Keywords
Perfluorooctanoate (PFOA) ; Plasma cholesterol ; Plasma triglycerides ; Lipoproteins ; Lipoprotein metabolism ; non-HDL-C/HDL-C ; APOE*3-Leiden.CETP mice
A recent article in Toxicology Reports by Rebholz et al. [26] reported increased plasma cholesterol levels (primarily as HDL) in C57BL/6 (male and female) and BALB/c (male only) mice given high fat (Westernized) diet which also contained PFOA. The authors concluded that PFOA ingestion leads to hypercholesterolemia and they stated these data were “consistent with human observational findings”. There are several key factors which have not been considered in their study and we offer this letter to identify this important information. In particular, we feel that this conclusion needs more balance, particularly with respect to the relevance and translatability of their findings to the human situation.
1. Difference in lipoprotein metabolism and responsiveness to hypolipidemic drugs between humans and mice
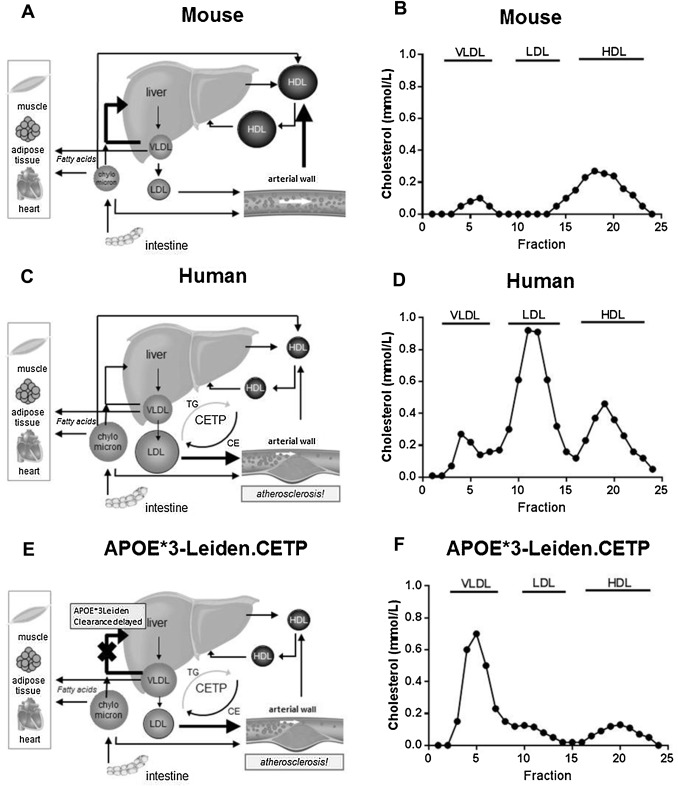
Conventional mouse strains used in preclinical biomedical and toxicological research, including the C57BL/6 mice and BALB/c mice studied by Rebholz et al., are considered not to be the most appropriate animal models to study modulation of lipoprotein metabolism, since lipolysis of TG-rich particles as chylomicrons and very low density lipoprotein (VLDL) and their remnants and clearance of the apoB-containing (non-HDL) lipoproteins via the apolipoproteinE-LDL-receptor (apoE-LDLR) pathway are fast processes as compared to humans [6] . Consequently, the mice have relatively low plasma TG and cholesterol levels with low levels of the atherogenic VLDL and LDL, and the majority of cholesterol is contained in HDL (Fig. 1 A,B). Severe dietary regimens with saturated fat and high amounts of cholesterol and cholic acid are required to increase the amount of non-HDL-C to some extent, but still lower than in humans [25] .
|
|
|
Fig. 1. Mice have a fast clearance of apoB-containing lipoproteins and do not express CETP (A), as a result the majority of plasma cholesterol is confined to HDL (B), with TC and TG levels of 1.5–2.0 and 0.2–0.3 mmol/L in C57BL/6 mice. Humans have a slower clearance of apoB-containing lipoproteins and do express CETP (C) and normolipidemic man have TC and TG levels of <5.2 and 0.5–1.5 mmol/L, respectively, and cholesterol consists mainly of non-HDL-C (VLDL-C/LDL-C) (D). The APOE*3-Leiden.CETP mouse has a lipoprotein profile similar as in FD patients and a lipoprotein metabolism similar to that in man (E), and on a chow diet TC and TG levels are 3.0–4.0 and 2.5–3.0 mmol/L, mainly confined to the non-HDL-C fraction (F). |
In contrast in humans, lipolysis is slower and removal of apoB-containing lipoproteins is delayed [6] . In addition, humans unlike mice possess an important player in lipoprotein metabolism, cholesterol ester transfer protein (CETP), which transfers cholesterol from HDL to (V)LDL in exchange for triglycerides, thereby increasing (V)LDL-C levels and decreasing HDL-C. Due to these differences, cholesterol is contained mainly in LDL and to a lesser extent in HDL (Fig. 1 C,D).
