Abstract
The future roles of biomass and carbohydrate for meeting needs of food/feed, renewable materials, and transportation fuels (biofuels) remain controversial due to numerous issues, such as increasing food and feed needs, constraints of natural resources (land, water, phosphate, biomass, etc.), and limitations of natural photosynthesis, as well as competing energy conversion pathways and technologies. The goal of this opinion article is to clarify the future roles of biomass and biorefineries using quantitative data other than adjective words. In most scenarios, human beings could have enough biomass resource from plant photosynthesis for meeting the three goals at the same time: feeding 9 billion people, providing renewable materials, and producing transportation biofuels that could replace nearly all fossil fuel-based liquid fuels used in the land transportation in 2050. Land transport means will pass through transitions from internal combustion engines plus liquid fuels, to hybrid systems, to hydrogen fuel cell vehicles (FCVs), while battery electric vehicles (BEVs) could play a minor role. Next generation biorefineries based on artificial photosynthesis featuring ultra-high energy efficiency and low-water consumption could produce a large amount of carbohydrate and/or other biocommodities from hydrogen/electricity and CO2. In conclusion, it is time to develop next generation biorefineries, which will efficiently utilize nonfood biomass for the coproduction of multiple products from biofuels, biochemicals, to food/feed, and even store electricity/hydrogen by fixing CO2 to carbon-containing chemicals and biofuels. Next generation biorefineries will address the food, biofuels, and environment trilemma at the same time.
Introduction
Modern civilization is the product of incessant utilization of natural resources on large scales: fossil fuels (e.g., oil, gas, and coal), renewable energy (e.g., biomass, wind, and solar), water, and land [1-4]. Among finite fossil fuels, cheap crude oil will run out first within next several decades [5, 6]. Therefore, it is a great scientific and engineering challenge to replace cheap oil with something that can be produced from renewable resources [7]. Feeding the world population from 7 billion now to 9 billion in 2050 [8] poses another challenge by considering constraints of natural resources – limited farming land supplies and emerging water crisis [9-11]. In addition, food security is closely related to issues of food distribution, geopolitical stability, cost volatility, and functional nutrition [12], which are not discussed here. Although water is renewable, the collective fresh water demand of human beings could exceed foreseen supply by ca. 40% in 2030 [13]. This water shortage could escalate food prices, disrupt energy production, constrict trade, create refugees, and undermine authority [13].
Biomass is defined as biological materials. Nearly, all biomass (i.e., plant, animal, and microbial) originates from CO2 fixation by natural photosynthesis. It has played important roles in human societies: (i) cereals from cultivated grains and grass from managed pastures are food and feed sources, respectively, accounting for approximately 2.0% of terrestrial net primary production (NPP); (ii) approximately, 2.3% of terrestrial biomass is directly burned for cooking and heating, especially in developing countries, or eventually converted to biogas as a secondary energy carrier; (iii) wood and other cellulosic materials accounting for approximately 1% of terrestrial NPP is used as construction materials and to make paper and renewable polymers (e.g., cellophane, rayon); and (iv) approximately, 0.2% of terrestrial NPP (e.g., corn kernels, sugarcane, and vegetable oil) is converted to liquid transportation biofuels in first generation biorefineries. It is important to retain biomass' irreplaceable roles as food/feed, construction materials, papers, and renewable polymers and then investigate whether there will be enough extra biomass resource to meet other needs.
Biofuels are defined as a secondary energy used in the transport sector, which is derived mainly from biomass [14-16]. First-generation biofuels include ethanol produced from sugarcane and starch-rich biomass (e.g., corn kernels, wheat, and aged cereals) and biodiesel produced from vegetable oils and animal fats. First-generation biofuels produced from food source receive severe criticisms because their impacts on the transportation fuels are minimal mainly due to limited feedstock supplies and their production has a minimum effect on a reduction of net greenhouse gas emissions [17, 18]. For example, it is estimated that replacing 5% of energy consumption through first generation biofuels could double water withdrawals for agriculture [13]. Clearly, the global production of first generation biofuels is not sustainable and is endangering current agricultural systems. Second-generation biofuels are produced mainly from nonfood biomass, such as cellulosic ethanol, butanol, fatty acid ethyl esters, methane, hydrogen, methanol, dimethylether, Fischer-Tropsch diesel, and bioelectricity [4, 19]. Because there are so many different energy conversion pathways (i.e., biological, thermochemical, and their hybrids) to converting nonfood biomass to a large variety of potential biofuels, which biofuels will become short-, middle- and long-term transportation fuels is a matter of vigorous debate [4, 19, 20]. Additionally, the future role of biomass in the nexus of energy, water, and food is not clear.
This article provides much-needed clarity on the desirability and feasibility of next generation biorefineries that will utilize nonfood biomass resource and/or even fix CO2 through artificial photosynthesis. Such biorefineries will meet needs of food/feed and biofuels while not endangering water security and maintaining biodiversity.
Appraisal facts
It is necessary to provide some quantitative data pertaining to energy production and consumption, resource availability, and constraints of natural resources and biosystems before the potential impacts of next generation biorefineries are predicted.
Energy status quo
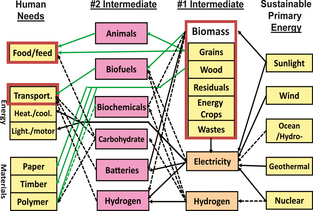
Generally speaking, energy demands determine energy production and conversion [4]. Typical energy systems are comprised of three basic components: primary (natural) energy sources, their conversion to secondary energies (i.e., #1 and #2 intermediates), and end applications from food/feed to energy to materials. In the past human societies, the simplest systems utilized one or two of energy sources (e.g., biomass) through few kinds of inefficient energy conversion for meeting basic needs – food/feed and cooking/heating. In contrast, modern societies can utilize numerous primary sources (e.g., fossil fuels, insolation, nuclear, and wind energy), convert them to a few energy carriers (e.g., electricity, hydrogen, and liquid fuels) with enhanced energy conversion efficiencies, and apply them in a myriad of ways to power complex high-energy societies [4, 21]. Figure 1 presents future pathways between basic needs and renewable primary energy without fossil fuels. In it, the needs of food/feed and renewable materials (i.e., paper, timber, and polymers) will have to be met by biomass and/or carbon-containing compounds made from artificial photosynthesis, while the energy needs (e.g., transport, heat/cooling) could be met through a variety of energy intermediates from numerous primary energy sources.
|
|
|
Figure 1. Human needs are and will be met from sustainable primary energies through numerous intermediates. Solid lines mean practical conversions; dash lines mean hypothetical conversions in the future. |
Table 1 presents status quo of the worlds energy production, where food/feed and wood consumption are included, because they are major energy consumption sections and their production greatly competes with the production of other energy for requiring water and land. Fossil fuels including oil, gas, and coal account for approximately 72% of the worlds energy consumption. Crude oil is the largest primary energy and its major usage is transportation fuels – gasoline, middle distillates (e.g., diesel), jet fuels, and fuel oil, accounting for more than 60% of oil consumption [22]. Renewable energy resources accounts for approximately 26% of the worlds energy consumption. Biomass is the largest utilized renewable energy source: heating fuel (i.e., 1.50 TW), 2.5 billion tons of food (i.e., 1.33 TW), and 3.5 billion cubic meters of wood (i.e., 1.28 TW). In all, the worlds energy consumption is estimated to be 18.2 TW. This value is approximately 20% higher than the widely used value of 15 TW in the literature [23] because the smaller value does include neither food/feed nor wood consumption for materials.
| Name | Power (TW) | Percentage | References |
|---|---|---|---|
| Fossil fuels | 13.10 | 72.02% | [22] |
| Oil | 5.22 | 28.70% | [22] |
| Gasoline | 1.20 | 6.60% | [22] |
| Middle distillates (diesel) | 1.79 | 9.84% | [22] |
| Jet fuel | 0.32 | 1.76% | [22] |
| Gas | 3.61 | 19.85% | [22] |
| Coal | 4.27 | 23.47% | [22] |
| Nuclear | 0.29 | 1.61% | [22] |
| Renewables | 4.80 | 26.39% | |
| Biomass - heating | 1.50 | 8.25% | [29] |
| Food/feeda | 1.33 | 7.31% | [11] |
| Woodb | 1.28 | 7.04% | [100] |
| Hydroelectricity | 0.39 | 2.15% | [22] |
| New renewablesc | 0.30 | 1.64% | [22, 32] |
| Total | 18.19 | 100.01% | |
Transportation fuels
Mobility reflects the level of civilization [4, 24, 25]. Human societies have passed through two transportation revolutions: from animal forces to external combustion engines to internal combustion engines (ICEs) [2, 16, 19, 26]. Affluent countries consume more transportation energy per capita than developing countries. For example, the global transport sector consumes approximately 20% of the energy produced (Table 1), and the transport sector in the United States consumes approximately 28% of the total energy [16].
