Abstract
Feline small cell lymphoma is associated with greater response to treatment and survival when compared to large cell lymphoma. Treatment-associated toxicity, response to rescue chemotherapy and prognostic factors are largely unknown. This retrospective study was performed to identify treatment-associated toxicity, response to rescue chemotherapy and treatment outcome for cats diagnosed with small cell lymphoma of various anatomic locations. Medical records from 56 cats were evaluated. All cats were treated with glucocorticoid and chlorambucil with discontinuation of treatment recommended at 1 year if complete clinical response was documented. Chemotherapy toxicity was uncommon (33.9%) and generally mild. Grade III or IV hepatotoxicity was documented in 10.7% of patients. Overall response rate was 85.7% with glucocorticoid and chlorambucil. Median progression-free survival was 1078 days. Overall response rate for rescue chemotherapy was 59%. Reintroduction of prednisone and chlorambucil was associated with significantly longer survival than prednisone and lomustine (>1500 vs. 492 days, P = 0.01). Median overall survival times for cats with lymphoma of the gastrointestinal tract was not significantly different from those with extra-intestinal disease locations (1148 vs. 1375 days, P = 0.23). Median overall survival was 1317 days. Toxicity, other than hepatotoxicity was mild. Rescue chemotherapy with re-introduction of glucocorticoids and chlorambucil was most successful. Discontinuation of glucocorticoid and chlorambucil with subsequent reintroduction as rescue chemotherapy appears to be just as effective as continued administration in cats.
Introduction
Lymphoma is the most common feline malignancy, comprising up to one-third of all cancers in cats (Teske et al. 2002). Since the initiation of programs to control feline leukaemia virus (FeLV), the alimentary tract has become the most common site of lymphoma in our patients (Carreras et al. 2003; Louwerens et al. 2005; Kiselow et al. 2008; Lingard et al. 2009; Pohlman et al. 2009). A lymphoma classification system developed by the National Cancer Institute Working Formulation (NCIWF) and the Revised European-American Lymphoma/World Health Organization (REAL/WHO) for use in humans has been evaluated and found to be applicable for use in cats (Valli et al. 2000; Barrs & Beatty 2012). Based on histological assessment, lymphoma can be classified as high, intermediate or low grade (Waly et al. 2005). Using the NCIWF scheme, the majority of diagnosed lymphomas are classified as intermediate (35%) or high grade (54%) on histological assessment; however, approximately 10% of all lymphomas are composed of small, well differentiated neoplastic lymphocytes and are classified as low grade (Valli et al. 2000; Milner et al. 2005; Kiselow et al. 2008). This reported prevalence is not well established throughout the general feline population and may not represent the actual frequency of these varying grades of lymphoma (Russell et al. 2012) Recent studies suggest that low grade lymphomas represent a much higher proportion than previously identified, with prevalence rates as high as 37–75% (Fondacaro et al. 1999; Pohlman et al. 2009; Moore et al. 2012). The existing literature is not standardized in techniques used to diagnose lymphoma; therefore the relative prevalence of low grade lymphomas may be affected by the predominance of cytological or histological samples included in prior reports. It is well established, however, that these low grade, small cell lymphomas are associated with greater response to treatment, disease-free interval and overall survival time when compared with large cell lymphoma (Fondacaro et al. 1999; Kiselow et al. 2008; Lingard et al. 2009; Stein et al. 2010). Standard treatment includes chlorambucil and glucocorticoids. Median survival times ranging from 447 to 967 days have been reported for cats treated with this protocol, documenting response rates between 69–96% and median remission durations ranging from 567 to 897 days for those that respond (Fondacaro et al. 1999; Kiselow et al. 2008; Lingard et al. 2009; Stein et al. 2010).
Although clinicopathological findings and response to treatment have been previously described and are well established (Carreras et al. 2003; Kiselow et al. 2008; Lingard et al. 2009), limited literature exists regarding ability to discontinue chemotherapy, effectiveness of rescue chemotherapy, or the treatment outcome associated with extra-intestinal disease location. At the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania as well as the Hope Advanced Veterinary Center, patients diagnosed with small cell lymphoma and treated with standard of care glucocorticoid and chlorambucil are discontinued from therapy at 1 year if determined to have had a complete clinical response at the time of restaging. This modified treatment protocol has not previously been reported in the literature, as continued administration is otherwise the standard of care (Fondacaro et al. 1999; Kiselow et al. 2008; Lingard et al. 2009; Stein et al. 2010).
Previously, rare treatment associated toxicities have been described, including vomiting, diarrhoea, anorexia, lethargy and leucopenia (Fondacaro et al. 1999). More recent literature also reports similar, rare adverse events including mild self-limiting gastrointestinal signs and myelosuppression (Lingard et al. 2009). Treatment delays as the result of thrombocytopenia and neutropenia have also been described; however, all of the reported myelosuppression resolved following delay (Stein et al. 2010). Idiosyncratic liver enzyme elevations have also been reported in human patients receiving chronic chlorambucil chemotherapy (Chabner & Longo 2006), however, to the authors knowledge, no other chemotherapy-related toxicities have previously been reported in cats.
Rescue chemotherapy has rarely been described for the treatment of feline small cell lymphoma. Cats receiving rescue chemotherapy with cyclophosphamide following treatment with chlorambucil and prednisone were reported to have significantly longer disease-free intervals (24 vs. 16.3 months) and survival times (29 vs. 18.8 months) than cats that did not receive rescue chemotherapy with cyclophosphamide (Fondacaro et al. 1999). A more recent report found an overall response rate of 100% for cyclophosphamide as a rescue agent with median remission duration of 241 days (Stein et al. 2010). Rescue chemotherapy with doxorubicin has been evaluated in feline lymphoma (Oberthaler et al. 2009), but low overall response rates (22%) and complete remission rates (9%) proved this agent ineffective in the rescue setting. However, cell size was determined to be predictive of response to treatment in this study and all small to intermediate cell lymphoma patients (n = 3) were documented to respond to rescue treatment with doxorubicin (complete remission (CR) = 1, partial remission (PR) = 2). No exclusively small cell lymphoma cases were included in this study. Lomustine was found to markedly improve the median progression-free interval for cats with small to intermediate cell lymphoma over cats with large cell lymphoma (169 vs. 39 days) (Dutelle et al. 2012). However, no studies have compared lomustine to cyclophosphamide or to reintroduction of chlorambucil and glucocorticoid to evaluate the effectiveness of these various rescue protocols in cats with small cell lymphoma. To the authors knowledge, the standardized discontinuation of glucocorticoid and chlorambucil at a recommended time point following documentation of disease remission as well as the reintroduction as a first line rescue protocol has also not yet been reported.