2. A translational mouse model that closely mimics human lipoprotein metabolism is available
To develop a mouse model with a more human-like lipoprotein metabolism for pharmacological and toxicological research, the APOE*3-Leiden transgenic mouse was generated by the introduction of a genomic human DNA construct carrying the mutant APOE*3-Leiden gene, the APOCI gene, and all known regulatory elements, obtained from a patient with Familial Dysbetalipoproteinemia (FD) [23] . These mice were cross-bred with mice expressing human CETP under control of its natural flanking regulatory DNA-sequences [12] to obtain the APOE*3-Leiden.CETP mouse, as a humanized model for FD and mixed dyslipoproteinemia [29] . While normal wild-type mice have a very rapid clearance of apoB-containing lipoproteins, APOE*3-Leiden(.CETP) mice have an impaired clearance and increased TG level, and are thereby mimicking the slow clearance observed in humans, particularly in patients with FD [6] , [28] and [15] . Importantly, as compared to the widely used hyperlipidemic and atherogenic apoE- and LDLR-deficient (E0/0 and LDLR0/0) mice, the APOE*3-Leiden(.CETP) mice possess an intact but delayed apoE-LDLR-mediated clearance, which is essential for the proper, human-like response on hypolipidemic drugs [1] . APOE*3-Leiden.CETP mice respond well to dietary intervention using human-relevant (Westernized) diets with increases in plasma cholesterol and TG and these lipids can be titrated to levels mimicking those in humans.
As background, FD or type III hyperlipoproteinemia is characterized by elevated levels of plasma cholesterol and an increased ratio of cholesterol to TG in the VLDL and intermediate-density lipoprotein (IDL) fractions, resulting in the appearance of β-VLDL particles ([15] ). Similarly as in FD patients, in APOE*3-Leiden and APOE*3-Leiden.CETP mice the major part of plasma cholesterol is contained in the VLDL and VLDL-remnant particles, leading to formation of β-VLDL particles, which is further increased by cholesterol feeding [23] and [29] (Fig. 1 E,F).
With respect to response to hypolidemic drugs and contrary to wildtype or E0/0 and LDLR0/0 mice and other species [17] , the APOE*3-Leiden(.CETP) mice respond in a similar way to statins as humans do, both with respect to direction and magnitude of the change with decreases in the apoB-containing lipoproteins up to 55% ([5] , [14] and [8] ). In addition, the extent of lipid-lowering and change in lipoprotein profile observed with fibrates ([16] , [10] and [9] ), niacin ([11] , [18] and [9] ) and the new anti-PCSK9 therapy with monoclonal antibodies ([19] and [1] ) in APOE*3Leiden(.CETP) mice is comparable to that of hyperlipidemic and FD patients.
3. Effect of PFOA on lipoprotein metabolism in relevant animal models and human clinical study
To explore the effects of PFOA on lipoprotein metabolism in this mouse model, we recently started to investigate the effects of environmentally and occupationally relevant PFOA exposure in male APOE*3-Leiden.CETP mice fed a Western-type diet. Our preliminary data showed that dietary intake of PFOA reduced plasma TG and cholesterol levels with the cholesterol reduction confined to the apoB-containing (non-HDL-C) lipoproteins.
In support of the translatability of the APOE*3-Leiden.CETP mouse for investigation of the mechanistic background of alterations in lipid and lipoprotein metabolism, the reduction in non-HDL-C found in our study as well as data reported by Loveless et al. [22] is consistent with results from a phase I trial with PFOA in humans. In that study, 41 cancer patients were given ammonium PFOA doses of up to 1200 mg/week for a median of 6.5 weeks, which resulted in a reduction of non-HDL-C as a treatment effect [24] .