Vehicles running on land constitute the largest type of transportation energy consumption. They have some special requirements, such as high energy storage capacity in a small container, high power output, affordable fuel, affordable vehicle, low costs for rebuilding the relevant infrastructure, fast charging or refilling of the fuel, and high safety [16]. Such strict requirements lead to the outcome – that ICEs along with high-energy density liquid fuels are the dominant transport means [4]. However, the depletion of crude oil, rising prices of crude oil, the accumulation of greenhouse gases, and concerns of national energy security are motivating the development of new sustainable transport means.
Food and feed
Food is fundamental to human well-being and development [27]. Henry Kissinger, a former US Secretary of State, said “control oil and you control nations; control food and you control the people.” In 5000 years of Chinese history, a lack of food supplies frequently resulted in dynasty shifts. Increasing food production is believed to effectively alleviate global food insecurity and stabilize societies.
The global energy market in terms of calories is approximately 13 times the food and feed market (Table 1). The ratios of the overall energy to food/feed are higher than 20 or even 40 in affluent countries and lower than 10 in developing countries [1, 28]. Therefore, the production of food/feed could be not as important in developed countries as in developing countries from a perspective of energy production and consumption.
Food and feed production is water-intensive. A simple rule of thumb is that it takes a half to one L of water to grow one calorie of cereals, depending on cultivation conditions and cereal types [13]. For example, the production of one kilogram of wheat requires the use of 1300 kg of water on average. Meat production, on average, requires about ten times the water per calorie than that of plants. For example, 10,000–20,000 kg of water are required to produce 1 kg of beef [13].
Currently, human beings consume approximately 2.5 billion tons of dry weight of harvested crops include approximately 2.3 billion tons of cereals (e.g., rice, wheat, and corn kernels) and grass from managed pastures [28]. Cultivated plants used for food and feed account for approximately 1.5% of the worlds NPP, which is calculated from the data of Tables 1 and 2.
| Renewable energy | Resource (TW) [1, 24, 29, 32] |
Resource potential (TW)
|
Utilization percentage |
|---|---|---|---|
| Surface insolation | 87,000 | 50.7 | 0.06% |
| Wind | 870 | 19.1 | 2.2% |
| Wave/Tide | 63.7 | 1.6 | 2.5% |
| Geothermal energy | 32 | 15.9 | 49.6% |
| Hydroelectric energy | 7.2 | 1.0 | 13.9% |
| Photosynthesis | 90 | ||
| Land | 65 | 7.99 | 12.3% |
| Ocean | 25 |
Growing population and continuous consumption growth per capita mean that the global demand for food/feed will increase by 50–100% in 2050 [9, 11]. Food security is inextricably linked to growing pressure on land, water, and energy resources [10]. Recent events of drought, large-scale land investments, and high energy prices underscore the worlds food security. In addition, issues of food distribution, geopolitical stability, cost volatility, and functional nutrition could lead to hunger in some areas [12].
Natural resources
Renewable energy
Three major types of renewable energies are solar radiation, geothermal energy, and tidal energy. The six transformations of solar radiation are wind, wind-generated ocean waves, ocean currents, hydro energy, thermal difference between the oceans surface and deep water, and biomass [29, 30]. Not all renewable energy sources can be utilized. For example, very low energy concentration (nonpoint) energies in terms of W/m2, such as ocean thermal differences, currents, and biomass in ocean, are difficult to collect and utilize economically [4]. Additionally, some fraction of energy resources cannot be utilized economically. For example, it is estimated that only 2.2% of wind energy resource could be utilized in the future (Table 2). Similarly, most biomass on lands cannot be economically collected and utilized due to high collection and transportation costs and/or environmental concerns. Approximately, 12.3% of biomass could be utilized, nearly double to current biomass consumption (i.e., 4.11 TW) [19, 31]. This data suggest that biomass resource may not be as large as expected.
Solar radiation is the largest renewable energy source (Table 2). Approximately, 170 petawatt (PW, 1015 W) radiation reaches Earth and approximately 30% is immediately reflected and scattered in the upper atmosphere [24, 32]. Once the radiation enters the atmosphere, a complex series of reflections and absorptions take place. Thirty-one petawatt insolation is converted to thermal energy in the atmosphere and the remaining solar radiation at the surface is approximately 87 PW [24, 32], approximately 5000 times of the worlds energy consumption. Of 87 PW surface radiation, 38 PW becomes thermal energy in the land and ocean, 41 PW contributes to evaporating water, and 5 PW diffuse radiation is reflected off the surface and escapes into space, and a very small fraction goes to photosynthesis [32]. Earths land surface, ocean, and atmosphere absorb solar radiation, and this raises their temperature and evaporates water, causing atmospheric circulation or convection. When the wet air reaches a high altitude where the temperature is low, water vapor condenses into clouds and then to rain/snow onto the Earths surface, completing the water cycle. The latent heat of water condensation amplifies convection, producing atmospheric phenomena, such as wind, hurricanes, and cyclones [24, 32].
Water
Although it is renewable, water has no substitutes or alternative. Agriculture consumes approximately 3100 billion tons of water, accounting for 71% of fresh water withdrawals today for the production of approximately 2.5 billion tons of food [13]. Industrial withdrawals and domestic withdrawals account for 16% and 14%, respectively [13]. The changes in population growth from 7 billion to 8 billion in the next two decades, economic growth and urbanization, accompanied with increased food demand per capita will intensify global water consumption. It is expected that the collective demand of the humans for water will exceed foreseen supply by about 40% in 2030 [13]. Compared to availability of land and energy consumed, water is the biggest limiting factor in the worlds ability to feed a growing population [13].
Land
The total arable land on Earth is 4.2 billion hectares [28]. Approximately, a third of arable land is being cultivated [28]. In reality, the potential to convert the remaining land is limited because most uncultivated land plays vital ecological roles [28]. Half of potential arable land is available only in seven countries (i.e., Brazil, Democratic Republic of the Congo, Angola, Sudan, Argentina, Columbia, and Bolivia) [28]. On the other extreme, South Asia and the Near East/North Africa have no spare land [13]. Overall, the worlds net amount of arable land could expand an additional seventy million hectares, being 5% [13]. Also, aggressive expansion of agricultural lands from forest and grassland will impair biodiversity and release a large amount of new CO2 emissions [18, 33].
The issues of energy, water, and land used for the food production have been interwoven ranging from ensuring access to services, to environmental impacts, to price volatility [34]. Systematic analysis and paradigm-shifting solutions are highly required to address challenges of the energy–food–water nexus.