Therefore, the purpose of this study was to document treatment-associated toxicity, treatment outcome of extraintestinal-disease locations and response to rescue chemotherapy, specifically reintroduction glucocorticoid and chlorambucil, for cats diagnosed with small cell lymphoma of various anatomic locations treated with standard of care chemotherapy.
Methods
Medical records from the Matthew J. Ryan Veterinary Hospital at the University of Pennsylvania in Philadelphia, Pennsylvania (MJR-VHUP) as well as the Hope Advanced Veterinary Center in Vienna, Virginia (HAVC) were searched for cases of confirmed small cell lymphoma from January 2000 to January 2010. Confirmation of diagnosis was determined by histopathology consistent with small cell lymphoma. Diagnosis through cytology was acceptable in three cases with the addition of polymerase chain reaction for antigen receptor rearrangement (PARR) or flow cytometry for confirmation. Additional inclusion criteria involved complete medical records and treatment with standard chemotherapy protocol. All cats were required to receive a glucocorticoid and chlorambucil as a first line chemotherapy protocol. A discontinuation of the treatment protocol was recommended at 1 year after initiation of chemotherapy if restaging diagnostics were consistent with a complete clinical response, following standard protocol for feline small cell lymphoma at MJR-VHUP and HAVC. Absence of presenting clinical signs as well as resolution of abnormal physical exam findings (i.e. thickened intestinal segments, enlarged peripheral lymph nodes) and abdominal ultrasound results (i.e. intestinal wall thickening, enlarged mesenteric lymph nodes) when available, determined if complete clinical response was achieved at restaging. Patients determined as not having a complete clinical response at 1 year restaging continued on chemotherapy.
Cats with confirmed intermediate or large cell lymphoma concurrently with a diagnosis of small cell lymphoma were excluded, as were cats with a concurrent diagnosis of other neoplasia. Information collected from medical records included signallment, clinical signs related to the diagnosis of lymphoma, duration of signs, treatments prior to diagnosis, staging results, biopsy description, PARR or flow results, cytological description, anatomic sites affected, initial chemotherapy protocol, response and duration of treatment, chemotherapy-related toxicity, type of rescue chemotherapy, response to rescue agents and outcome. All toxicities that occurred during the original treatment protocol were recorded and then divided into gastrointestinal, haematopoietic or hepatic groupings. Toxicities were graded according to previously published and accepted criteria for adverse events (VCOG-CTCAE) (Vail 2004). Results of post-mortem examination were recorded when available.
Disease was defined as gastrointestinal in location if pathology confirmed small cell lymphoma within the stomach, small intestines or large intestines on any sample from an individual patient. Disease was only defined as extra-intestinal if pathology samples confirmed small cell lymphoma in any other anatomic site outside of the gastrointestinal tract without concurrent disease present within the stomach, small intestines, or large intestines. Board-certified pathologists at the University of Pennsylvania as well as outside laboratories, interpreted submitted tissue samples.
Response to therapy was defined as complete clinical response (100% resolution of clinical signs for ≥30 days), partial clinical response (≥50% but <100% resolution of clinical signs for ≥30 days) or non-responder (<50% improvement of clinical signs or response of <30 days), utilizing the same previously established parameters (Kiselow et al. 2008) based on clinician assessment of clinical signs. Response to therapy at 30 and 90 days post initiation of treatment as well as at completion of the recommended chemotherapy protocol was recorded. Overall survival time was calculated from the time of diagnosis to the time of death. Progression-free survival was calculated from the time of diagnosis to the date of documented progression of disease or death. Disease progression was documented with return of presenting clinical signs and concurrent abnormal physical exam findings (i.e. thickened intestinal segments, enlarged peripheral lymph nodes) and diagnostics (i.e. abdominal ultrasound, aspirates) when available. Rescue specific survival for patients receiving rescue therapy was defined as the time from initiation of any rescue protocol to the time of death due to any cause. Cats still alive or lost to follow-up at the time of statistical analysis were censored from analysis.
Kaplan–Meier product-limit method was used to estimate overall survival, progression-free survival and rescue specific survival. The Log Rank test was used to compare survival between cats with gastrointestinal and extra-intestinal disease locations as well as rescue specific survival among the different rescue chemotherapy protocols used. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed by Stat13 computer software (College Station, TX, USA).
Results
Fifty-six cats were identified with confirmed small cell lymphoma during the study period (Table 1). Six of these cases were identified at HAVC with the remainder identified through MJR-VHUP.