4. Non-HDL-C/LDL-C-lowering versus HDL-C-raising on cardiovascular risk in clinical outcome studies and relevant animal models
The increase of plasma cholesterol reported by Rebholz et al., was confined to HDL-C, whereas we and others found a decrease in (V)LDL-C (non-HDL-C) after PFOA exposure. The question then arises how these alterations in lipoprotein metabolism translate into cardiovascular risk. LDL-C is recognized as a primary causal risk factor in cardiovascular disease as evidenced from many experimental, epidemiological and genetic studies [27] . In addition, intervention trials with statin therapy confirm a reduced incidence of coronary heart disease as a consequence of cholesterol-lowering in LDL [2] and [21] and recent trials indicate that intensive lipid-lowering with statins may be more beneficial in risk reduction than less intensive (or standard) therapy [3] . According to results from the latter meta-analysis, every 1 mmol/L (40 mg/dL) reduction in LDL-C is associated with a 22% reduction in the risk of major vascular events suggesting that a 2–3 mmol/L reduction in LDL-C would correspond with a 40–50% reduction in events.
Epidemiological studies consistently report an inverse association between coronary heart disease risk and HDL-C: Results from 4 prospective epidemiologic studies indicate that an increase of 1 mg/dL (0.03 mM) in HDL-C is associated with a 2–3% reduction in risk [7] .
Besides its major role in reverse cholesterol transport, HDL has also been described to have anti-inflammatory, anti-oxidant, anti-platelet and vasodilatory properties and may therefore have a protective role in coronary heart disease [13] . Several therapeutic approaches aimed at raising HDL-C levels have since been investigated. However, undisputed proof for causality of low HDL-C in cardiovascular disease is lacking and clinical trials aimed at raising HDL-C to prevent disease (AIM-HIGH, HPS2-THRIVE, dal-OUTCOMES) have failed to meet their primary goals [13] . In addition, data from Mendelian randomization studies show that HDL-C and myocardial infarction risk are not causally related [13] .
For a systematic review and meta-analysis of relevant preclinical studies and clinical trials on the contribution of non-HDL-C/LDL-C-lowering versus HDL-C-raising we refer to Kühnast et al. [20] . In this review we investigated the effects of established and novel treatment strategies, specifically targeting HDL, on inhibition of atherosclerosis development in CETP-expressing animals, since CETP is a crucial gene involved in HDL-C metabolism and implicated in the mechanisms by which most therapies modulate HDL-C [4] . In addition, we conducted a meta-analysis to evaluate the potential effects of these treatment strategies on the prevention of clinical events in randomized controlled trials, focusing specifically on the contribution of non-HDL-C/LDL-C-lowering versus HDL-C-raising. We conclude that the protective role of lowering LDL-C and non-HDL-C is well-established. The contribution of raising HDL-C on inhibition of atherosclerosis and the prevention of cardiovascular disease remains undefined and may be dependent on the mode of action of HDL-C-modification. Similar outcome data were found for the prevention of clinical events in randomized controlled trials and on inhibition of atherosclerosis in relevant, CETP-expressing, animals emphasizing the validity/translatability of these animal models to the human situation [20] .
5. Concluding remarks
Rebholz et al. conclude “To summarize, we have shown that C57BL/6 and male BALB/c mice exposed to PFOA have significantly increased plasma cholesterol levels when fed Westernized diets, consistent with human observational findings at the national [refs 39,14 therein] and community levels [refs 16,19,60 therein] and in adolescent [refs 18,20 therein] and occupational [refs 44,56,57,9 therein] cohorts”. As discussed above, there are a number of issues with the outcome of their study and extrapolation of the data from a study in wildtype mice to the human situation. The choice of the mouse model should be carefully considered, given its inherent differences in lipoprotein metabolism and distribution of cholesterol among lipoproteins between wildtype mice and man, as well as the differences in changes in lipoproteins induced by PFOA and the potential cardiovascular risk raised by these changes. For all the reasons articulated above we felt it is premature for Rebholz et al. to conclude that PFOA causes hypercholesterolemia in these mice fed Westernized diets.
Funding
This work was funded in part by an unrestricted educational grant from 3M Corporation to TNO-Metabolic Health Research but represents an independent opinion of the authors.