Natural photosynthesis
Natural photosynthesis comprises a set of photochemical and redox reactions, called the “light reactions” and a sequence of enzymatic synthesis reactions, called “light-independent reactions” [17, 35-37]. In the light reactions, photosynthetic pigments (e.g., chlorophyll molecules) absorb approximately 47% of the light of the sun called “photosynthetic active radiation,” but do not include green light, UV, and IR irradiation [37, 38]. The adsorbed energy is transferred to the reaction centers where the primary charge separation and transmembrane transport of electrons occurs. Subsequent electron- and proton-transfer reactions lead to the synthesis of ATP from ADP and inorganic phosphate and NADPH synthesis from NADP+. In theory, eight photons are required to reduce two molecules of NADP+ to NADPH. In reality, approximately 9.4 photons are consumed, that is, 11.8% of the energy of sunlight can be converted to the form of NADPH, which is close to the efficiency limit of the photosynthetic production of biohydrogen under optimal insolation [17, 39]. Light reactions have the highest photosynthesis efficiency at relatively low light intensities. The efficiency is saturated at 20% of full sunlight and decreases greatly at high light intensities. In addition, high light intensities lead to photo damage of a central protein subunit of the photosynthetic apparatus. The energy efficiency of light-independent reactions are limited by (i) low chemical synthesis efficiency of the enzyme RuBisCO for taking up low-concentration CO2 from air and removing 2-phosphoglycolate; (ii) availability of sufficient amounts of water that is not met during much of the day and of fertilizers, and (iii) respiration of living organisms [17, 38, 40]. Light reactions operate on very short time scales from femtoseconds to milliseconds, while light-independent reactions operate over a timespan of seconds to hours [35, 36]. As a result, natural plant photosynthesis has low theoretical energy efficiencies from solar energy to chemical energy of 4.6 and 6.0% for C3 and C4 plants, respectively [38]. Although global efficiency of plant photosynthesis is 0.2%, the global primary biomass production is approximately five times the worlds energy consumption (Tables 1 and 2).
Best energy efficiencies for well-fertilized and well-watered crops are between 2% and 3% [28]. In the past decades mainly due to the green revolution, yields of crops have increased by approximately three times [11]. Now global means of corn, wheat, and rice are 3.5, 2.0, and 2.5 ton/ha, respectively [28]. The highest corn, wheat, and rice harvest records are 22, 15.2, and 15.2 ton/ha, but such high crop productivities are achieved at the costs of high energy inputs, such as fertilizers, insecticides, and water [28]. As crop yields increases, the ratio of photosynthetic energy captured to energy spent on crop cultivation has decreased [41]. For example, ca. 50% fertilizers or even 70% used for cultivating high-yield crops in the United States and China cannot be utilized, resulting in serious nonpoint water pollution from farmland [42]. Therefore, it raises a challenge: how to increase crop yields while simultaneously decreasing energy consumption and utilizing natural resources, such as water and phosphate, more efficiently.
Key questions to clarify
The following addresses four key questions pertaining to the energy–food–water nexus and clarifies the roles of next generation biorefineries in the sustainability revolution.
Could we have enough biomass to feed the world?
There is no doubt that the production of food is more important than the production of energy and materials. Prior to the green revolution, the production of food was the first priority for human beings for several thousand years. For example, the former Soviet Union and United States investigated the production of single-cell proteins from crude oil. When the food supplies are abundant, the prices of food decrease greatly and the prices of crude oil soars, the production of liquid transportation fuels (i.e., ethanol and biodiesel) from food sources is in practice, especially the United States and Brazil. However, it is discouraged or even prohibited to expand the production capacity of first generation biofuels in most countries, such as China and the European Union, mainly due to the concern of food security.
How to meet increasing food needs is becoming a global challenge [10, 11, 43, 44]. Because the production of 2.5 billion tons of food has utilized ~30% arable lands and ~70% freshwater withdrawals, it is difficult to greatly increase agricultural lands and increase water withdrawals. Therefore, a group of scientists [9] suggests a variety of solutions to address food security: (i) closing yield gaps on underperforming lands, (ii) increasing agricultural resource efficiency, and (iii) increasing food delivery by shifting diets and reducing food waste, while halting agricultural land expansion. For example, several studies find that about one-third to one half of food is never consumed [45, 46]. For example, developing countries usually lose more than 40% of food postharvest or during processing, while industrialized countries often lose more than 40% of food at the retail or consumer levels [45]. On the other hand, some plant biologists, big plant companies, and policy makers promoted the genetically modified (GM) crops as a future solution [47]. However, long-term impacts of GM cereals on human health are not clear and their wide application is in heated debates [47-51].
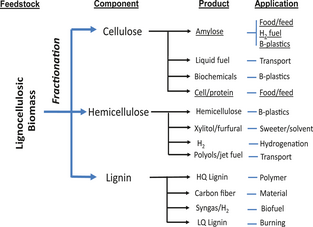
Here, a paradigm-shifting solution is proposed – enzymatic biotransformation of cellulose to synthetic starch in next generation cellulosic biorefineries [52]. Via biomass fractionating [53], a variety of multiple products could be produced from major lignocellulosic components: cellulose, hemicellulose, and lignin (Fig. 2). I demonstrate simultaneous enzymatic biotransformation and fermentation (SEBF) that can transform cellulosic materials to starch, ethanol, and single-cell protein in one vessel in the presence of cascade enzymes isolated from bacterium, fungus, and plant sources, and a typical ethanol-producing yeast. Our data showed that up to 30% of the anhydroglucose units in cellulose were converted to synthetic starch; the remaining units were hydrolyzed to glucose suitable for yeast fermentation that can produce ethanol. This cellulose to starch biotransformation could be scaled up by increasing the stability of two key enzymes – cellobiose phosphorylase and starch phosphorylase because this process does not involve any labile coenzymes (e.g., CoA and NAD[P]); no glucose is wasted; neither energy nor costly reagents is added. The stability of both cellobiose phosphorylase [54] and starch phosphorylase [55] can be enhanced greatly by protein engineering. Also, starch production from cellulose mediated by enzymes rather than GM organisms may avoid potential negative impacts of GM cereals and prevent bioethics debate.
|
|
|
Figure 2. Next generation biorefineries based on fractionated lignocellulosic components for the production of multiple products for meeting different needs from biofuels to biochemicals to food/feed. |
Cellulose resource is approximately 40 times the starch produced by cultivated crops. Every ton of cereals harvested is usually accompanied by the production of at least two tons of cellulose-rich crop residues, most of which are not utilized [56]. In addition to the use of agricultural and forest residues (e.g., straws, corn stover, and wood dust), growing dedicated bioenergy crops could greatly increase biomass availability. Dedicated bioenergy crops usually have much higher productivities (e.g., approximately 40–80 ton/ha/y [57-59]), have much higher water utilization efficiency, require less energy-related inputs, such as fertilizers, insecticides, and herbicides, tolerate harsher environments, and could not require annual seedling, compared to cultivated starch-rich crops. Dedicated bioenergy crops can grow on low-quality arable land.
The Department of Energy (DOE) of the United States has summarized three distinct goals associated with potential bioenergy crops: (i) maximizing the total amount of biomass produced per hectare per year, (ii) producing sustainable biomass with minimal inputs (e.g., pesticides, fertilizers, seeds, and harvesting), and (iii) maximizing the amount of biofuels that can be produced per unit of biomass [60]. A yield of ca. 50 dry tons per hectare per year may be considered as a reasonable target in an area with adequate rainfall and good soil [60], which is about 15–25 times average yields of cultivated cereals. In addition to well-studies bioenergy crops, such as switchgrass, poplar, and Miscathanus [59, 61, 62], this study recommends two new promising bioenergy plants – bamboo and common reed. Although both of them have been cultivated and harvested in some areas, they are often ignored by most. Bamboos are giant woody, tree-like, perennial evergreen grasses [58]. They have been cultivated in East Asia and South East Asia [63]. Phyllostachys pubescens (Moso bamboo) grows in a subtropical monsoon climate but it can withstand as low as −20°C in winter. It can be cultivated in marginal lands, such as mountain valley, foot of mountain, and gentle slope. The bamboo productivity is highly dependent on soil, water, and climate conditions. The highest average yearly biomass productivity during 10-year plantation is approximately 76 tons of dry culms/ha/y, which can be easily collected [64]. Phragmites australis (common reed) is a widespread perennial grass that grows in wetlands or near inland water ways [57]. Although it is harvested for thatched roofs, ropes, baskets, and pulping feedstock, the common reed is more typically considered an invasive weed due to its vigorous growth and difficulty of eradication. Common reed could be used as a bioenergy crept due to three unique features: (i) high biomass productivity (e.g., ca. 45–71 tons/ha/y), (ii) low inputs needed for planting, such as water, fertilizers, and pesticides, and (iii) removal of phosphorus- and nitrogen-containing pollutants in water ways [57].