| Variable | Category | n | % |
|---|---|---|---|
| Breed | Domestic short-haired | 47 | 83.9 |
| Domestic long-haired | 4 | 7.1 | |
| Maine coon | 2 | 3.6 | |
| Domestic medium-haired | 1 | 1.8 | |
| Siamese | 1 | 1.8 | |
| Ocicat | 1 | 1.8 | |
| Gender | Male castrated | 32 | 57.1 |
| Male intact | 2 | 3.6 | |
| Female spayed | 22 | 39.3 | |
| Median age | 12.3 years (3.8–16.5) | ||
| Median weight | 4.78 kg (2.6–10.0) | ||
| Location | Gastrointestinal tract only | 37 | 66.1 |
| Gastrointestinal tract, other sites | 12 | 21.4 | |
| Liver only | 4 | 7.1 | |
| Other* | 3 | 5.4 | |
| Organ involvement | Small intestines | 63 | 69.2 |
| Liver | 11 | 12.1 | |
| Lymph nodes | 9 | 9.9 | |
| Stomach | 6 | 6.6 | |
| Spleen | 1 | 1.1 | |
| Paw | 1 | 1.1 | |
| Initial presenting complaints | Vomiting | 27 | 24.3 |
| Weight loss | 24 | 21.6 | |
| Anorexia | 22 | 19.8 | |
| Diarrhoea | 12 | 10.8 | |
| Lethargy | 10 | 9.0 | |
| Elevated liver enzymes | 6 | 5.4 | |
| Haematomesis/haematochezia | 3 | 2.7 | |
| other† | 7 | 6.3 | |
| Supportive medications prior to diagnosis | Oral antibiotics (other than metronidazole) | 18 | 21.8 |
| Metronidazole | 18 | 21.8 | |
| Diet trial | 9 | 10.8 | |
| Prednisone or budesonide | 7 | 8.4 | |
| Famotidine or sucralfate | 6 | 7.2 | |
| Cobalamin injection | 4 | 4.8 | |
| Ursodiol or SAMe | 4 | 4.8 | |
| Appetite stimulant | 4 | 4.8 | |
| Deworming agent | 3 | 3.6 | |
| Lactulose | 3 | 3.6 | |
| Maropitant | 2 | 2.4 | |
| Injectable steroid | 2 | 2.4 | |
| Tylosin | 1 | 1.2 | |
| Diphenhydramine | 1 | 1.2 | |
| Subcutaneous fluids | 1 | 1.2 | |
Patient demographics for 56 patients included in the study. *Other sites included paw of thoracic limb, lymph nodes only, spleen only. †Other presenting complaints included increased appetite, pancreatitis, leucocytosis, polyuria/polydipsia, swollen paw, enlarged lymph nodes and abdominal mass. | |||
The most common initial presenting complaints included vomiting [n = 27 (24.3%)], weight loss (n = 24 (21.6%)] and anorexia (n = 22 (19.8%)]. Forty-five cats (80.3%) were reported to have had a chronic history of clinical signs and 33 cats (58.9%) received some type of supportive treatment prior to diagnosis. The most common supportive medications included oral antibiotics other than metronidazole (n = 18), metronidazole (n = 18) or a diet trial (n = 9). Nine patients received steroids prior to diagnosis (prednisone or budesonide: n = 7, injectable steroid: n = 2). Twenty-one cats (37.5%) had more than one clinical sign at the time of diagnosis and 22 (39.2%) received more than one supportive treatment prior to diagnosis (Table 1).
Results of complete blood count and serum biochemistry analysis were available for 53 (94.6%) cats. Forty-one (73.2%) cats had results of urinalysis available for evaluation. Results of abdominal ultrasound [n = 52 (92.8%)], chest radiographs [n = 42 (75.0%)], feline leukemia virus (FeLV) (n = 23 (41.1%)] and feline immunodeficiency virus (FIV) [n = 22 (39.3%)] testing were also available for most patients. Other common diagnostic tests performed at the time of diagnosis included serum total T4 concentration [n = 34 (61.0%)], feline pancreatic lipase immunoreactivity [n = 16 (28.6%)], serum cobalamin concentration [n = 7 (12.5%)], serum folate concentration [n = 6 (10.7%)] and serum trypsin-like immunoreactivity [n = 4 (7.1%)].
The most common haematological abnormalities included a mature neutrophilia (19.4%) and anaemia (12.9%). Common serum chemistry abnormalities included elevated liver enzymes (AST 32.0%, ALT 24.5%, ALP 18.9%, TBili 9.4%) as well as elevated BUN (18.9%) and creatinine (13.2%). Serum total T4 concentrations were normal in 28 of 35 (80.0%) patients and serum cobalamin was decreased in 3 of 8 (37.5%) patients. FeLV and FIV testing was negative in all cats. Ultrasonographic findings included thickened intestines [n = 37 (71.2%)], enlarged mesenteric lymph nodes [n = 30 (57.7%)], enlarged spleen or liver [n = 21 (40.4%)], prominent pancreas [n = 19 (36.5%)] and splenic or liver nodules [n = 15 (28.8%)]. A measureable intestinal mass was identified in 3 (5.8%) patients and peritoneal effusion was evident in 10 (19.2%) cats.
Histopathological diagnosis of lymphoma was available in 53 (94.6%) cases. Biopsy samples included full thickness (n = 32), endoscopic (n = 19) or needle biopsy specimens (n = 2). Tissues sampled for histopathology included the jejunum, duodenum, ileum, stomach, lymph nodes, large intestines, liver and spleen. The liver was the only organ sampled by needle biopsy technique. The remaining three (5.4%) cases had a combination of cytology and confirmatory testing for diagnosis. PARR (n = 2) and flow cytometry (n = 1) were used to confirm the cytological suspicion of small cell lymphoma in three cases. In the first case, aspirates of a forelimb paw swelling revealed a monomorphic population of small lymphocytes, described as lymphocytic infiltrate. PARR analysis was performed on the cytology sample and confirmed a clonal B-cell population, consistent with lymphoma. In the second case, aspirates from mandibular and popliteal lymph nodes revealed lymphoid hyperplasia, with a concerning population of monomorphic small lymphocytes. Further testing with PARR confirmed a clonal T-cell population, consistent with lymphoma. Aspirates performed on the liver, spleen and a mesenteric lymph node in the third patient revealed increased numbers of small lymphocytes, similar in appearance to those evaluated on peripheral lymphocytosis (18 740 lymphocytes μL−1 at the time of diagnosis). Flow cytometry performed on peripheral blood confirmed a marked expansion of CD4 positive T-cells, providing enough supportive evidence for initiation of treatment for small cell lymphoma. Forty-nine patients (87.5%) were therefore categorized as having lymphoma located within the gastrointestinal tract. Seven patients (12.5%) were categorized with purely extra-intestinal lymphoma, based on the results of pathology samples and grouping descriptions listed above.
Thirty-eight patients (66.6%) had available dosing information, whereas the dosages from 19 patients were unable to be recorded due to inconsistent reporting. Twenty-eight (75.7%) of 37 patients with available steroid dosing information received prednisone or prednisolone at a dose of 5 mg PO every 24 h. Three cats (8.1%) received budesonide at 1 mg PO every 24 h and two cats (5.4%) received prednisone or prednisolone at a dose of 10 mg PO every 24 h. The remaining four cats received varying doses of prednisone or prednisolone [5 mg PO every 12 h (n = 1), 5 mg PO every 48 h (n = 1), 2.5 mg PO every 12 h (n = 1), 7.5 mg PO every 24 h (n = 1)]. Twenty-one (56.8%) of 37 patients with available chemotherapy dosing information received chlorambucil at a dose of 2 mg PO every other day. Nine (24.3%) cats received a dose of 2 mg PO Monday, Wednesday, Friday and 6 (16.2%) cats at a dose of 2 mg PO every 72 h. One cat received 2 mg PO every 24 h. Median duration of treatment was 236 days (mean 331 days) with a range of 7–1111 days.
Toxicity was uncommon and generally mild, with 19 cats (33.9%) experiencing adverse events related to chemotherapy. A total of 25 events were documented, most commonly low-grade (Grade I and II in 72.7%, Grade III in 27.3%) myelosuppression in 44.0% of patients (Table 2). Fifty-two percent of the adverse events required treatment delays to address the toxicity, whereas 32.0% required discontinuation of prednisone and chlorambucil and subsequent initiation of a different chemotherapy protocol to continue treating their disease. Four percent required a dose reduction for resolution of toxicity and 4% were documented to have complete resolution without any specific therapy. The remaining adverse events (8.0%) required only supportive care for resolution of toxicity.
| Organ of toxicity | Adverse events | Percentage total adverse events (%) | VCOG Grade I II III IV V |
|---|---|---|---|
| Bone marrow | 11 | 44.0 | 4 4 3 0 0 |
| Gastrointestinal | 7 | 28.0 | 3 4 0 0 0 |
| Liver | 7 | 28.0 | 0 0 1 6 0 |
| Adverse events documented for chemotherapy-related toxicity. Standard VECOG-CTCAE criteria was used to grade and report adverse events. Twenty-five total events were observed in 19 total patients. Gastrointestinal toxicity and myelosuppression were mild, whereas heptatotoxicity was more severe. | |||
Moderate to severe elevations in liver enzymes were documented in six (10.7%) patients receiving chemotherapy. Grade IV hepatotoxicity occurred in all of these patients with one patient experiencing both Grade III and Grade IV hepatotoxicity. Only one patient had hepatic involvement of lymphoma based upon the results of liver biopsies. Liver enzymes were elevated in this patient at the time of diagnosis [alanine aminotransferase 2035 U L−1 (33–152), alkaline phosphatase 678 U L−1 (22–87), total bilirubin 1.0 mg dL−1 (0.1–0.8)] but showed initial improvement 142 days after treatment with prednisone and chlorambucil [ALT 629 U L−1 (33–152), ALP 171 U L−1 (22–87), total bilirubin 0.7 mg dL−1 (0.1–0.8)]. Recurrence of the elevated liver enzymes was noted 192 days into treatment with chlorambucil chemotherapy [ALT 1120 U L−1 (33–152), ALP 200 U L−1 (22–87), total bilirubin 0.6 mg dL−1 (0.1–0.8)], however, these elevations improved after discontinuation of chlorambucil [ALT 590 U L−1 (33–152), ALP 221 U L−1 (22–87), total bilirubin 0.6 (0.1–0.8)]. The other five patients had disease localized to the gastrointestinal tract (n = 4) without hepatic involvement, or confined to the paw of the thoracic limb (n = 1). Of these patients, three had mildly elevated liver enzymes at the time of diagnosis [≤ALT 207 U L−1 (33–152), ≤AST 84 U L−1 (1–37), ≤ALP 139 U L−1 (22–87)] and all went on to have liver aspirates performed prior to initiation of therapy. Aspirates were most consistent with cholangitis in two cases and vacuolar change in the last. Only one of these patients went on to have biopsies performed on the affected liver, revealing cholangitis, with no evidence of lymphoma. The patient with documented vacuolar change at the time of diagnosis experienced both the Grade III [AST 86 U L−1 (1–37)] and Grade IV [ALT 313 U L−1 (33–152)] hepatotoxicity, which resolved without treatment delay or discontinuation of therapy (no treatment initiated following documentation of toxicity). The remaining five patients who experienced Grade IV hepatotoxicity required discontinuation of chlorambucil, which ultimately lead to resolution of liver enzyme elevation. Average time to resolution for the six patients was 76.4 days (range 6–228 days). The patient with documented hepatic lymphoma never experienced complete resolution of liver enzyme elevation despite initial improvement during treatment, and was lost to follow-up 179 days after documentation of hepatotoxicity (369 days from diagnosis).
At 30 days, 46 cats (82.1%) were found to have had a response to treatment based on improvement (57.8% partial clinical response) or resolution (22.8% complete clinical response) of clinical signs. By 90 days, 48 (85.7%) cats had responded to chemotherapy (50.8% complete clinical response, 35.0% partial clinical response). Eleven cats (19.6%) were determined to be non-responders at the 30-day time point, with five cats (8.9%) determined to be non-responders at day 90. Two other cats were killed, one at day 84 due to neurological signs thought to be unrelated to the lymphoma based on neurological evaluation and results of magnetic resonance imaging of the brain with tentative diagnosis of dysmyelinating or neurodegenerative disease and the second at day 15 due to a progressive decrease in quality of life following diagnosis. Twenty-three (41.1%) patients developed progressive disease during the follow-up period, with a median progression-free survival of 1078 days (range 7–2479 days). Location of lymphoma, gastrointestinal vs. extraintestinal, was not found to be associated with progression-free survival (P = 0.23).
Fifty-three (94.6%) patients had available information regarding the reason for first-line prednisone and chlorambucil protocol discontinuation. Eighteen patients (34.0%) were discontinued because they were determined to be in a clinical remission and completed their intended 12-month treatment protocol. Fourteen patients (26.4%) were discontinued due to progressive disease. Four patients (7.5%) were discontinued due to protocol toxicity and two (3.8%) due to owner non-compliance. Unfortunately, 15 (28.3%) patients were lost to follow-up or had died (n = 2) prior to this data collection time point and cause for protocol discontinuation was unable to be determined.
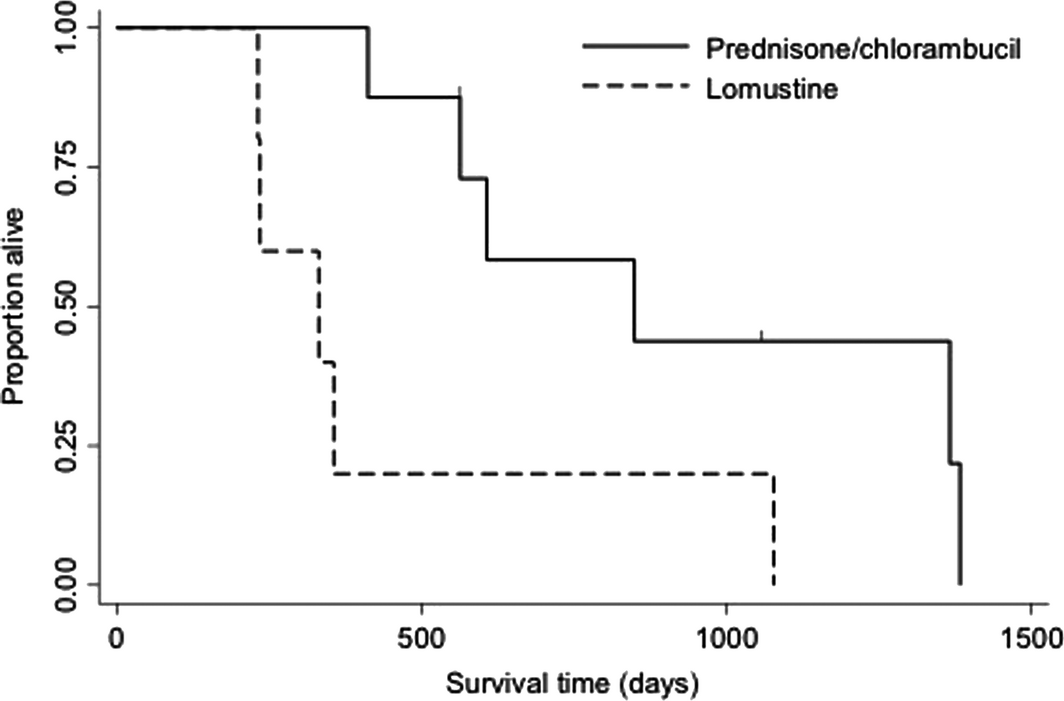
Twenty-two patients (39.3%) went on to receive at least one rescue protocol following documentation of disease progression. Nine patients (16.1%) went on to receive more than one rescue protocol. The most common first-line rescue protocol was reintroduction of prednisone and chlorambucil (n = 9), followed by a COP-based (cyclophosphamide, vincristine [Oncovin ®], prednisone) protocol (n = 6), prednisone and lomustine (n = 5), or prednisone and cyclophosphamide (n = 2). Overall response rate for first attempt rescue chemotherapy was 59% (45% complete clinical response, 14% partial clinical response). Median rescue specific survival for all cats was 861 days [range 0–2262 days; 95% CI (121, 1271)]. Progression-free survival of cats receiving reintroduction of prednisone and chlorambucil was significantly longer than prednisone and lomustine rescue [850 days (range 0–901) vs. 332 days (range 8–861), P = 0.02] (Fig. 1). Cats receiving reintroduction prednisone and chlorambucil were discontinued from the initial protocol following completion of protocol (66.7%), owner non-compliance (22.2%) or disease progression (11.1%). Cats receiving prednisone and lomustine rescue were discontinued from the initial protocol due to disease progression in all cases.
|
|
|
Figure 1. Kaplan–Meier curve depicting progression-free survival time for 14 cats that received rescue therapy with prednisone and chlorambucil (n = 9) or prednisone and lomustine (n = 5). Progression free was significantly longer in patients receiving prednisone and chlorambucil (median 850 days vs. 332 days, P = 0.02). |
Four patients (7.1%) had a secondary neoplasia diagnosed during the follow-up period. Three patients (5.4%) had a cytologically confirmed diagnosis of large cell lymphoma, achieved through aspirates of the liver and spleen (n = 2), kidneys (n = 1) and pleural effusion (n = 1). All cats had histopathological diagnosis of small cell lymphoma confined to their gastrointestinal tract [duodenum (2), ileum (2), jejunum (1)] 522, 681 and 1495 days prior to the diagnosis of large cell lymphoma. Two cats were receiving prednisone and chlorambucil at the time of diagnosis of large cell lymphoma and the one patient was receiving only budesonide. Most recent restaging diagnostics performed prior to the diagnosis of large cell lymphoma was available from two patients and abdominal ultrasound results revealed normal appearing gastrointestinal tracts as well as small intestinal wall thickening in both. One patient was diagnosed with colonic mucinous adenocarcinoma 1126 days after histopathological diagnosis of hepatic lymphoma. A colonic mass was discovered on abdominal ultrasound and exploratory surgery with biopsies revealed carcinoma. This patient went on to live 222 days after diagnosis with additional chemotherapy treatment.
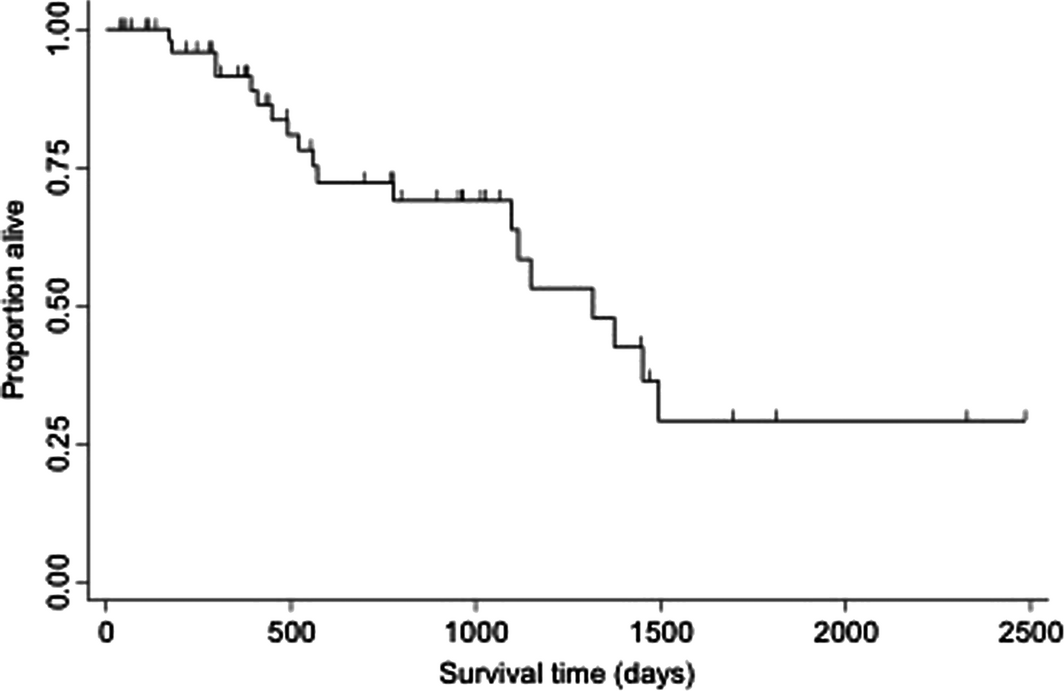
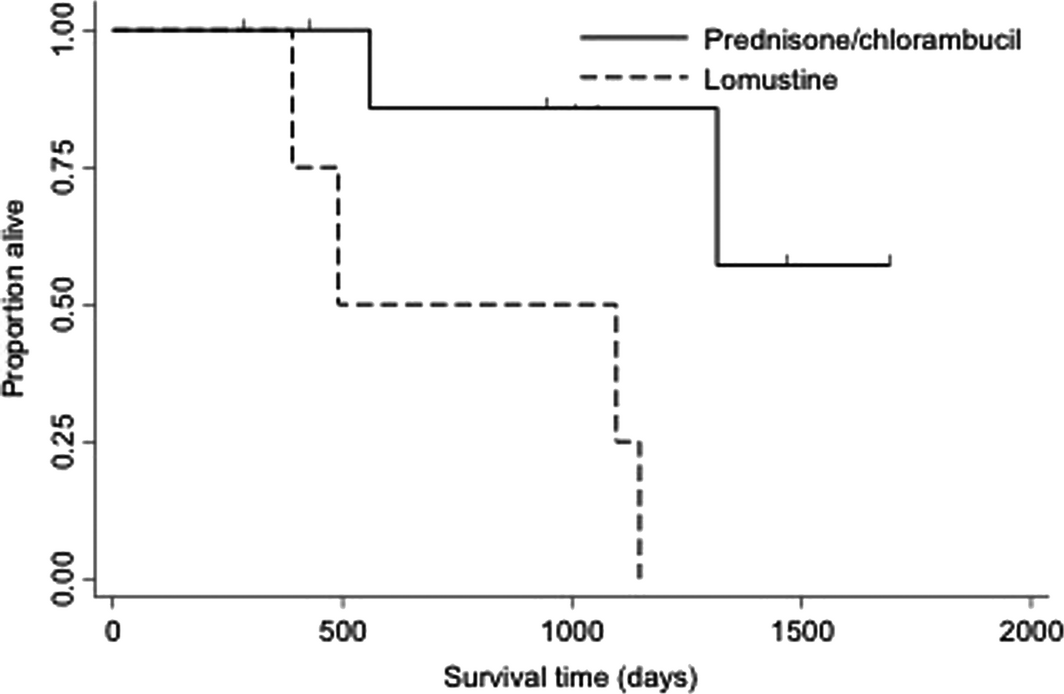
Overall median survival time for all cats with small cell lymphoma was 1317 days (range 15–2479 days) (Fig. 2). Median overall survival times for cats with small cell lymphoma confined to the gastrointestinal tract compared to extra-intestinal locations was 1148 (range 15–2479) and 1375 (range 208–1805) days (P = 0.23). Overall median survival time for cats receiving at least one rescue protocol was not significantly different than cats that did not receive any rescue therapy [1317 days (range 281–2322) vs. 1495 days (range 29–2479), P = 0.97]. Overall median survival time for cats receiving reintroduction prednisone and chlorambucil rescue was significantly longer compared to prednisone and lomustine rescue [>1500 days (range 425–1688) vs. 492 days (range 281–1148), P = 0.01] (Fig. 3). Fourteen cats (25.0%) were alive at the end of the study. Twenty-three cats were lost to follow-up at completion of the study. Of the 19 cats that died, 10 died due to tumour-related causes and nine due to non-tumour related causes. Four patients underwent post-mortem examination. Three of these patients were found to have evidence of small cell lymphoma present within their gastrointestinal tract as well as the liver. One patient had no evidence of lymphoma.
|
|
|
Figure 2. Kaplan–Meier curve depicting overall survival time for all 56 cats treated with glucocorticoid and chlorambucil chemotherapy for small cell lymphoma. |
|
|
|
Figure 3. Kaplan–Meier curve depicting overall survival time for 14 cats that received prednisone/chlorambucil rescue vs. prednisone/lomustine rescue chemotherapy. Cats that received prednisone/chlorambucil chemotherapy lived significantly longer than cats that received prednisone/lomustine as first attempt rescue chemotherapy (median >1500 days vs. 492 days, P = 0.01). |
Discussion
Patient demographics from our study are similar to those previously reported. Presenting clinical signs that lead to a diagnosis of small cell lymphoma were variable but similar to prior literature (Fondacaro et al. 1999; Carreras et al. 2003; Kiselow et al. 2008) with vomiting, weight loss and anorexia being most common. However, a small proportion (5.4%) of patients presented for investigation of elevated liver enzymes as their sole presenting complaint.
Our study revealed a favourable prognosis for cats with small cell lymphoma treated with the above-mentioned modified protocol, as the progression-free survival and overall survival times reported in this study are consistent with, or longer than, prior reports (Fondacaro et al. 1999; Carreras et al. 2003; Kiselow et al. 2008; Stein et al. 2010). The ability to discontinue the original protocol at a defined time period following documentation of complete clinical response has not previously been reported in the literature and our study supports the success of this practice.
Extra-intestinal lymphoma location was also included in this study and was not found to be significantly associated with overall treatment response or survival time when compared with gastrointestinal locations. Although previous reports have included cases with extra-intestinal disease location and cited similar results (Kiselow et al. 2008), these reports did not isolate purely extra-intestinal cases without gastrointestinal involvement for statistical analysis. Despite the small number of cases in this study, the behaviour of purely extra-intestinal lymphoma without any gastrointestinal involvement has not previously been described.
In our study, all patients were treated with a glucocorticoid and chlorambucil as a first-line chemotherapy protocol. This chemotherapy protocol was first proposed by Fondacaro et al. who suggested using cytotoxic agents more typical of those used for indolent neoplasia, which has since been proven effective in multiple reports (Fondacaro et al. 1999; Carreras et al. 2003; Kiselow et al. 2008). According to previous studies, the toxicity profile associated with long-term chlorambucil use in cats with lymphoma is mild. The majority of reported side effects include self-limiting gastrointestinal toxicity or myelosuppression, most often thrombocytopenia and neutropenia (Lingard et al. 2009; Stein et al. 2010). Neurotoxicity, specifically myoclonus (Benitah et al. 2003), has also been documented with chlorambucil use in a cat with lymphoma.
Chemotherapy-related toxicity was uncommon in our study, similar to historic reports of mostly low-grade myelosuppression (Fondacaro et al. 1999; Lingard et al. 2009; Stein et al. 2010). However, over 10% of patients experienced moderate to severe hepatotoxicity at some time point during the treatment period. Patients that had hepatic aspirates performed at the time of enzyme elevation (66.6%) revealed cytological findings consistent with vacuolar change and cholangitis, supporting treatment-related toxicity over infiltration of lymphoma as the cause of enzyme elevation. Only one of these patients went on to have biopsies performed on the affected liver, revealing only cholangitis, with no evidence of lymphoma. All but one patient experienced resolution of liver enzyme elevation following discontinuation of chlorambucil, further supporting treatment-related toxicity. The patient with persistently elevated liver enzymes was the only patient with confirmed hepatic lymphoma and showed an initial improvement in liver enzymes after treatment with prednisone and chlorambucil. Despite recurrence of the elevated liver enzymes during treatment with chlorambucil chemotherapy, resolution of these values was documented with discontinuation of chlorambucil. The improvement in original liver enzyme elevations along with the resolution of elevated values following discontinuation of chemotherapy supports treatment-related toxicity in this patient despite the lack of complete resolution of elevated enzymes.
Anecdotally, elevations in liver enzymes are occasionally observed in canine and feline patients treated with chlorambucil; however, this chemotherapy-related toxicity has not previously been reported for cats undergoing treatment for small cell lymphoma. As only one patient with documented hepatotoxicity was confirmed to have hepatic involvement of lymphoma and the remaining five cats had improvement in liver enzyme elevations when chlorambucil was discontinued, results suggest that this toxicity was drug related. Although only a small percentage of our patients experienced this toxicity, our study required pathological confirmation of small cell lymphoma for inclusion. It is possible that if all cats receiving chlorambucil chemotherapy were included into this study, a higher incidence of hepatotoxicity may have been documented. Similar idiosyncratic liver enzyme elevations have been documented in human patients receiving chlorambucil chemotherapy. This toxicity is generally self-limiting, although severe in grade, and thought to be a product of the extensive hepatic metabolism of this drug (Chabner & Longo 2006). Additional studies are necessary to better qualify the nature of hepatotoxicity associated with chlorambucil use in cats.
Administration of rescue chemotherapy did not significantly affect overall survival in our study. A previous study (Fondacaro et al. 1999) disputed this finding and reported that disease-free interval and median survival time were significantly increased in cats receiving rescue therapy with cyclophosphamide (24, 29 months) vs. cats that did not receive rescue (16.3, 18.8 months) at the time of disease progression. Similar reports have not since been published, and limited literature exists regarding alternative recommended rescue protocols. Rescue therapy with cyclophosphamide (Stein et al. 2010), lomustine (Dutelle et al. 2012) and doxorubicin (Oberthaler et al. 2009) have been reported with progression-free intervals and median survival times ranging from 169–241 days and 14.6 months respectively. However, these papers included intermediate and large cell lymphomas and did not always comment on the role of rescue therapy as a factor related to overall survival. In fact, the only patients who responded to doxorubicin were those of the small to intermediate cell type, a factor determined to be significant to response. The success of reintroduction prednisone and chlorambucil as a first line rescue therapy, following discontinuation of the original prednisone and chlorambucil protocol, was found to be more effective when compared to prednisone and lomustine rescue chemotherapy in our study, a finding that has not yet been reported. However, all patients that received lomustine rescue were unable to complete first line prednisone and chlorambucil due to progressive disease, whereas most patients receiving rescue reintroduction prednisone and chlorambucil completed the original protocol, and were discontinued due to complete clinical response. Therefore, it is likely that patients who failed to respond to first line prednisone and chlorambucil therapy had more aggressive or more resistant tumour populations, making comparison of results of these rescue protocols difficult, and an obvious limitation to the study. Toxicity was also not standardly recorded in our study following rescue protocols so the effect of rescue chemotherapy-related toxicity on overall survival between these populations could not be evaluated.
The lack of standardized medical records, thorough staging information, and consistent recheck and restaging diagnostics as well as available dosing information for all patients are limitations to this study given the retrospective nature. Dosing information, when available, was also varied, ranging from 2.3 to 14 mg (m−2 week−1). The dose intensity of chemotherapy may be less likely to alter overall patient outcomes in cases of indolent neoplasia such as that in our study population, however, this is another obvious limitation of our study. The reliance on clinician and owner interpretation of resolution of clinical signs for patient response assessment is also a limitation to response assessment in this study; however, such assessment is standardly reported in prior literature in the evaluation of treatment of feline small cell lymphoma. The large amount of patients lost to follow-up at completion of the study was also disappointing, and may have affected overall survival time as well as the incidence of toxicity and secondary malignancies. The limited dosing information available may also have affected our ability to completely assess chemotherapy-related toxicity and any potential correlation between toxicity and dosage or frequency. Nine cats that were lost to follow-up received rescue protocols. These cats were ultimately censored from statistical analysis, which may have altered results of rescue response assessment, further complicating interpretation of results. In this study, histological samples were available for most patients; however, a few samples were confirmed through a combination of flow cytometry, PARRs and cytology. This lack of standardized pathological sampling may have also influenced our outcome assessment, as comparison of histological subtypes or immunophenotypes were unavailable and unable to be compared between patients.
Conclusions
The results of our study support the use of glucocorticoids and chlorambucil for the treatment of feline small cell lymphoma across various anatomic locations as well as in first attempt rescue therapy settings supported by favourable response rates. The discontinuation of glucocorticoid and chloram-bucil with subsequent reintroduction as rescue chemotherapy appears to be just as effective as continued administration in cats with small cell lymphoma. Location of lymphoma did not affect survival and extraintestinal lymphoma appears to have a similar biological behaviour and response to treatment as the well-described gastrointestinal form. Infrequent hepatotoxicity appears to be treatment related and reversible with discontinuation of chlorambucil.
Source of funding
This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
Conflicts of interest
The authors report no conflicts of interest.
Contributions
No additional contributions were made to this study.
References
- Barrs V.R. & Beatty J.A. (2012) Feline alimentary lymphoma: 1. Classification, risk factors, clinical signs and non-invasive diagnostics. Journal of Feline Medicine and Surgery14, 182–190.
- Benitah N., de Lorimier L.P., Gasper M. & Kitchell B.E. (2003) Chlorambucil-induced myoclonus in a cat with lymphoma. Journal of the American Animal Hospital Association39, 283–287.
- Carreras J.K., Goldschmidt M., Lamb M., McLear R.C., Drobatz K.J. & Sorenmo K.U. (2003) Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000). Journal of Veterinary Internal Medicine17, 326–331.
- Chabner B.A. & Longo D.L. (2006) Cancer Chemotherapy and Biotherapy Principles and Practice. 4th edn. Lippincott Williams and Wilkins: Philadelphia.
- Dutelle A.L., Bulman-Fleming J.C., Lewis C.A. & Rosenberg M.P. (2012) Evaluation of lomustine as a rescue agent for cats with resistant lymphoma. Journal of Feline Medicine and Surgery14, 694–700.
- Fondacaro J.V., Richter K.P. & Carpenter J.L. (1999) Feline gastrointestinal lymphoma: 67 cases (1988–1996). European Journal of Comparative Gastroenterology4, 5–11.
- Kiselow M.A., Rassnick K.M., McDonough S.P., Goldstein R.E., Simpson K.W., Weinkle T.K.et al. (2008) Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005). Journal of the American Veterinary Medical Association232, 405–410.
- Lingard A.E., Briscoe K., Beatty J.A., Moore A.S., Crowley A.M., Krockenberger M.et al. (2009) Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. Journal of Feline Medicine and Surgery11, 692–700.
- Louwerens M., London C.A., Pedersen N.C. & Lyons L.A. (2005) Feline lymphoma in the post-feline leukemia virus era. Journal of Veterinary Internal Medicine19, 329–335.
- Milner R.J., Peyton J., Cooke K., Gallagher A., Gordon P. & Hester J. (2005) Response rates and survival times for cats with lymphoma treated with the University of Wisconsin-Madison chemotherapy protocol: 38 cases (1996–2003). Journal of the American Veterinary Medical Association227, 1118–1122.
- Moore P.F., Rodriguez-Bertos A. & Kass P.H. (2012) Feline gastrointestinal lymphoma mucosal architecture, immunophenotype, and molecular clonality. Veterinary Pathology49, 658–668.
- Oberthaler K.T., Mauldin E., McManus P.M., Shofer F.S. & Sorenmo K.U. (2009) Rescue therapy with doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma: a retrospective study of 23 cases. Journal of Feline Medicine and Surgery11, 259–265.
- Pohlman L.M., Higginbotham M.L., Welles E.G. & Johnson C.M. (2009) Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Veterinary Pathology46, 259–268.
- Russell K.J., Beatty J.A., Dhand N., Gunew M., Lingard A.E., Baral R.M.et al. (2012) Feline low-grade alimentary lymphoma: how common is it?Journal of Feline Medicine and Surgery14, 910–912.
- Stein T.J., Pellin M., Steinberg H. & Chun R. (2010) Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. Journal of the American Animal Hospital Association46, 413–417.
- Teske E., van Straten G., van Noort R. & Rutteman G.R. (2002) Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. Journal of Veterinary Internal Medicine16, 179–186.
- Vail D.M. (2004) Veterinary Co-operative Oncology Group: consensus statement. Veterinary and Comparative Oncology2, 194–213.
- Valli V.E., Jacobs R.M., Norris A., Couto C.G., Morrison W.B., McCaw D.et al. (2000) The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. Journal of Veterinary Diagnostic Investigation12, 295–306.
- Waly N.E., Gruffydd-Jones T.J., Stokes C.R. & Day M.J. (2005) Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats. Journal of Comparative Pathology133, 253–260.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?