Conflict of interest
Hans M.G. Princen and Elsbet J. Pieterman report grants from 3M Company during the conduct of the study and outside the submitted work. Marianne G. Pouwer has nothing to disclose.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [1] B. Ason, J.W.A. van der Hoorn, J. Chan, E. Lee, E.J. Pieterman, K. Khanh Nguyen, M. Di, S. Shetterly, J. Tang, W.C. Yeh, M. Schwarz, J.W. Jukema, R. Scott, S.M. Wasserman, H.M.G. Princen, S. Jackson; PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol and atherosclerosis in the absence of ApoE; J. Lipid Res., 55 (2014), pp. 2370–2379
- [2] C. Baigent, A. Keech, P.M. Kearney, L. Blackwell, G. Buck, C. Pollicino, A. Kirby, T. Sourjina, R. Peto, R. Collins, R. Simes, Cholesterol Treatment Trialists’ Collaboration; Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins; Lancet, 366 (2005), pp. 1267–1278
- [3] C. Baigent, L. Blackwell, J. Emberson, L.E. Holland, C. Reit, N. Bhala, R. Peto, E.H. Barnes, A. Keech, J. Simes, R. Collins, Cholesterol Treatment Trialists’ Collaboration; Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials; Lancet, 376 (2010), pp. 1670–1681
- [4] M.J. Chapman, W. Le Goff, M. Guerin, A. Kontush; Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins fibrates, niacin, and cholesteryl ester transfer protein inhibitors; Eur. Heart J., 31 (2010), pp. 149–164
- [5] D.J.M. Delsing, J.W. Jukema, M. van de Wiel, J.J. Emeis, A. van der Laarse, L.M. Havekes, H.M.G. Princen; Differential effects of amlodipine and atorvastatin treatment and their combination on atherosclerosis in ApoE*3-Leiden transgenic mice; J. Cardiovasc. Pharmacol., 42 (2003), pp. 63–70
- [6] J.M. Dietschy, S.D. Turley, D.K. Spady; Role of liver in the maintenance of cholesterol and low-density lipoprotein homeostasis in different animal species including humans; J. Lipid Res., 34 (1993), pp. 1637–1659
- [7] D.J. Gordon, J.L. Probstfield, R.J. Garrison, J.D. Neaton, W.P. Castelli, J.D. Knoke, D.R. Jacobs, S. Bangdiwala, H.A. Tyroler; High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies; Circulation, 79 (1989), pp. 8–15
- [8] W. De Haan, J. de Vries-van der Weij, J.W.A. van der Hoorn, T. Gautier, C.C. van der Hoogt, M. Westerterp, J.A. Romijn, J.W. Jukema, L.M. Havekes, H.M.G. Princen, P.C.N. Rensen; Torcetrapib does not reduce atherosclerosis beyond atorvastatin and induces more proinflammatory lesions than atorvastatin; Circulation, 117 (2008), pp. 2515–2522
- [9] A.M. Van den Hoek, J.W.A. van der Hoorn, A.C. Maas, R.M. van den Hoogen, A. van Nieuwkoop, S. Droog, E.H. Offerman, E.J. Pieterman, L.M. Havekes, H.M.G. Princen; APOE*3Leiden.CETP transgenic mice as model for pharmaceutical treatment of the metabolic syndrome; Diabetes Obes. Metab., 16 (2014), pp. 537–544
- [10] C.C. Van der Hoogt, W. de Haan, M. Westerterp, M. Hoekstra, G.M. Dallinga-Thie, J.A. Romijn, H.M.G. Princen, J.W. Jukema, L.M. Havekes, P.C.N. Rensen; Fenofibrate increases HDL-cholesterol by reducing cholesteryl ester transfer protein expression; J. Lipid Res., 48 (2007), pp. 1763–1771
- [11] J.W.A. Van der Hoorn, W. de Haan, J.F.P. Berbée, L.M. Havekes, J.W. Jukema, P.C.N. Rensen, H.M.G. Princen; Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice; Arterioscler. Thromb. Vasc. Biol., 28 (2008), pp. 2016–2022
- [12] X.C. Jiang, L.B. Agellon, A. Walsh, J.L. Breshlow, A. Tall; Dietary-cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice- dependence on natural flanking sequences; J. Clin. Invest., 90 (1992), pp. 1290–1295
- [13] B.A. Kingwell, M.J. Chapman, A. Kontush, N.E. Miller; HDL-targeted therapies: progress: failures and future; Nat. Rev. Drug Discov., 13 (2014), pp. 445–464
- [14] R. Kleemann, H.M.G. Princen, J.J. Emeis, J.W. Jukema, R.D. Fontijn, A.J.G. Horrevoets, T. Kooistra, L.M. Havekes; Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice; Circulation, 108 (2003), pp. 1368–1374
- [15] P. De Knijff, A.M. van den Maagdenberg, A.F. Stalenhoef, J.A. Gevers Leuven, P.N. Demacker, L.P. Kuyt, R.R. Frants, L.M. Havekes; Familial dysbetalipoproteinemia associated with apolipoprotein E3-Leiden in an extended multigeneration pedigree; J. Clin. Invest., 88 (1991), pp. 643–655
- [16] T. Kooistra, L. Verschuren, J. de Vries-van der Weij, W. Koenig, K. Toet, H.M.G. Princen, R. Kleemann; Fenofibrate reduces atherogenesis in ApoE*3Leiden mice: evidence for multiple antiatherogenic effects besides lowering plasma cholesterol; Arterioscler. Thromb. Vasc. Biol., 26 (2006), pp. 2322–2330
- [17] B.R. Krause, H.M.G. Princen; Lack of predictability of classical animal models for hypolipidemic activity: a good time for mice?; Atherosclerosis, 140 (1998), pp. 15–24
- [18] S. Kühnast, M.C. Louwe, M.M. Heemskerk, E.J. Pieterman, J.B. van Klinken, S.A. van den Berg, J.W. Smit, L.M. Havekes, P.C. Rensen, J.W. van der Hoorn, H.M.G. Princen, J.W. Jukema; Niacin reduces atherosclerosis development in APOE*3Leiden.CETP mice mainly by reducing nonHDL-cholesterol; PLoS One, 8 (6) (2013), p. e66467
- [19] S. Kühnast, J.W. van der Hoorn, E.J. Pieterman, A.M. van den Hoek, W.J. Sasiela, V. Gusarova, A. Peyman, H.L. Schäfer, J.W. Jukema, H.M.G. Princen; Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin; J. Lipid Res., 55 (2014), pp. 2103–2112
- [20] S. Kühnast, M. Fiocco, J.W.A. van der Hoorn, H.M.G. Princen, J.W. Jukema; Innovative pharmaceutical interventions in cardiovascular disease: focusing on the contribution of non-HDL-C/LDL-C-lowering versus HDL-C-raising. A systematic review and meta-analysis of relevant preclinical studies and clinical trials; Eur. J. Pharmacol., 763 (2015), pp. 48–63
- [21] S. Lewington, G. Whitlock, R. Clarke, P. Sherliker, J. Emberson, J. Halsey, N. Qizilbash, R. Peto, R. Collins, Prospective Studies Collaboration; Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths; Lancet, 370 (2007), pp. 1829–1839
- [22] S.E. Loveless, C. Finlay, N.E. Everds, S.R. Frame, P.J. Gilles, J.C. OConnor, C.R. Powley, G.L. Kennedy; Comparative responses of rats and mice exposed to linear/branched linear, or branched ammonium perfluorooctanoate (APFO); Toxicology, 220 (2006), pp. 203–217
- [23] A.M. Van den Maagdenberg, M.H. Hofker, P.J. Krimpenfort, I. de Bruijn, B. van Vlijmen, H. van der Boom, L.M. Havekes, R.R. Frants; Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia; J. Biol. Chem., 268 (1993), pp. 10540–105451
- [24] I.R. MacPherson, D. Bissett, R.D. Petty, B. Tait, L.M. Samuel, J. MacDonals, M. Smith, J.A. Birse-Archbold, A.L. Barnett, C.R. Wolf, C.R. Elcombe, A. Jeynes-Ellis, T.R.J. Evans; A first-in-human phase I clinical trial of CXR1002 in patients with advanced cancer; J. Clin. Oncol. (2011), p. 29
- [25] P.M. Nishina, S. Lowe, J. Verstuyft, J.K. Naggert, F.A. Kuypers, B. Paigen; Effects of dietary fats from animal and plant sources on diet-induced fatty streak lesions in C57BL/6J mice; J. Lipid Res., 34 (1993), pp. 1413–1422
- [26] S.L. Rebholz, T. Jones, R.L. Herrick, C. Xie, A.M. Calafat, S.M. Pinney, L.A. Woollett; Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice; Toxicol. Rep., 3 (2016), pp. 46–54
- [27] P.M. Ridker; LDL cholesterol: controversies and future therapeutic directions; Lancet, 384 (2014), pp. 607–617
- [28] M.R. Wardell, K.H. Weisgraber, L.M. Havekes, S.C. Rall; Apolipoprotein E3-Leiden contains a seven-amino acid insertion that is a tandem repeat of residues 121–127; J. Biol. Chem., 264 (1989), pp. 21205–21210
- [29] M. Westerterp, C.C. van der Hoogt, W. de Haan, E.H. Offerman, G.M. Dallinga-Thie, W. Jukema, L.M. Havekes, P.C.N. Rensen; Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice; Arterioscler. Thromb. Vasc. Biol., 26 (2006), pp. 2552–2559
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?