Intensive irrigation for cultivating dedicated bioenergy crops could not be recommended. Since it consumes approximately three and one orders of magnitude water based on energy content more than the production of oil from traditional oil drilling and advanced oil recovery, respectively [13], the production of biomass is believed to increase usage of freshwater [65, 66]. This issue has raised concerns about the increase in water stress, particularly in countries that are already facing water shortage [67]. Therefore, cultivating future dedicated bioenergy crops must take in account water consumption.
In a word, the cost-effective transformation of nonfood cellulose to starch could not only revolutionize agriculture by promoting the cultivation of plants chosen for rapid growth rather than those optimized for starch production [68-70] but also could maintain biodiversity and minimize agricultures environmental footprint [71]. Also, wide implementation of cellulosic biorefineries would decrease postharvest food loss, especially for developing countries, so to increase overall food/feed availability [72].
What powertrain and fuel will become the dominant transport means in the future?
A number of scenarios (Fig. 1) can and could bridge between renewable primary energy and transportation energy demand through four powertrain systems: (i) ICEs and/or hybrid electric vehicles (HEVs) that burn liquid biofuels and compressed methane [19, 73], (ii) BEVs that run on electricity stored in rechargeable batteries, where electricity can be generated from sun radiation, tide, geothermal, wind, and nuclear energy [74], (iii) hydrogen FCVs that run on stored hydrogen through proton exchange membrane (PEM) fuel cells and electric motor [73], and (iv) sugar fuel cell vehicles (SFCVs) that run on stored sugar as a high-density hydrogen carrier based on FCVs [25]. Powertrain systems for vehicles must meet all of the following criteria: high energy storage capacity in a small container, high power output, economically competitive fuel, affordable vehicle, fast charging or refilling of the fuel, and high safety [16].
Table 3 compares the gravimetric energy densities of liquid fuels, stored hydrogen, rechargeable batteries, and capacitors, as well as kinetic energy output densities on wheels through different powertrain systems. The energy storage densities in a decreasing order are diesel, gasoline, butanol, ethanol, methanol, sugar, stored hydrogen, rechargeable batteries, and capacitors. Liquid gasoline and diesel plus their respective ICEs have kinetic energy output densities of 6.50 and 8.32 MJ/kg, respectively. When ICEs energy efficiencies are increased through hybrid electric systems, HEV-gas, and HEV-diesel can drive farther. Conventional hydrogen storage means have lower energy storage densities from 5.0 to 9.3 MJ/kg or even lower, resulting in shorter driving distance of FCVs compared to vehicles based on ICEs if the same weight fuel tank is used. Therefore, the DOE strongly encourages to develop novel high-density hydrogen storage means and provides the H-prize cash award [16]. Rechargeable batteries have at least one order magnitude lower energy storage densities than liquid fuels and stored hydrogen (Table 3). As a result, BEVs have very short driving distances. The energy densities of capacitors are very low, limiting its application in the transport sector.
| Name | Gravimetric energy density (MJ/kg) | Kinetic energy output (MJ/kg) | Powertrain (efficiency, %) |
|---|---|---|---|
| |||
| H2 without container | 143 | NA | NA |
| Diesel | 46.2 | 8.32 | ICE-diesel (18%) |
| 17.09 | HEV-diesel (37%) | ||
| Gasoline | 46.4 | 6.50 | ICE-gas (14%) |
| 14.38 | HEV-gas (31%) | ||
| Butanol | 36.6 | 5.12 | ICE-gas (14%) |
| 11.35 | HEV-gas (31%) | ||
| Ethanol | 30 | 4.20 | ICE-gas (14%) |
| 9.30 | HEV-gas (31%) | ||
| 11.10 | HEV-diesel (37%) | ||
| Methanol | 19.7 | 6.90 | DMFC (35%) |
| Starch/Cellulose | 17.0 | 8.16 | Sugar-H2-PEMFC/Motor (48%) |
| 8% H2 mass including container | 11.4 | 5.13 | PEMFC/Motor (45%) |
| Cryo-compressed H2 including container | 9.3 | 4.19 | PEMFC/Motor (45%) |
| Compressed H2 (700 bars) including container | 6.0 | 2.70 | PEMFC/Motor (45%) |
| 4% H2 mass including container | 5.7 | 2.57 | PEMFC/Motor (45%) |
| Compressed H2 (350 bars) including container | 5.0 | 2.25 | PEMFC/Motor (45%) |
| Lithium ion rechargeable battery | 0.56 | 0.381 | BEV (68%) |
| NiMnH rechargeable battery | 0.36 | 0.245 | BEV (68%) |
| Lead acid rechargeable battery | 0.14 | 0.095 | BEV (68%) |
| Ultra-capacitor | 0.02 | 0.016 | Motor (80%) |
| Super-capacitor | 0.01 | 0.008 | Motor (80%) |
Battery electric vehicles will not be a dominant future transport means. For example, the International Energy Agency and several studies predict that BEVs will play a minor role in the future [74, 75]. Rechargeable lithium (Li) batteries have energy densities of approximately 150 Wh/kg (i.e., 0.56 MJ/kg), resulting in very short driving distances for BEVs [76, 77]. If the energy densities of lithium batteries were increased by 5–10-fold [78, 79], other issues, such as safety, recharging time, and lifetime, could still prohibit their wide use in personal vehicles. In reality, future energy densities of rechargeable lithium batteries are expected to increase by twofold in next decades [76, 77] rather than 5–10 times by considering the configuration of Li batteries and its combustion energy (i.e., 43.1 MJ/kg lithium) [4]. Although developing lithium-air batteries are expected to have very high energy densities but the regeneration of lithium oxidize to lithium by electricity is energy intensive. Therefore, metal-air batteries are not suitable in the transport sector.
In addition to low energy densities of Li batteries, BEVs have other weaknesses. First, the recharging cycles and lifetime of high-density lithium batteries is approximately 1000 time and 2–3 years, respectively. Both are much shorter than requirement of the major car components lasting at least 10 years. (Think of lithium ion batteries in cellphones and laptops.) Second, lithium ion batteries are still costly for vehicles although its production costs could be decreased by several-fold. It is not realistic to believe that battery costs would be drastically decreased following Moores Law because it is impossible to exponentially both decrease material consumption in batteries and increase battery performance according to the basic physical limits of materials. Third, Li batteries require a long recharging time. Although ultra-fast charging batteries have been developed [80], these capacitor-like batteries are made at the cost of decreasing energy storage densities [81]. Fourth, a huge infrastructure investment could be needed to upgrade the electrical grid, install sockets for fast recharge, and build power stations [21]. Fifth, disposing and recycling a large number of used rechargeable batteries could be another environmental challenge [21]. Sixth, the energy density loss rates of rechargeable batteries depend on temperature; for example, standard loss rates per year are 6% at 0°C, 20% at 25°C, and 35% at 40°C [21]. Seventh, whether there is enough low-cost lithium for BEVs is not a certain thing. Goodenough, a pioneer of lithium batteries, pointed out that the principal challenges facing the development of rechargeable batteries for BEVs are cost, safety, energy density (voltage × capacity), rate of charge/discharge, and service life [82]. Due to BEVs' unique features such as cleanness and quietness, BEVs will still be popular in some special markets, for example, in golf courts. In a word, a complete switch to all battery electric cars is utterly unrealistic [21] by considering the above problems and the likelihood that better competing technologies will appear and mature.
This study suggests another paradigm-shifting solution for the future vehicles – SFCVs. Based on FCVs, carbohydrate (shorthand, CH2O) is suggested to be a high-density hydrogen carrier so that its use could address hydrogen storage, distribution, and safety issues [40, 83-85]. In the hypothetical SFCV, an on-board biotransformer containing numerous thermoenzymes and (biomimetic) co-enzymes that can achieve the reaction of CH2O + H2O → 2H2 + CO2 [86, 87]. Because enzymes are 100% selective, work under moderate reaction conditions, and generate highly pure hydrogen, carbohydrates have a gravimetric density of 8.33 H2 mass% for the carbohydrate/water slurry [16, 25]. During the past several years, we have increased enzymatic hydrogen generation rates to approximately 160 mmole H2/L/h by nearly 800-fold (in preparation for publication). We anticipate to increase reaction rates by another 30-fold within next several years so that the on-board biotransformer will be small enough to store in a SFCV [16, 40].
In a word, HEVs based on ICEs are believed to be a short- and middle-term solution before FCVs [73]. SFCVs could be a good solution to address the problems of FCVs from hydrogen production, storage, distribution, infrastructure, and safety. SFCVs could have several advantages over BEVs: much higher energy storage densities, faster refilling rates, better safety, and less environmental burdens [19, 40].
Could we have enough extra biomass source to drive vehicles and feed the world?
As shown in Table 1, two irreplaceable applications of biomass resource are food/feed (1.33 TW) and wood for materials (1.28 TW). Compared to all terrestrial biomass resource (65 TW), the current biomass utilization efficiency is 6.32% and it is expected that biomass utilization efficiency will be increased to up to 12.3% [31]. This value is also partially supported by the DOE and USUAs a billion ton report [88].Two liquid fuels used for land transportation are gasoline (1.2 TW) and middle distillates (1.79 TW). Since the global average ICE-gas and ICE-diesel have fuel-to-wheel efficiencies of approximately 14% and 23%, respectively [19], the global kinetic energy output on wheels is 0.58 TW.
When we increase biomass utilization efficiency from 6.32% now to 12.3% in 2050, this study provides quantitative predictions for the worst, best, and most likely scenarios for the year 2050 based on different assumptions. In the worst scenarios, food/feed needs, wood consumption, and biomass for burning could increase by 100%, 50%, and 50%, respectively. At the same time, total biomass resource could be constant. Therefore, the remaining biomass source that could be collected and utilized will be 1.17 TW. The land transportation energy in terms of kinetic energy could increase to 0.85 TW from 0.58 TW based on an annual growth rate of 1%.
In the best scenarios, food/feed needs and wood consumption could increase by 50% and 20%, respectively. Slow growth in wood consumption could be attributed to less use of papers in affluent countries and better recycling. Biomass for burning could be decreased to half due to an increase in burning efficiency in developing countries [24]. At the same time, total biomass resource could increase to 94.9 TW at an annual growth rate of 1% due to (i) rising CO2 levels in the atmosphere that fertilizes plant productivity [19, 38] and (ii) dedicated high-yield bioenergy crops [88]. Therefore, the biomass resource will be 7.52 TW. The land transportation energy in terms of kinetic energy could increase to 0.70 TW based on an annual growth rate of 0.5%.
In the most likely scenarios, food/feed needs, wood consumption, and biomass for burning could increase by 70%, 35%, and 0%, respectively. Food/feed production from cultivated cereals could increase to 1.66 TW; the remaining food/feed need (0.60 TW) could be supplemented with synthetic starch made from biorefineries. At the same time, total biomass resource could increase to 78.6 TW at an annual growth rate of 0.5%. Therefore, the remaining biomass resource will be 4.84 TW. The land transportation energy in terms of kinetic energy could increase to 0.76 TW based on an annual growth rate of 0.7%.
The last uncertainty is the biomass-to-wheel (BTW) efficiency of future land transport means. The worst scenario is based on current ICE-gas (ethanol) system (BTW = 7%), while the best could be SFCVs (BTW = 27%). Several transitional powertrains could be HEV-gas (BTW = 20.7%), HEV-diesel (BTW = 24.8%), and FCV (BTW = 22%). In the 2050 market, it is likely that the transport sector could constitute different transportation means so that an average BTW efficiencies could range from 11% to 20%.
Table 4 presents the analysis for the future biomass and biofuels roles. In the worst scenarios, biomass could play a significant role in replacing approximately 10–25% transportation fuel need. On the contrast, in the best scenarios, biomass could be sufficient to meet all land transportation energy need plus a large surplus. In the most likely scenarios, biofuels made from biomass could replace at least 50% to nearly 100% land transportation fuel need. The above analysis suggests that (i) we must increase powertrain system efficiency so to decrease biomass consumption, (ii) we must develop next generation biorefineries because it not only produce biofuels but also could produce food/feed and biochemicals, and (iii) we must utilize agricultural and forest residuals and then grow dedicated water-saving bioenergy crops by spatial segregation of food/feed and energy-producing areas by continuing producing food on established and productive agricultural land while growing dedicated energy crops on marginal land [89].
| Worst 2050 | Best 2050 | Highly possible 2050 | ||||||
|---|---|---|---|---|---|---|---|---|
| Name | Power (TW) | Assumption | Name | Power (TW) | Assumption | Name | Power (TW) | Assumption |
| Food/Feed | 2.66 | 100% gain | Food/Feed | 2.00 | 50% gain | Food/Feed | 2.26 | 70% gain |
| Food/Feed crops | 2.66 | 100% gain | Food/Feed crops | 1.86 | 40% gain | Food/Feed crops | 1.66 | 35% gain in crop |
| New food/Feed | 0.00 | NA | New food/Feed | 0.13 | New food/Feed | 0.60 | ||
| Wood | 1.92 | 50% gain | Wood | 1.54 | 20% gain | Wood | 1.66 | 30% gain |
| Burning | 2.25 | 50% gain | Burning | 0.75 | 50% decrease | Burning | 1.50 | No change |
| Total land biomass | 65 | No change | Total land biomass | 94.87 | 1% gain/year | Total biomass resource | 78.56 | 0.5% gain/year |
| Available biomass | 1.17 | 12.3% biomass use | Available biomass | 7.52 | 12.3% biomass use | Available biomass | 4.84 | 12.3% biomass use |
| Land kinetic energy | 0.85 | 1% gain/year | Land kinetic energy | 0.70 | 0.5% gain/year | Land transportation use | 0.76 | 0.7% gain/year |
| Scenario | Land fuel replacement | Scenario | Land fuel replacement | Scenario | Land fuel replacement | |||
| S1: ICE-gas (ethanol) | 9.6% | BTW = 7% | S4: HEV-gas (ethanol) | 156% | BTW = 15% | S7: ICE/HEV-gas (ethanol) | 54.6% | BTW = 11% |
| S2: HEV-gas (ethanol) | 20.7% | BTW = 15% | S5: FCV | 229% | BTW = 22% | S8: HEV-gas (ethanol) | 74.4% | BTW = 15% |
| S3: HEV-diesel (ethanol) | 24.8% | BTW = 18% | S6: SFCV | 280% | BTW = 27% | S9: SFCV/SFC/HEV | 99.2% | BTW = 20% |
Could we surpass natural photosynthesis?
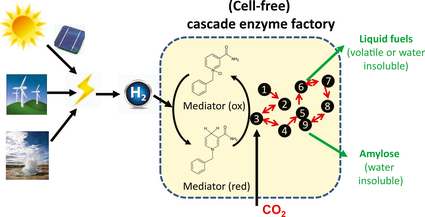
This study suggests developing next generation biorefineries by integrating high-efficiency solar cells or other electricity-generating systems, water electrolysis, with biological CO2 fixation mediated by cell-free synthetic cascade enzymes (Fig. 3). This cell-free biosystem is believed to work based on the design principles of synthetic biology, knowledge in the literature, and thermodynamics analysis [40, 90]. This hypothetical system could have numerous advantages. First, solar cells have much broader light adsorption spectrum and higher efficiencies than plant pigments. Also, the efficiency of solar cells, unlike plants, does not change in response to insolation variation. Also, it is easy to concentrate nonpoint insolation to a point energy – electricity. Second, hydrogen generated by water electrolysis at daytime can be stored for a few hours so that it can be consumed at a constant synthesis rate for the biological CO2 fixation process at night. Therefore, it is easy to regulate and match changed-rate electricity generation and constant-rate biosynthesis process. Third, the products of artificial photosynthesis are carefully chosen: water-insoluble amylose, volatile alcohols, or water-insoluble fatty alcohols. So the product separation costs could be minimal. Fourth, ultra-high energy efficiency from hydrogen or electricity and CO2 to chemical energy could be achieved, much better than natural processes mediated by living organisms that dissipate energy by respiration [91-93]. Table 5 presents the comparison between natural photosynthesis and artificial photosynthesis. Validation experiments and practical application of these systems will require worldwide collaborative efforts from biologists, chemists, electrochemists, and engineers [90] (Note: It is important to fix high concentration CO2 generated from power stations rather than to capture atmospheric CO2 because the latter requires extremely high energy inputs, resulting in economical infeasibility [94]).
| Natural photosynthesis | Artificial photosynthesis | |
|---|---|---|
| Solar to chemical efficiency | Theoretical ~4–10% | Theoretical, 51% |
| Practical: ~0.2–2% | Practical: >10% | |
| Sunlight spectrum (e.g., 350–2350 nm) | Only 48% (only 400–700 nm) | Whole spectrum adsorption by solar cells (63%, theoretical; 42% highest; 18%, commercial) |
| Light-harvesting efficiency | Low under nonoptimal conditions | Nearly constant independent of insolation |
| Chemical synthesis pathway | Unmatched reaction rates between light reactions and dark reactions | Constant synthesis rate from stored hydrogen |
| Chemical synthesis efficiency | 12–18 ATP + 6NAD(P)H equivalent consumed per hexose synthesis for utilizing low level CO2 | ATP-neutral synthesis pathway by using high levels CO2 from power stations |
| Respiration | ~50% loss | Not applicable |
| Complicated regulation between primary and secondary metabolisms | A (small) fraction of chemical energy could flux to product | 99+% of energy could flux to product because of insulation of biocatalyst synthesis (e.g., cell growth) from product formation |
| Product separation costs | Separate intracellular product from aqueous cells | Generate water-insoluble product (e.g., amylose or fatty alcohols) or volatile products |
| Large water consumption | 500 + kg of water needed for 1 kg of carbohydrate produced | 0.6 kg water needed for 1 kg of carbohydrate synthesized |
| Contamination | Use of weedicides | Not applicable |
| Operation time | Daytime only | 24/7 |
| Temperature | Modest | Well-controlled bioreactors |
| Land resource | Limited due to the combined requirements of temperature, water and insolation | Nearly everywhere by separating solar harvesting systems from product synthesis systems |
| Waste generated | Nonpoint pollutants | Point pollutants from fermenters |
|
|
|
Figure 3. Next generation biorefineries based on artificial photosynthesis that can fix CO2 and hydrogen to starch or other compounds. The enzymes involved in the synthetic enzymatic pathway responsible for CO2 fixation are suggested in the reference [40], which are different from all natural CO2 fixation pathways [99]. Also, the enzymes responsible for product formation are subject to change. |
In a word, next generation biorefineries based on artificial photosynthesis would not only bridge the current and future primary energy utilization systems aimed at facilitating electricity and hydrogen storage but also address such sustainability challenges such as renewable biofuel and chemical production, CO2 utilization, and fresh water conservation [90]. Its large-scale implementation would foster the switch from fossil fuel-based resources to renewable bioresources.
Recommendations
First, the development of next generation biorefineries based on nonfood biomass is a must rather than an option because they will produce a variety of products that cannot be substituted by other renewable resources, such as transportation fuels, biochemicals, and food/feed. With respect to biomass fractionating and biorefining technologies, the production of multiple products in next generation biorefineries will be of importance to their economic viability because natural biomass feedstock contains multiple components (Fig. 2). With respect to feedstock, we need start utilizing the ready agricultural and forest residues before we grow dedicated bioenergy crops on a large scale. Also, it is strongly recommended not to change current agricultural lands used for food/feed production to the production of bioenergy crops, which could lead to food shortage. With respect to biofuels, biofuels must be produced from sugars through anaerobic fermentation because a fraction of sugar in aerobic fermentation is wasted, resulting in low energy efficiencies [20, 95]. The failure of Amyriss and LS9s efforts on biofuels production is a good example – hopeless aerobic fermentation.
Second, it is extremely important to develop more energy efficiency powertrain systems from ICEs to HEVs to FCVs to SFCVs. Increasing energy utilization efficiency is a megatrend for human societies [2, 4, 24]. Higher energy efficiency means less primary energy consumption and lower environmental footprints.
Third, it is important to develop next generation biorefineries based on artificial photosynthesis that can produce carbon-containing compounds from CO2 and H2/electricity. Large-scale implementation of artificial photosynthesis would address such sustainability challenges as electricity and hydrogen storage, CO2 utilization, fresh water conservation, and maintenance of a small closed ecosystem for human survival in emergency situations [90].
Fourth, to address food security, it is recommended (i) not to increase the production capacity of first generation biofuels, and (ii) not to grow GM cereals as the future food source. Human beings will have enough food/feed without GMs cereals by increasing traditional crop productivity, decreasing food waste, enhancing food distribution, and producing synthetic starch from nonfood cellulosic resource, and even producing amylose through artificial photosynthesis. Additionally, potential negative impacts of GM cereals on human health should not be underestimated because systematic long-term studies are not available and may not be conductive. For example, negative effects of saturated fat are recently realized after its long utilization [96, 97]. Cotton seed oil was once used to replace vegetable oil as food. After years, it was found that the use of cottonseed oil resulted in low fertility in males [98]. Similarly, chronic negative effects of tobacco were realized in 1960s after its use for several thousand years. Therefore, it is not necessary to take risk in consuming GM cereals but its benefits to food security are not irreplaceable.
In a word, biomass sugar isolated from nonfood biomass and/or produced from artificial photosynthesis could play an irreplaceable role in the sustainability revolution by providing food/feed, renewable materials, and transportation biofuels in the future.
Acknowledgments
This study was supported by the Biological Systems Engineering Department of Virginia Tech, the CALS Biodesign and Bioprocessing Research Center, and Shell GameChanger Program.
Conflict of Interest
The authors declare competing financial interests. The enzymatic sugar-to-hydrogen technology is protected by the US patent 8211681. The enzymatic transformation of non-food biomass to edible starch is under protection of provisional patent disclosure filed by Virginia Tech. PZ has a financial interest in CFB9 and Gate Fuels.
References
- MacKay, D. J. C. 2009. Sustainable energy – without the hot air. UIT Cambridge Ltd., Cambridge, U.K.
- Smil, V. 2008. Energy in nature and society. MIT Press, Cambridge, MA.
- Wei, J. 2012. Great inventions that changed the world. John Wiley & Sons, Inc., Hoboken, NJ.
- Zhang, Y.-H. P.2011. What is vital (and not vital) to advance economically-competitive biofuels production. Process Biochem.46:2091–2110.
- Smil, V. 2008. Oil: a beginners guide. Oneworld Publications, Oxford, U.K.
- Sorrell, S., J. Speirs, R. Bentley, A. Brandt, and R. Miller. 2010. Global oil depletion: a review of the evidence. Energy Policy38:5290–5295.
- Kerr, R. A., and R. F. Service. 2005. What can replace cheap oil – and when. Science309:101.
- Sibly, R. M., and J. Hone. 2002. Population growth rate and its determinants: an overview. Phil. Trans. R. Soc. Lond. B Biol. Sci.357:1153–1170.
- Foley, J. A., N. Ramankutty, K. A. Brauman, E. S. Cassidy, J. S. Gerber, M. Johnston, et al. 2011. Solutions for a cultivated planet. Nature478:337–342.
- Godfray, H. C. J.2011. Food and biodiversity. Science333:1231–1232.
- Godfray, H. C. J., J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, et al. 2010. Food security: the challenge of feeding 9 billion people. Science327:812–818.
- Lappé, F. M., J. Collins, P. Rosset, and L. Esparza. 1998. World hunger: twelve myths. Grove Press, New York, NY.
- The World Economic Forum Water Initiative. 2011. Water security: the water-food-energy-climate nexus. Island Press, Washington, DC.
- Lynd, L. R.2010. Bioenergy: in search of clarity. Energy Environ. Sci.3:1150–1152.
- Vertès, A. A., M. Inui, and H. Yukawa. 2006. Implementing biofuels on a global scale. Nat. Biotechnol.24:761–764.
- Zhang, Y.-H. P.2010. Renewable carbohydrates are a potential high density hydrogen carrier. Int. J. Hydrogen Energy35:10334–10342.
- Michel, H.2012. Editorial: The Nonsense of Biofuels. Angew. Chem. Int. Ed.51:2516–2518.
- Searchinger, T., R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, et al. 2008. Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science319:1238–1240.
- Huang, W. D., and Y.-H. P. Zhang. 2011. Energy efficiency analysis: biomass-to-wheel efficiency related with biofuels production, fuel distribution, and powertrain systems. PLoS ONE6:e22113.
- Huang, W. D., and Y.-H. P. Zhang. 2011. Analysis of biofuels production from sugar based on three criteria: thermodynamics, bioenergetics, and product separation. Energy Environ. Sci.4:784–792.
- Smil, V. 2010. Energy myths and realities: bringing science to the energy policy debate. The AEI Press, Washington, DC.
- International Energy Agency (IEA). 2012. Key World Energy Statistics 2012. Available at http://www.iea.org/publications/freepublications/publication/kwes.pdf (accessed 2012).
- Lewis, N. S., and D. G. Nocera. 2006. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA103:15729–15735.
- Smil, V. 1999. Energies: an illustrated guide to the biosphere and civilization. The MIT Press, Cambridge, MA.
- Zhang, Y.-H. P.2009. A sweet out-of-the-box solution to the hydrogen economy: is the sugar-powered car science fiction?Energy Environ. Sci.2:272–282.
- Zhang, Y.-H. P.2008. Reviving the carbohydrate economy via multi-product biorefineries. J. Ind. Microbiol. Biotechnol.35:367–375.
- Misselhorn, A., P. Aggarwal, P. Ericksen, P. Gregory, L. Horn-Phathanothai, J. Ingram, et al. 2012. A vision for attaining food security. Curr. Opin. Environ. Sustainability4:7–17.
- Smil, V. 2000. Feeding the world: a challenge for the twenty-first century. MIT Press, Cambridge, MA.
- Hoffert, M. I., K. Caldeira, G. Benford, D. R. Criswell, C. Green, H. Herzog, et al. 2002. Advanced technology paths to global climate stability: energy for a greenhouse planet. Science298:981–987.
- Smil, V.2010. Energy Transitions: History, Requirements. Prospects, ABC-CLIO, LLC, Santa Barbara, CA.
- Worldwatch Institute. 2009. State of the world 2009: into a warming world. Available at http://www.worldwatch.org/node/5984 (accessed 2009).
- Hermann, W. A.2006. Quantifying global exergy resources. Energy31:1685–1702.
- Fargione, J., J. Hill, D. Tilman, S. Polasky, and P. Hawthorne. 2008. Land clearing and the biofuel carbon debt. Science319:1235–1238.
- Bazilian, M., H. Rogner, M. Howells, S. Hermann, D. Arent, D. Gielen, et al. 2011. Considering the energy, water and food nexus: Towards an integrated modelling approach. Energy Policy39:7896–7906.
- Blankenship, R. E., D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, et al. 2011. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science332:805–809.
- Williams, P. J. L., and L. M. L. Laurens. 2010. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci.3:554–590.
- Zhang, Y.-H. P., C. You, H. Chen, and R. Feng. 2012. Surpassing Photosynthesis: High-Efficiency and Scalable CO2 Utilization through Artificial Photosynthesis. ACS Sym. Ser. Recent Advances in Post-Combustion CO2Capture chemistry. 1097:275–292.
- Zhu, X.-G., S. P. Long, and D. R. Ort. 2008. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass?Curr. Opin. Biotechnol.19:153–159.
- Weyer, K., D. Bush, A. Darzins, and B. Willson. 2009. Theoretical maximum algal oil production. Bioenergy Res.3:204–213.
- Zhang, Y.-H. P.2011. Simpler is better: high-yield and potential low-cost biofuels production through cell-free synthetic pathway biotransformation (SyPaB). ACS Catal.1:998–1009.
- Gregory, P. J., and T. S. George. 2011. Feeding nine billion: the challenge to sustainable crop production. J. Exp. Bot.62:5233–5239.
- Braskerud, B. C.2002. Factors affecting phosphorus retention in small constructed wetlands treating agricultural non-point source pollution. Ecol. Eng.19:41–61.
- Godfray, H. C. J.2011. Food for thought. Proc. Natl Acad. Sci. USA108:19845–19846.
- Tilman, D., C. Balzer, J. Hill, and B. L. Befort. 2011. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA108:20260–20264.
- Gustavsson, J., C. Cederberg, U. Sonesson, R. van Otterdijk, and A. Meybeck. 2011. Global food losses and food wastein Section 3.2 of international congress “Save Food” (FAO, rural infrastructure and agro-industries division). Available at: http://www.fao.org/fileadmin/user_upload/suistainability/pdf/Global_Food_Losses_and_Food_Waste.pdf (accessed May 2011).
- Parfitt, J., M. Barthel, and S. Macnaughton. 2010. Food waste within food supply chains: quantification and potential for change to 2050. Philos. Trans. R. Soc. Lond. B Biol. Sci.365:3065–3081.
- Bruce, T. J. A.2012. GM as a route for delivery of sustainable crop protection. J. Exp. Bot.63:537–541.
- Bagla, P.2012. Negative Report on GM Crops Shakes Governments Food Agenda. Science337:789.
- Balmford, A., R. Green, and B. Phalan. 2012. What conservationists need to know about farming. Proc. R. Soc. B: Biological Sciences279:2714–2724.
- Beatty, P. H., and A. G. Good. 2011. Future Prospects for Cereals That Fix Nitrogen. Science333:416–417.
- Fedoroff, N. V. 2013. Will common sense prevail?Trends Genet. doi: 10.1016/j.tig.2012.09.002
- Zhang, Y.-H. P., H. G. Chen, C. You, and R. L. Feng. 2012. Feeding the world: two out-of-the-box solutions. in The 243rd ACS national meeting, division of AGFD 258, Available at: http://abstracts.acs.org/chem/243nm/program/view.php. (accessed March 29, 2012).
- Sathitsuksanoh, N., A. George, and Y.-H. P. Zhang. 2013. New lignocellulose pretreatments by using cellulose solvents: a review. J. Chem. Technol. Biotechnol.88:169–180.
- Ye, X., C. Zhang, and Y.-H. P. Zhang. 2012. Engineering a large protein by combined rational and random approaches: stabilizing the Clostridium thermocellum cellobiose phosphorylase. Mol. BioSyst.8:1815–1823.
- Yanase, M., H. Takata, K. Fujii, T. Takaha, and T. Kuriki. 2005. Cumulative Effect of Amino Acid Replacements Results in Enhanced Thermostability of Potato Type L α-Glucan Phosphorylase. Appl. Environ. Microbiol.71:5433–5439.
- Tuck, C. O., E. Pérez, I. T. Horváth, R. A. Sheldon, and M. Poliakoff. 2012. Valorization of Biomass: Deriving More Value from Waste. Science337:695–699.
- Sathitsuksanoh, N., Z. Zhu, N. Templeton, J. Rollin, S. Harvey, and Y.-H. P. Zhang. 2009. Saccharification of a potential bioenergy crop, Phragmites australis (common reed), by lignocellulose fractionation followed by enzymatic hydrolysis at decreased cellulase loadings. Ind. Eng. Chem. Res.48:6441–6447.
- Sathitsuksanoh, N., Z. Zhu, T.-J. Ho, M.-D. Bai, and Y.-H. P. Zhang. 2010. Bamboo saccharification through cellulose solvent-based biomass pretreatment followed by enzymatic hydrolysis at ultra-low cellulase loadings. Bioresour. Technol.101:4926–4929.
- Sathitsuksanoh, N., Z. Zhu, and Y.-H. P. Zhang. 2012. Cellulose solvent- and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour. Technol.117:228–233.
- DOE. 2006. Office of energy efficiency and renewable energy, office of science: breaking the biological barriers to cellulosic ethanol: a joint research agenda. a research roadmap resulting from the biomass to biofuels workshop. Available at http://www.doegenomestolife.org/biofuels/ (accessed June, 2006).
- Clifton-Brown, J. C., B. Neilson, I. Lewandowski, and M. B. Jones. 2000. The modelled productivity of Miscanthus×giganteus (GREEF et DEU) in Ireland. Ind. Crops Prod.12:97–109.
- Murnen, H. K., V. Balan, S. P. S. Chundawat, and B. Bals. 2007. daCostaSousa L, Dale BE: Optimization of ammonia fiber expansion (AFEX) pretreatment and enzymatic hydrolysis of Miscanthus x giganteus to fermentable sugars. Biotechnol. Prog.23:846–850.
- Gratani, L., M. F. Crescente, L. Varone, G. Fabrini, and E. Digiulio. 2008. Growth pattern and photosynthetic activity of different bamboo species growing in the Botanical Garden of Rome. Flora203:77–84.
- Shanmughavel, P., and K. Francis. 2001. Physiology of bamboo. Scientific Publishers, India.
- Batidzirai, B., E. M. W. Smeets, and A. P. C. Faaij. 2012. Harmonising bioenergy resource potentials—Methodological lessons from review of state of the art bioenergy potential assessments. Renew. Sustain. Energy Rev.16:6598–6630.
- Bernardi, A., S. Giarola, and F. Bezzo. 2012. Optimizing the economics and the carbon and water footprints of bioethanol supply chains. Biofuels, Bioprod. Biorefin.6:656–672.
- Gheewala, S. H., G. Berndes, and G. Jewitt. 2011. The bioenergy and water nexus. Biofuels, Bioprod. Biorefin.5:353–360.
- Casillas, C. E., and D. M. Kammen. 2010. The Energy-Poverty-Climate Nexus. Science330:1181–1182.
- French, C. E.2009. Synthetic biology and biomass conversion: a match made in heaven?J. Roy. Soc. Interface6:S547–S558.
- Sheppard, A. W., I. Gillespie, M. Hirsch, and C. Begley. 2011. Biosecurity and sustainability within the growing global bioeconomy. Curr. Opin. Environ. Sustainability3:4–10.
- Somerville, C., H. Youngs, C. Taylor, S. C. Davis, and S. P. Long. 2010. Feedstocks for Lignocellulosic Biofuels. Science329:790–792.
- Lynd, L. R., and J. Woods. 2011. Perspective: A new hope for Africa. Nature474:S20–S21.
- Demirdoven, N., and J. Deutch. 2004. Hybrid cars now, fuel cell cars later. Science305:974–976.
- Thomas, C. E.2009. Fuel cell and battery electric vehicles compared. Int. J. Hydrogen Energy34:6005–6020.
- Melamu, R., and H. von Blottnitz. 2009. A comparison of environmental benefits of transport and electricity applications of carbohydrate derived ethanol and hydrogen. Int. J. Hydrogen Energy34:1126–1134.
- Armand, M., and J. M. Tarascon. 2008. Building better batteries. Nature451:652–657.
- Tarascon, J. M., and M. Armand. 2001. Issues and challenges facing rechargeable lithium batteries. Nature414:359–367.
- Chan, C. K., H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins, et al. 2008. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol.3:31–35.
- Hassoun, J., and B. Scrosati. 2010. A High-Performance Polymer Tin Sulfur Lithium Ion Battery. Angew. Chem. Int. Ed.49:2371–2374.
- Kang, K., Y. S. Meng, J. Breger, C. P. Grey, and G. Ceder. 2006. Electrodes with high power and high capacity for rechargeable lithium batteries. Science311:977–980.
- Zaghib, K., J. B. Goodenough, A. Mauger, and C. Julien. 2009. Unsupported claims of ultrafast charging of LiFePO4 Li-ion batteries. J. Power Sources194:1021–1023.
- Goodenough, J. B., and Y. Kim. 2010. Challenges for Rechargeable Li Batteries. Chem. Mater.22:587–603.
- Zhang, Y.-H. P.2010. Production of biocommodities and bioelectricity by cell-free synthetic enzymatic pathway biotransformations: Challenges and opportunities. Biotechnol. Bioeng.105:663–677.
- Zhang, Y.-H. P., J.-B. Sun, and J.-J. Zhong. 2010. Biofuel production by in vitro synthetic pathway transformation. Curr. Opin. Biotechnol.21:663–669.
- Zhang, Y.-H. P., S. Myung, C. You, Z. G. Zhu, and J. Rollin. 2011. Toward low-cost biomanufacturing through cell-free synthetic biology: bottom-up design. J. Mater. Chem.21:18877–18886.
- Ye, X., Y. Wang, R. C. Hopkins, M. W. W. Adams, B. R. Evans, J. R. Mielenz, et al. 2009. Spontaneous high-yield production of hydrogen from cellulosic materials and water catalyzed by enzyme cocktails. ChemSusChem2:149–152.
- Zhang, Y.-H. P., B. R. Evans, J. R. Mielenz, R. C. Hopkins, and M. W. W. Adams. 2007. High-yield hydrogen production from starch and water by a synthetic enzymatic pathway. PLoS ONE2:e456.
- Perlack, R. D., L. L. Wright, R. L. Graham, B. J. Stokes, and D. C. Erbach. 2005. Biomass as feedstock for a bioenergy and bioproducts industries: The technical feasibility of a billion-ton annual supply. Oak Ridge National Laboratory, Oak Ridge, TN.
- Dauber, J., C. Brown, A. L. Fernando, J. Finnan, E. Krasuska, J. Ponitka, et al. 2012. Bioenergy from “surplus” land: environmental and socio-economic implications. BioRisk7:5–50.
- Zhang, Y.-H. P., and W.-D. Huang. 2012. Constructing the electricity-carbohydrate-hydrogen cycle for a sustainability revolution. Trends Biotechnol.30:301–306.
- Li, H., P. H. Opgenorth, D. G. Wernick, S. Rogers, T.-Y. Wu, W. Higashide, et al. 2012. Integrated Electromicrobial Conversion of CO2 to Higher Alcohols. Science335:1596.
- Magnuson, A., M. Anderlund, O. Johansson, P. Lindblad, R. Lomoth, T. Polivka, et al. 2009. Biomimetic and Microbial Approaches to Solar Fuel Generation. Acc. Chem. Res.42:1899–1909.
- Nevin, K. P., T. L. Woodard, A. E. Franks, Z. M. Summers, and D. R. Lovley. 2010. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio1:e00103–00110.
- House, K. Z., A. C. Baclig, M. Ranjan, E. A. van Nierop, J. Wilcox, and H. J. Herzog. 2011. Economic and energetic analysis of capturing CO2 from ambient air. Proc. Natl Acad. Sci. USA51:20428–20433.
- Bastian, S., X. Liu, J. T. Meyerowitz, C. D. Snow, M. M. Y. Chen, and F. H. Arnold. 2011. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab. Eng.13:345–352.
- Mozaffarian, D., R. Micha, and S. Wallace. 2010. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med.7:e1000252.
- Siri-Tarino, P. W., Q. Sun, F. B. Hu, and R. M. