 (2009), pp. 364–372
- [10] C.M. Daumerie, L.A. Woollett, J.M. Dietschy; Fatty acids regulate hepatic low density lipoprotein receptor activity through redistribution of intracellular cholesterol pools; Proc. Natl. Acad. Sci. U. S. A., 89 (1992), pp. 10797–10801
- [11] A.O. DeSilva, D.C.G. Muir, S.A. Marbury; Distribution of perfluorocarboxylate isomers in select samples from the North American environment; Environ. Toxicol. Chem., 28 (2009), pp. 1801–1814
- [12] J.M. Dietschy, S.D. Turley, D.K. Spady; Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans; J. Lipid Res., 34 (1993), pp. 1637–1659
- [13] E.A. Emmett, F.S. Shofer, H. Zhang, D.J. Freeman, C. Desai, L.M. Shaw; Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources; J. Occup. Environ. Med., 48 (2006), pp. 759–770
- [14] K.T. Eriksen, O. Raascchou-Nielsen, J.K. McLaughlin, L. Lipworth, A. Tjonneland, K. Overvad, M. Sorensen; Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population; PLoS One, 8 (2013), p. e56j969
- [15] N.E. Everds, G.L. Kennedy; Serum perfluorooctanoic acid (PFOA) concentrations in normal and hyperlipidemic female hamsters dosed orally with ammonium perfluorooctanoate (APFO) for up to 30 days; Toxicol. Rep., 2 (2015), pp. 70–77
- [16] N. Fitz-Simon, T. Fletcher, M.I. Luster, K. Steenland, A.M. Calafat, K. Kato, B. Armstrong; Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluoroocanesulfonic acid; Epidemology, 24 (2013), pp. 569–576
- [17] T. Fletcher, T.S. Galloway, D. Melzer, P. Holcroft, R. Cipelli, L.C. Pilling, D. Mondal, M.I. Luster, L.W. Harries; Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans; Environ. Int., 57–58 (2013), pp. 2–10
- [18] S.J. Frisbee, A. Shankar, S.S. Knox, K. Steenland, D.A. Savitz, T. Fletcher, A.M. Ducatman; Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8Health Project; Arch. Biochem. Biophys., 164 (2010), pp. 860–869
- [19] Y. Fu, T. Wang, Q. Fu, P. Wang, Y. Lu; Associations between serum concentrations of perfluoroalkyl acids and serum lipid levels in a Chinese population; Exotoxicol. Environ. Saf., 106 (2014), pp. 246–252
- [20] S.D. Geiger, J. Xiao, A. Ducatman, S. Frisbee, K. Innes, A. Shankar; The association between PFOA, PFOS and serum lipid levels in adolescents; Chemosphere, 98 (2014), pp. 78–83
- [21] F.D. Gilland, J.S. Mandel; Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men; Am J. Ind. Med., 29 (1996), pp. 560–568
- [22] B. Haughom, O. Spydevold; The mechanism underlying the hypolipemic effect of perfluorooctanoic acid (PFOA), perfluorooctane sulphonic acid (PFOSA) and clofibric acid; Biochim. Biophys. Acta, 1128 (1992), pp. 67–72
- [23] J.D. Horton, J.L. Goldstein, M.S. Brown; SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver; J. Clin. Invest., 109 (2002), pp. 1125–1131
- [24] U. Intrasuksri, S.M. Rangwala, M. O'Brien, D.J. Noonan, D.R. Feller; Mechanisms of peroxisome proliferation by perfluorooctanoic acid and endogenous fatty acids; Gen. Pharmacol., 31 (1998), pp. 187–197
- [25] R.B. Jain; Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003-2008; Int. J. Hyg. Environ. Health, 217 (2014), pp. 52–61
- [26] A.A. Jensen, H. Leffers; Emerging endocrine disrupters: perfluoroalkylated substances; Int. J. Androl., 31 (2008), pp. 161–169
- [27] K. Kato, B.J. Basden, L.L. Needham, A.M. Calafat; Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals; J. Chromatogr. A., 1218 (2011), pp. 2133–2137
- [28] N. Kudo, H. Mizuguchi, A. Yamamoto, Y. Kawashima; Alterations by perfluorooctanoic acid of glycerolipid metabolism in rat liver; Chem. Biol. Int., 118 (1999), pp. 69–83
- [29] C. Lau, K. Anitole, C. Hoese, D. Lai, A. Pfanles-Hutchens, J. Seed; Perfluoroalkyl acids: a review of monitoring and toxicological findings; Toxicol. Sci., 99 (2007), pp. 366–394
- [30] R.C. LeBoeuf, M. Caldwell, E. Kirk; Regulation by nutritional status of lipids and apolipoproteins A-I, A-II, and A-IV in inbred mice; J. Lipid Res., 35 (1994), pp. 121–133
- [31] M.H. Lichtenberg, C.S. Wilke, J.A. McConihay, N.A. Granholm, L.A. Woollett; Yolk sac cholesteryl ester secretion rates can be manipulated in the golden syrian hamster: effect of yolk sac cholesterol concentrations; Biochim. Biophys. Acta, 1735 (2005), pp. 214–221
- [32] C. Liu, X. Zhang, H. Chang, P.H. Jones, S. Wiseman, J. Nalle, M. Hecker, J.P. Giesy, B. Zhou; Effects of fluorotelomer alcohol 8:2 FTOH on steroidogenesis in H295R cells: targeting the cAMP signalling cascade; Toxicol. Appl. Pharmacol., 247 (2010), pp. 222–228
- [33] C.M. Lo, A. King, L.C. Samuelson, T.L. Kindel, T. Rider, R.J. Jandacek, H.E. Raybould, S.C. Woods, P. Tso; Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity; Gastroenterology, 138 (2010), pp. 1997–2005
- [34] M.J. Lopez-Espinosa, T. Fletcher, B. Armstrong, B. Genser, K. Dhatariya, D. Mondal, A. Ducatman, G. Leonardi; Association of perfluoroocantoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant; Environ. Sci. Technol., 45 (2011), pp. 8160–8166
- [35] S.E. Loveless, C. Finlay, N.E. Everds, S.R. Frame, P.J. Gilles, J.C. O'Connor, C.R. Powley, G.L. Kennedy; Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO); Toxicology, 220 (2006), pp. 203–217
- [36] M.T. Martin, R.J. Breman, W. Hu, E. Ayanoglu, C. Lau, H. Rem, C.R. Wood, J.C. Corton, R.J. Kavlock, D.J. Dix; Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity; Toxicol. Sci., 97 (2007), pp. 595–613
- [37] T. Nadagawa, D.H. Ramdhan, N. Tanaka, H. Naito, H. Tamada, Y. Ito, Y. Li, Y. Hayashi, N. Yamagishi, Y. Yanagiba, T. Aoyama, F.J. Gonzalez, T. Nakajima; Modulation of ammonium perfluorooctanoate-induced hepatic damage by genetically different PPARα in mice; Arch. Toxicol., 86 (2012), pp. 63–74
- [38] T. Nakamur, Y. Ito, Y. Yanagiba, D.H. Ramdhan, Y. Dono, H. Naito, Y. Hayashi, Y. Li, T. Aoyama, F.J. Conzalez, T. Nakajima; Microgram-order ammonium perfluorooctanoate may activate mouse peroxisome proliferator-activated receptor alpha, but not human PPARalpha; Toxicology, 265 (2009), pp. 27–33
- [39] J.W. Nelson, E.E. Hatch, T.F. Webster; Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population; Environ. Health Perspect., 118 (2010), pp. 197–202
- [40] NHANES. http://www.cdc.gov/exposurereport/ .
- [41] C.W. Noorlander, S.P. van Leeuwen, J.D. Te Biesebeek, M.J. Mengelers, M.J. Zeilmaker; Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in the Netherlands; J. Agric. Food Chem., 59 (2011), pp. 7496–7505
- [42] F. Oliaei, D. Kriens, R. Weber, A. Watson; PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota USA; Environ. Sci. Pollut. Res. Int., 20 (2013), pp. 1977–1992
- [43] G.W. Olsen, J.M. Burris, M.M. Burlew, J.H. Mandel; Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluoroocatnoate production workers; Drug Chem. Toxicol., 23 (2000), pp. 608–620
- [44] G.W. Olsen, J.M. Burris, M.M. Burlew, J.H. Mandel; Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations; J. Occup. Environ. Med., 45 (2003), pp. 260–270
- [45] G.W. Olsen, J.M. Burris, D.J. Dhresman, J.W. Froehlich, A.M. Seacat, J.L. Butenhoff, L.R. Zobel; Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers; Enciron. Health Perspect., 115 (2007), pp. 1298–1305
- [46] G.W. Olsen, D.J. Ehresman, B.D. Buehrer, B.A. Gibson, J.L. Butenhoff, L.R. Zobel; Longitudinal assessment of lipid and hepatic clinical parameters in workers involved iwth the demolition of perfluoroalkyl manufacturing facilities; J. Occup. Environ. Med., 54 (2012), pp. 974–983
- [47] G.W. Olsen, L.R. Zobel; Assesment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorchemical production workers; Int. Arch. Occup. Environ. Health, 8 (2007), pp. 231–246
- [48] L.K. Olson, Y.S. Tan, Y. Zhao, M.D. Aupperlee, S.Z. Haslam; Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness; Int. J. Obes., 34 (2010), pp. 1415–1426
- [49] Y. Osono, L.A. Woollett, J. Herz, J.M. Dietschy; Role of the low density lipoprotein receptor in the flux of cholesterol across the tissues of the mouse; J. Clin. Invest., 95 (1995), pp. 1124–1132
- [50] S. Peng, L. Yan, J. Zhang, Z. Wang, M. Tian, H. Shen; An integrated metabonomics and transcriptomics approach to understanding metabolic pathway disturbance induced by perfluorooctanoic acid; J. Pharm. Biomed. Anal., 86 (2013), pp. 56–64
- [51] S.M. Pinney, F.M. Biro, G.C. Windham, R.L. Herrick, L. Yaghjyan, A.M. Calafat, P. Succop, H. Sucharew, K.M. Ball, K. Kato, L.H. Kushi, R. Bornschein; Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA; Environ. Pollut., 184 (2014), pp. 327–334
- [52] M.R. Qazi, M.R. Abedi, B.D. Nelson, J.W. DePierre, M. Abedi-Valugerdi; Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice; Int. Immunopharmacol., 10 (2010), pp. 1420–1427
- [53] M.B. Rosen, K.P. Das, C.R. Wood, C.J. Wolf, B.D. Abbott, C. Lau; Evaluation of perfluoroalkyl acid activity using primary mouse and human hepatocytes; Toxicology, 308 (2013), pp. 129–137
- [54] M.B. Rosen, J.S. Lee, H. Ren, B. Vallanat, J. Liu, M.P. Waalkes, B.D. Abbott, C. Laun, J.C. Corton; Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR; Toxicol. Sci., 103 (2008), pp. 46–56
- [55] M.B. Rosen, B.D. Abbott, D.C. Wolf, J.C. Corton, C.R. Wood, J.E. Schmid, K.P. Das, R.D. Zehr, E.T. Blair, C. Lau; Gene expression profiling in the livers of wild-type and PPARalpha-null mice exposed to perfluorooctanoic acid; Toxicol Pathol., 36 (2008), pp. 592–607
- [56] C.J. Sakr, K.H. Kreckmann, J.W. Green, P.J. Gilles, J.L. Reynolds, R.C. Leonard; Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers; J. Occup. Environ. Med., 49 (2007), pp. 1086–1096
- [57] C.J. Sakr, R.C. Leonard, K.H. Kreckmann, M.D. Slade, M.R. Cullen; Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate; J. Occup. Environ. Med., 49 (2007), pp. 872–879
- [58] Z. Shi, L. Ding, H. Zhang, Y. Feng, M. Xu, J. Dai; Chronic expsoure to perfluorododecanoic acid disrupts testicular steroidogenesis and the expression of related genes in male rats; Toxicol. Lett., 188 (2009), pp. 192–200
- [59] D.K. Spady, L.A. Woollett, J.M. Dietschy; Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids; Annu. Rev. Nutr., 13 (1993), pp. 355–381
- [60] K. Steenland, S. Tinker, S. Frisbee, A. Ducatman, V. Vaccarino; Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant; Am. J. Epidemiol., 170 (2009), pp. 1268–1278
- [61] X. Tan, G. Xie, X. Sun, Q. Li, W. Zhong, P. Qiao, X. Sun, W. Jia, Z. Zhou; High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways; PLoS One, 8 (2013), p. e61409
- [62] S.D. Turley, D.K. Spady, J.M. Dietschy; Identification of a metabolic difference accounting for the hyper- and hyporesponder phenotypes of cynomologus monkey; J. Lipid Res., 38 (1997), pp. 1598–1611
- [63] S.D. Turley, D.K. Spady, J.M. Dietschy; Identification of a metbolic difference accounting for the hyper- and hyporesponder phenotypes of cynomolgus monkey; J. Lipid Res., 38 (1997), pp. 1598–1611
- [64] S.K. Turley, M. Schwarz, D.K. Spady, J.M. Dietschy; Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets; Hepatology, 28 (1998), pp. 1088–1094
- [65] S.K. Turley, M. Schwarz; Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets; Hepatology, 28 (1998), p. 1088
- [66] J. Wang, Y. Zhang, W. Zhang, Y. Jin, J. Dai; Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199-3p in workers of a fluorochemical plant and nearby residents; Environ. Sci. Technol., 46 (2012), pp. 9274–9281
- [67] S.S. White, A.M. Calafat, Z. Kuklenyik, L. Vilaneuva, R.D. Zehr, L. Helfant, M.J. Strynar, A.B. Lindstrom, J.R. Thibodeux, C.R. Wood, S.E. Fenton; Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring; Toxicol. Sci., 96 (2007), pp. 133–144
- [68] S.S. White, S.E. Fenton, E.P. Hines; Endocrine disrupting properties of perfluorooctanoic acid; J. Steroid Biochem. Mol. Biol., 127 (2011), pp. 16–26
- [69] S.S. White, K. Kato, L.T. Jia, B.J. Basden, A.M. Calafat, E.P. Hines, J.P. Stanko, C.J. Wolf, B.D. Abbott, S.E. Fenton; Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures; Reprod. Toxicol., 27 (2009), pp. 289–298
- [70] S.S. White, J.P. Stanko, K. Kato, A.M. Calafat, E.P. Hines, S.E. Fenton; Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice; Environ. Health Perspect., 119 (2011), pp. 1070–1076
- [71] A. Winquist, K. Steenland; Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts; Environ. Health Perspect., 122 (2014), pp. 1299–1305
- [72] L.A. Woollett, D.K. Spady, J.M. Dietschy; Mechanisms by which saturated triacylglycerols elevate the plasma low density lipoprotein-cholesterol concentration in hamsters. Differential effects of fatty acid chain length; J. Clin. Invest., 84 (1989), pp. 119–128
- [73] S. Yan, J. Wang, W. Zhang, J. Dai; Circulating microRNA profiles altered in mice after 28 d exposure to perfluorooctanoic acid; Toxicol. Lett., 224 (2014), pp. 24–31
- [74] C. Yang, Y.S. Tan, J.R. Harkema, S.Z. Haslam; Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains; Reprod. Toxicol., 27 (2009), pp. 299–306
- [75] X.W. Zeng, Z. Zian, B. Emo, M. Vaughn, J. Bao, X.D. Qin, Y. Zhu, J. Li, Y.L. Lee, G.H. Dong; Association of polyfluoroalkyl chemical exposure with serum lipids in children; Sci. Total Environ., 512–513 (2015), pp. 364–370
- [76] Y. Zhao, Y.S. Tan, M.J. Strynar, G. Perez, S.Z. Haslam, C. Yang; Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice; Reprod. Toxicol., 33 (2012), pp. 563–576
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?