Abstract
Background and aims
The aim of this study was to investigate the effect of a glucagon-like peptide-1 receptor agonist (GLP-1RA), exenatide, on clozapine-associated glucose dysregulation in mice.
Materials and methods
We randomly separated B6 male mice into four groups (A to D). Mice in groups C and D received a daily oral dose of 13.5 mg/kg body weight of clozapine for 4 months. Mice in groups B and D received 1 μg of exenatide daily. The body weight and blood glucose before and 2 h after clozapine treatment were measured twice a week. Intraperitoneal glucose tolerance test (IPGTT) scores and the amount of daily food intake were recorded. The pancreases of the mice were removed for insulin content (PIC) measurement and histological examination after sacrifice.
Results
The mean non-fasting blood glucose levels were not different, and the mean changes in blood glucose 2 h after oral clozapine were 0 ± 4, −40 ± 2, 25 ± 3, and −39 ± 2, in groups A to D, respectively. There was no significant difference in daily calorie intake or area under the curve of IPGTT among the four groups. At sacrifice, the PIC of mice treated with clozapine was significantly lower than that of the control mice, however the PIC was completely restored in the mice treated with exenatide. Histological examination of the pancreas revealed that exenatide treatment reversed the clozapine-induced apoptosis of islet cells.
Conclusion
Our results provide preclinical evidence of a pharmaceutical role of GLP-1RA in managing glucose dysregulation in schizophrenic patients under long-term atypical antipsychotic treatments.
Keywords
Clozapine ; Exenatide ; Glucose dysregulation ; Beta cell ; Apoptosis
Chemical compounds studied in this article
Exenatide (PubChem CID: 16158469) ; Clozapine (PubChem CID: 2818)
1. Introduction
Atypical or second-generation antipsychotic medications are thought to be more effective with fewer extrapyramidal side effects than conventional antipsychotic in the treatment of schizophrenia and other psychiatric disorders. However, patients taking clozapine, olanzapine and quetiapine have been reported to have a significantly increased risk of developing diabetes than those not taking antipsychotics and those taking conventional antipsychotics such as haloperidol [1] , [2] , [3] and [4] . Although it is widely assumed that the development of diabetes in psychiatric patients taking atypical antipsychotics is caused by peripheral insulin resistance following excessive weight gain, the mechanism of action by which atypical antipsychotics induce glucose dysregulation has yet to be elucidated [5] and [6] . In rodent models, the short-term administration of clozapine and quetiapine has been shown to cause acute and reversible derangement of glucose metabolism via suppressing glucagon-like peptide-1 (GLP-1) and elevating glucagon secretion which subsequently increases hepatic gluconeogenesis [7] and [8] . Whether anti-diabetic drugs are effective in reversing the adverse effects of atypical antipsychotics on glucose metabolism dysregulation is largely unknown. Lifestyle interventions are the fundamental strategy to prevent the onset of diabetes and manage hyperglycemia in high-risk subjects such as those with obesity, impaired glucose tolerance and metabolic syndrome. However, lifestyle interventions per se are not adequate to prevent the development of diabetes in schizophrenic patients who are taking atypical antipsychotics [9] . Moreover, metformin, the corner-stone drug in the management of type 2 diabetes, can only partially reverse derangements of atypical antipsychotic-associated glucose metabolism [10] . Therefore, new pharmaceutical strategies are needed to manage glucose dysregulation in patients who are taking atypical antipsychotics. GLP-1 receptor agonists have been shown to inhibit apoptosis and enhance the proliferation of pancreatic islet beta cells and to suppress glucagon secretion, along with other beta cell protective functions such as weight reduction and anti-inflammatory properties [11] and [12] . However there is a paucity of preclinical evidence for the beneficial effect of GLP-1 receptor agonists on atypical antipsychotic-associated glucose dysregulation. In this study, we investigated the effect of administering clozapine for 4 months on glucose variability and pancreatic islet beta cell mass, and studied the effect of a GLP-1 receptor agonist, exenatide, on derangements of clozapine-associated glucose metabolism.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice (8–12 weeks old) were obtained from a local breeder, and three to five mice were housed in each cage and fed pelleted food and tap water ad libitum. The animal room had an automatic light cycle with 12 h of light and 12 h of dark. The animals were treated humanely in accordance with the laboratory animal guidelines of Chang-Gung Memorial Hospital.
2.2. Experimental designs
We randomly separated healthy B6 male mice into four groups (A–D). Mice in groups C and D were given a daily oral dose of 13.5 mg/kg body weight of clozapine in a volume of 250 μL for 4 months, while mice in the groups A and B received the same volume of distilled water. Mice in groups B and D received a daily subcutaneous injection of 1 μg of exenatide 1 h before the oral administration of clozapine or the vehicle. Body weight and non-fasting random blood glucose level at 8:00–9:00 a.m. (basal BG) and blood glucose 2 h after the administration of clozapine or the vehicle (2-h BG) were recorded twice a week. The acute effect of clozapine on glucose derangement (ΔBG) was expressed as the difference in subtracting basal BG from 2-h BG on the same day. The long-term change in body weight (ΔBW) was calculated as the difference in average body weight between the first week and the sixteenth week. Bilateral gastrocnemius and peri -renal fat pads of all mice were removed after sacrifice and weighed.
2.3. Daily food intake
At 2 and 14 weeks of the experiment, six mice from each group were placed separately in metabolic cages and the amount of daily chow intake was measured for 3 consecutive days. The average daily chow intake over the 3 days was used in analysis and expressed as g/kg body weight/day.
2.4. Islet function according to the intraperitoneal glucose tolerance test
At 14 weeks, each mouse in each group received an intraperitoneal glucose tolerance test (IPGTT) using 1.5 g/kg of body weight of dextrose solution. The area under the curve (AUC, minute × mg/dL) was determined by the whole blood glucose concentration measured before and 30, 60, 90 and 120 min after the dextrose injection.
2.5. Quantitation of serum levels of glucagon and chemokines
At the end of 14 weeks, sera were collected at basal (before exenatide) and 2 h after clozapine administration to measure glucagon concentrations using a Glucagon EIA kit from Phoenix Pharmaceutical Inc. (Burlingame, CA, USA). The basal sera samples were also subjected to quantitative analysis for chemokines including GM-CSF, RANTES, MCP-1, MCP -3, MIP-1α and MIP-1β using a FlowCytomix multiplex kit (eBioscience, San Diego, CA, USA).
2.6. The apoptotic effect of clozapine on an insulin-secreting cell line
RINm5F cells were cultured in RPMI-1640 medium containing 100 mg/dL glucose and clozapine at concentrations of 0, 1, 10 and 25 μM. At 16 h following cultivation, the cells were harvested and assayed for apoptosis by flow cytometry based on the binding of PE-annexin V and 7-amino-actinomycin (7-AAD) using an apoptosis detection kit according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). The results were expressed as the percentage of PE-annexin V-negative and 7-AAD-negative cells (viable, no apoptosis), PE-annexin V-positive but 7-AAD-negative cells (early apoptosis), and PE-annexin V-positive and 7-AAD-positive cells (late apoptosis, dead). Data are expressed as mean ± standard error.
2.7. Histology of pancreatic islet beta cells
Mice from each group were randomly selected and their pancreases removed for histological examination at 2 weeks and 4 months after sacrifice. The apoptotic and proliferative nuclei of islet cells of paraffin-embedded pancreatic sections were detected using a TUNEL Apoptosis Detection Kit (GenScrip, Piscataway, NJ, USA), and immunohistochemical analysis was performed using an anti-Ki67 antibody (ab15580, Abcam Inc., Cambridge, MA, USA). For each mouse, approximately 2000 nuclei of pancreatic islet sections were counted and the results were expressed as the percentage of positive nuclei.
2.8. Pancreatic insulin content
All mice in each group were sacrificed at the end of 4 months, and the pancreases were removed and the insulin content (PIC) determined using an acid-ethanol extraction and insulin kit (# RI-13k, LINCO Research, Inc., St. Charles, MO, USA).
2.9. Statistics
Data were expressed as means ± standard error. Statistical differences between means were analyzed using paired or unpaired Student’s t -tests or one-way ANOVA as appropriate. A p value of less than 0.05 was considered to be statistically significant.
3. Results
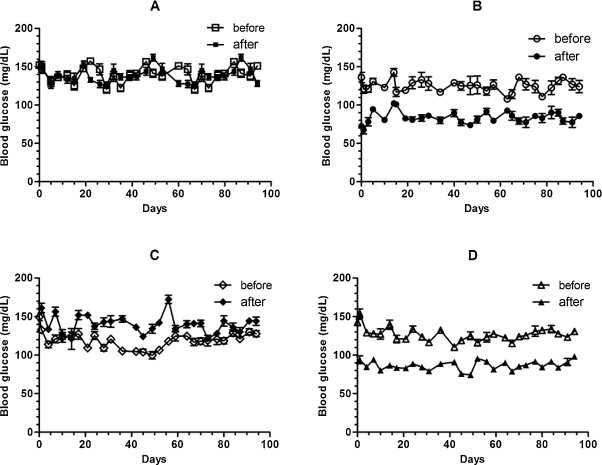
An acute effect of clozapine on glucose derangement was reflected by a rapid and significant increase in glucose concentration after administration (Table 1 , ΔBG, group A vs. C, 0 ± 4 vs. 25 ± 3 mg/dL, p < 0.001; Fig. 1 A and C). The administration of exenatide not only rapidly suppressed the blood glucose level (Table 1 , group A vs. B, 0 ± 4 vs. −40 ± 2 mg/dL, p < 0.001; Fig. 1 B) but also effectively reversed the clozapine-induced acute elevation of blood glucose concentration (Table 1 , group C vs. D, 25 ± 3 vs. −39 ± 2 mg/dL, p < 0.001; Fig. 1 D). There was no significant difference in mean average non-fasting BG level over the whole experimental period among the four groups (Table 1 , p > 0.05). In addition, there was no significant difference in the function of islet beta cells as evaluated by IPGTT at the 14th week among the four groups (Table 1 , IPGTT, one-way ANOVA, p > 0.05).
| n | Clozapine | GLP-1RA | Mean of average ΔBG | Mean of non-fasted random BG (mg/dL) | IPGTT (AUC, min × mg/dL) | |
|---|---|---|---|---|---|---|
| A | 15 | − | − | 0±4a,b,c | 135±3 | 21208±845 |
| B | 15 | − | + | −40± 2a | 125±2 | 19484±687 |
| C | 15 | + | − | 25±3b,d | 122±1 | 21440±848 |
| D | 15 | + | + | −39±2c,d | 127±2 | 20568±645 |
|
|
|
Fig. 1. Effect of exenatide on clozapine treatment-associated glucose metabolism derangement. We randomly separated healthy B6 male mice into four groups (A to D). The mice in groups C and D were given a daily oral dose of 13.5 mg/kg body weight of clozapine in a volume of 250 μL for 4 months, while those in groups A and B received the same volume of distilled water. The mice in groups B and D received a daily subcutaneous injection of 1 μg of exenatide 1 h before the oral administration of clozapine or the vehicle. Non-fasting random blood glucose levels at 8:00-9:00 a.m. (solid markers) and blood glucose levels 2 h after the administration of clozapine or the vehicle (blank markers) were recorded twice a week. |
The mean basal body weights were 27.2 ± 0.5, 27.0 ± 0.6, 26.9 ± 0.5 and 27.1 ± 0.5 g in groups A–D, respectively (one-way ANOVA, p > 0.05). At the end of 4 months, the control mice had gained significantly more body weight than the mice that were treated with clozapine and/or exenatide (Table 2 , ΔBW, groups A vs. B, A vs. C, and A vs. D, p < 0.05). The administration of exenatide did not affect the clozapine-associated lower gain in body weight or lean mass, but further reduced the peri -renal fat pad mass ( Table 2 , peri -renal fat pad, group C vs. D, 0.216 ± 0.025 vs. 0.100 ± 0.011 g, p < 0.05). The amount of chow intake did not significantly differ among the four groups at the 2nd and 14th weeks (2nd week, A: 14.4 ± 0.4, B: 13.8 ± 0.6, C: 14.0 ± 0.8, D: 13.6 ± 0.9 g/kg body-weight/day, p > 0.05; 14th week, A: 12.4 ± 0.6, B: 12.8 ± 0.7, C: 11.7 ± 0.7, D: 13.2 ± 0.4 g/kg body-weight/day, p > 0.05).
| n | Clozapine | GLP-1RA | ΔBW (g) | Amount of chow intake (g/kg BW/day) | Gastrocnemius (g) | Peri-renal fat pad (g) | |
|---|---|---|---|---|---|---|---|
| A | 12 | − | − | 7.11±1.11a,b,c | 12.4±0.6 | 0.385±0.014d,e,f | 0.451±0.010g,h,i |
| B | 12 | − | + | 5.55±0.54a | 12.8±0.7 | 0.354±0.010d | 0.285±0.011g |
| C | 12 | + | − | 5.03±0.31b | 11.7±0.7 | 0.341±0.005e | 0.216±0.025h,j |
| D | 12 | + | + | 5.45±0.35c | 13.2±0.4 | 0.337±0.006f | 0.100±0.011i,j |
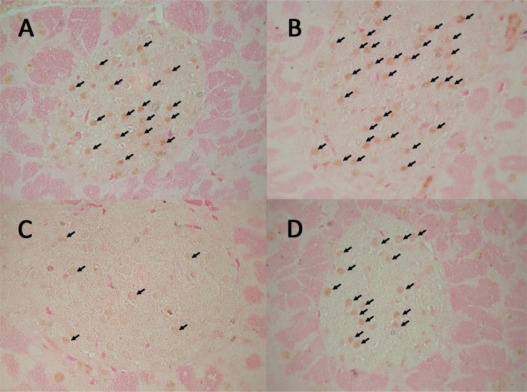
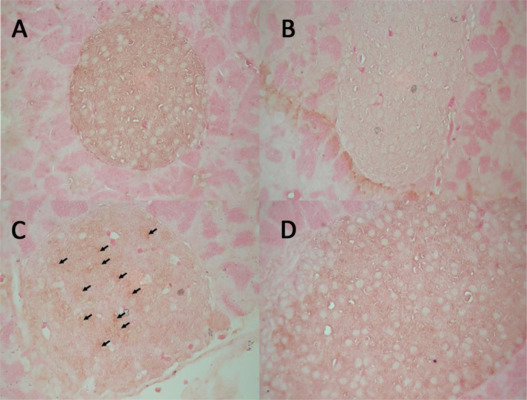
Compared with the control mice, the administration of clozapine resulted in a significant reduction in PIC at the end of 4 months (Table 3 , PIC, group A vs. C, p < 0.01). The mice treated with exenatide had a higher PIC than the control mice (Table 3 , PIC, group A vs. B, p < 0.05), and exenatide treatment also restored the clozapine-associated reduction in PIC (Table 3 , PIC, group C vs. D, p < 0.01). The immunohistochemical analysis of the pancreatic islet cells revealed that the administration of clozapine resulted in an increase in apoptosis and a decrease in proliferation markers, and this occurred as early as 2 weeks after the start of the experiment but it attenuated at 4 months of the experiment (Table 3 and Fig. 2 and Fig. 3 ).
| Clozapine | GLP-1RA | PIC (μg) | 2 weeks (n = 3) | 16 weeks (n = 3) | |||
|---|---|---|---|---|---|---|---|
| Ki67 (%) | TUNEL (%) | Ki67 (%) | TUNEL (%) | ||||
| A | − | − | 8.5±0.8a,b | 9±4d | 0f | 9±3 | 0 |
| B | − | + | 10.5±0.6a | 12±5 | 0 | 10±4 | 0 |
| C | + | − | 5.1±0.3b,c | 2±1d,e | 5±2f,g | 7±2 | 0 |
| D | + | + | 7.5±0.8c | 8±3e | 0g | 9±3 | 0 |
|
|
|
Fig. 2. Effect of clozapine on the proliferation of pancreatic islet cells. Immunohistochemical analysis of the pancreatic islet cells 2 weeks after the start of the experiment revealed that the administration of clozapine resulted in a decrease in Ki67 positively-stained nuclei (arrows in group C), and that the administration of exenatide resulted in an increase in Ki67 positively-stained nuclei (arrows in groups B and D). |
|
|
|
Fig. 3. Effect of clozapine on the apoptosis of pancreatic islet cells. Immunohistochemical analysis of the pancreatic islet cells 2 weeks after the start of the experiment revealed that the administration of clozapine resulted in an increase in apoptotic nuclei (arrows in group C). No apoptotic nuclei were observed in the pancreatic islet cells of the control mice (group A) or the mice that received exenatide (groups B and D). |
At the end of 14 weeks, the basal serum glucagon concentrations in the mice in groups A–D were 15.3 ± 2.0 (n = 12), 13.0 ± 1.8 (n = 12), 12.4 ± 2.1 (n = 12) and 13.9 ± 1.0 (n = 12) ng/mL, respectively (p > 0.05 by one-way ANOVA). Two hours after administering clozapine, the serum glucagon levels were 15.7 ± 2.3 (n = 12) and 15.6 ± 1.6 (n = 12) ng/mL (p > 0.05) in the mice in groups C and D, respectively. The basal serum level of MCP -3 in the mice treated with clozapine was significantly reduced, and it was totally restored by the co-administration of exenatide ( Table 4 , MCP -3, groups A vs. C and C vs. D, p < 0.05). Other basal serum chemokines including RANTES, GM-CSF, MCP-1, MIP-1α and MIP-1β were not significantly different among the four groups (Table 4 ).
| Gr | n | Clozapine | GLP-1RA | RANTES (pg/mL) | GMCSF (pg/mL) | MCP-1 (pg/mL) | MCP-3 (pg/mL) | MIP-1α (pg/mL) | MIP-1β (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| A | 12 | − | − | 696±54 | 8.9±4.0 | 564±326 | 440±146a | 4.8±3.5 | 2.8±2.6 |
| B | 12 | − | + | 573±49 | 9.8±5.4 | 429±142 | 258±62 | 12.9±7.3 | 22.3±9.4 |
| C | 12 | + | − | 778±42 | 9.8±4.4 | 479±117 | 189±33a,b | 13.2±5.3 | 81.7±36.3 |
| D | 12 | + | + | 689±59 | 10.0±6.4 | 469±187 | 430±64b | 20.4±12.9 | 10.9±7.0 |
In RINm5F cell in-vitro cultivation study, the percentage of early and total apoptosis increased significantly after incubation with 1 μM clozapine for 16 h (Table 5 ). At a concentration of 10 μM clozapine, the late apoptosis of RINm5F cell also significantly increased (Table 5 ). Both clozapine-associated early and late apoptosis of RINm5F cell are dose-dependent (Table 5 ).
| Clozapine | n | Early apoptosis (%) | Late apoptosis (%) | Total apoptosis (%) |
|---|---|---|---|---|
| 0μM | 6 | 3.6±0.3 | 2.0±0.1 | 5.5±0.3 |
| 1μM | 6 | 4.7±0.2b | 2.1±0.2 | 6.8±0.3a |
| 10μM | 6 | 6.7±0.1c | 3.4±0.2c | 10.0±0.1c |
| 25μM | 6 | 8.6±0.2c | 3.8±0.3c | 12.3±0.4c |
4. Discussion and conclusions
Our results suggest that clozapine treatment resulted in a significant increase in apoptosis and inhibition of the proliferation of pancreatic islet cells, and that this occurred as early as 2 weeks after its administration. The anti-proliferative effect of clozapine on islet cells was attenuated at four months of treatment. Although the non-fasting random blood glucose level of the mice treated with clozapine did not increase significantly during the experimental period, the adverse effect of clozapine on islet beta cells was confirmed by a 40% reduction in PIC in the mice treated with clozapine for 4 months. Using a rat beta cell line, RINm5F, we found that clozapine directly increased the apoptosis of insulin-secreting cells in a dose-dependent manner. It has been reported that preservation of more than one-tenth of the mass of beta cells in a healthy pancreas is adequate to maintain normal glucose metabolism [13] . This may explain, at least partly, why the random non-fasting blood glucose levels in the mice treated with clozapine in this study did not increase significantly. Since the AUC of IPGTT did not significantly differ between the mice treated and not treated with clozapine, the glucose-stimulated insulin secretion of pancreatic islet beta cells did not seem to be disrupted by the long-term administration of clozapine.
A previous rat study reported that administration of a single dose of clozapine resulted in suppression of the serum level of GLP-1 and increase in glucagon level, thereby resulting in acute derangement of glucose metabolism [8] . Mechanisms such as the clozapine-induced activation of hepatic phosphorylase and G6Pase have been proposed to be involved in atypical antipsychotic-associated increases in the hepatic glucose output [14] . It also has been reported that clozapine is a potent acetylcholine muscarinic M3 receptor (M3R) antagonist and both pancreatic and hypothalamic and brainstem M3Rs regulate the insulin secretion and alter glucometabolic status of the body [15] . Another recent study reported that acute treatment with olanzapine caused glucose intolerance through the activation of hypothalamic adenosine 5′-monophosphate-activated protein kinase (AMPK) [16] . Clozapine has also been reported to significantly induce the production of proinflammatory cytokines in cultured insulin responsive cells, and by doing this clozapine may interfere in insulin action and result in peripheral insulin resistance [17] . Our results reveal that the long-term administration of clozapine did not increase the levels of proinflammatory chemokines. Only a significant decrease in MCP-3 was found in the mice treated with clozapine, which is compatible with a previous in-vitro cell culture study which found that clozapine reduced PMN chemotaxis [18] . Our results suggest that this mechanism of clozapine-associated derangements of glucose metabolism is multifactorial.
In this study, we did not find a significant difference in the serum level of glucagon between baseline and 2 h after the administration clozapine in the mice treated with clozapine for 4 months. Meanwhile, the clozapine-associated increase in glucose variability was larger in the first two months but it was getting smaller in the 4th month of treatment. Moreover, the clozapine-associated anti-proliferative effects on islet cells were also attenuated at the end of 4 months of treatment. In a rodent phencyclidine (PCP)-induced hyperlocomotion model, adult response to clozapine treatment is attenuated by adolescent clozapine exposure [19] . Moreover, repeated administration of clozapine has been reported to induce tolerance to clozapine-associated disruption of avoidance response in rodents [20] . The mechanism that causes clozapine tolerance in disruptive avoidance response and PCP-induced hyperlocomotion is thought to be related with serotonin 5-HT 2A/2C expressed in medial prefrontal cortex or nucleus accumbens shell [21] . However, the mechanism by which the mice were spared the long-term effect of clozapine-associated glucagon dysregulation and the anti-proliferative property on islet beta cells in this study is not clear.
Our results also revealed that the mice treated with clozapine had less body weight gain, less gastrocnemius muscle mass, and less peri -renal fat pad mass compared to the control mice. In the mice treated with clozapine, the body weight loss did not result from a loss of appetite, because the daily chow intake per kilogram body weight did not differ among the four groups. The mechanism for the lower increase in body weight, skeletal muscle and visceral fat mass in the mice treated with clozapine is unknown. Smith et al. reported that 10 μM of clozapine, which is approximate 10 times higher than the therapeutic concentration, resulted in a marginal decrease in glucose uptake in 3T3L1 adipocytes in a rodent model but did not reduce glucose uptake in soleus muscles in vitro [7] . We estimate that the serum concentration of clozapine in the mice orally fed with 13.5 mg/kg body weight clozapine was about 1 μM, which was not sufficient to reduce blood glucose disposal through skeletal muscles and visceral adipocytes. Therefore, the mechanism of action for changes in the mass of skeletal muscles and visceral fat in the mice treated with clozapine was not likely due to a direct effect of clozapine on glucose uptake in skeletal muscles and visceral adipocytes in this study. Moreover, the clozapine-associated acute increase in serum glucose level was not due to a reduction in glucose uptake of skeletal muscles or visceral fat cells. However, it is not clear whether clozapine-associated changes in lean muscle and visceral fat are also related to central AMPK and/or M3Rs activities [15] and [16] .
In conclusion, clozapine treatment resulted in acute derangements in glucose metabolism, decreased proliferation and increased apoptosis of pancreatic islet cells, which resulted in a reduction in PIC. With the administration of a GLP-1 receptor agonist, exenatide, the clozapine-associated enhancement of apoptosis, inhibition of islet cell proliferation and loss of pancreatic beta cell mass were completely restored. Moreover, by the administration of exenatide, the acute increase in serum glucose level was totally reversed in the mice treated with clozapine, and this may exert an additional protective effect on the cardiovascular system in schizophrenic patients, who are at high risk of major cardiovascular events [22] , [23] and [24] . Our results provide preclinical evidence of the pharmaceutical role of GLP-1 receptor agonists in managing glucose dysregulation in schizophrenic patients under long-term atypical antipsychotic treatment.
Conflict of interest
The authors declare no conflict of interest.
Transparency document
Transparency Document.
Acknowledgement
This work was supported by a grant from the Ministry of Science and Technology of Taiwan (MOST102-2314-B-182-020 )
References
- [1] M. Smith, D. Hopkins, R.C. Peveler, R.I.G. Holt, M. Woodward, K. Ismail; First-v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis; Br. J. Pshchiatry, 192 (2008), pp. 406–411
- [2] C.E. Koro, D.O. Fedder, G.J. L’Italien, S.S. Weiss, L.S. Magder, J. Kreyenbuhl, D.A. Revicki, R.W. Buchanan; Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study; BMJ, 325 (2002), pp. 243–247
- [3] S. Park, S.M. Hong, I.S. Ahn, S.H. Kim; Olanzapine, not resperidone, exacerbates beta-cell function and mass in ovariectomized diabetic rats and estrogen replacement reverses them; J. Psychopharmacol., 24 (2010), pp. 1105–1114
- [4] M. Sohn, J. Talbert, K. Blumenschein, D.C. Moga; Atypical antipsychotic initiation and the risk of type II diabetes in children and adolescents; Pharmacoepidemicol. Drug Saf., 24 (2015), pp. 583–591
- [5] T. Argo, R. Carnahan, M. Barnett, T.L. Holman, P.J. Perry; Diabetes prevalence estimates in schizophrenia and risk factor assessment; Ann. Clin. Psychiatry, 23 (2011), pp. 117–124
- [6] K. Kawabe, S. Ochi, Y. Yoshino, Y. Mori, H. Onuma, H. Osawa, Y. Hosoda, S. Ueno; Metabolic status and resistin in chronic schizophrenia over a 2-year period with continuous atypical antipsychotics; Ther. Adv. Psychopharmacol., 5 (2015), pp. 271–277
- [7] G.C. Smith, C. Chaussade, M. Vickers, J. Jensen, P.R. Shepherd; Atypical antipsychotic drugs induce derangements in glucose homeostasis by acutely increasing glucagon secretion and hepatic glucose output in the rat; Diabetologia, 51 (2008), pp. 2309–2317
- [8] G.C. Smith, M.H. Vickers, E. Cognard, P.R. Shepherd; Clozapine and quetiapine acutely reduce glucagon-like peptide-1 production and increase glucagon release in obese rats: implications for glucose metabolism and food choice behaviour; Schizophr. Res., 115 (2009), pp. 30–40
- [9] A. Bolger, N. Verdolini, M. Agius; Can metabolic side effects of antipsychotics be reversed by lifestyle changes; Psychiatr. Danub., 26 (Suppl. 1) (2014), pp. 330–335
- [10] G.J. Remington, C. Teo, V. Wilson, A. Chintoh, M. Guenette, Z. Ahsan, A. Giacca, M.K. Hahn; Metformin attenuates olanzapine-induced hepatic, but not peripheral insulin resistance; J. Endocrinol., 227 (2015), pp. 71–81
- [11] S. Xue, C. Wasserfall, M. Parker, S. McGrail, K. McGrail, M. Campbell-Thompson, D.A. Schatz, M.A. Atkinson, M.J. Haller; Exendin-4 treatment of nonobese diabetic mice increases beta-cell proliferation and fractional insulin reactive area; J. Diabetes Complications, 24 (2010), pp. 163–167
- [12] J.Y. Kim, D.M. Lim, C.I. Moon, K.J. Jo, S.K. Lee, H.W. Baik, K.H. Lee, K.W. Lee, K.Y. Park, B.J. Kim; Exendin-4 protects oxidative stress-induced β-cell apoptosis through reduced JNK and GSK3β activity; J. Korean Med. Sci., 25 (2010), pp. 1626–1632
- [13] H. Hosokawa, Y.A. Hosokawa, J.L. Leahy; Upregulated hexokinase activity in isolated islets from diabetic 90% pancreatectomized rats; Diabetes, 44 (1995), pp. 1328–1333
- [14] M.M. El-Seweidy, N.A. Sadik, M.M. Malek, R.S. Amin; Chronic effects of clozapine administration on insulin resistance in rats: evidence for adverse metabolic effects; Pathol. Res. Pract., 210 (2014), pp. 5–9
- [15] K. Weston-Green, X.F. Huang, C. Deng; Second generation antipsychotic-induced type 2 diabetes: a role for the muscarinic M3 receptor; CNS Drugs, 27 (2013), pp. 1069–1080
- [16] M. Ikegami, H. Ikeda, Y. Ishikawa, M. Ohsawa, T. Ohashi, M. Kai, A. Kamei, J. Kamei; Olanzapine induces glucose intolerance through the activation of AMPK in the mouse hypothalamus; Eur. J. Pharmacol., 718 (2013), pp. 376–382
- [17] V. Contreras-Shannon, D.L. Heart, R.M. Paredes, E. Navaira, G. Catano, S.K. Maffi, C. Walss-Bass; Clozapine-induced mitochondria alterations and inflamantion in brain and insulin-responsive cells; PLoS One, 8 (2013), p. e59012
- [18] M. Capannolo, I. Fasciani, S. Romeo, G. Aloisi, M. Rossi, P. Bellio, G. Celenza, B. Cinque, M.G. Cifone, M. Scarselli, R. Maggio; The atypical antipsychotic clozapine selectively inhibits interleukin 8 (IL-8)-induced neutrophil chemotaxis; Eur. Neuropsychopharmacol., 25 (2015), pp. 413–424
- [19] S. Chou, C. Davis, S. Jones, M. Li; Repeated effects of the neurotensin receptor agonist PD149163 in three animal tests of antipsychotic activity: assessing for tolerance and cross-tolerance to clozapine; Pharmacol. Biochem. Behav., 128 (2015), pp. 78–88
- [20] Q. Shu, G. Hu, M. Li; Adult response to olanzapine or clozapine treatment is altered by adolescent antipsychotic exposure: a preclinical test in the phencyclidine hyperlocomotion model; J. Psychopharmacol., 28 (2014), pp. 363–375
- [21] M. Feng, J. Gao, N. Sui, M. Li; Effects of central activation of serotonin 5-HT2A/2C or dopamine D 2/3 receptors on the acute and repeated effects of clozapine in the conditioned avoidance response test; Psychopharmacology (Berl.), 232 (2015), pp. 1219–1230
- [22] D.G. Haider, F. Mittermayer, A. Friedl, A. Batrice, M. Auinger, M. Wolzt, W.H. Horl; Postprandial blood glucose level in maintenance hemodialysis patients predicts post-transplant-diabetes-mellitus; J. Exp. Clin. Endocrinol. Diabetes, 118 (2010), pp. 200–204
- [23] F. Chen, M. Sha, Y. Wang, T. Wu, W. Shan, J. Liu, W. Zhou, Y. Zhu, Y. Sun, Y. Shi, D. Bleich, X. Han; Transcription factor Ets-1 links glucotoxicity to pancreatic beta cell dysfunction through inhibiting PDX-1 expression in rodent models; Diabetologia, 59 (2016), pp. 316–324
- [24] S.M. Reddy, C.T. Goudie, M. Agius; The metabolic syndrome in untreated schizophrenia patients: prevalence and putative mechanisms; Psychiatr. Danub., 25 (Suppl. 2) (2013), pp. S94–98
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?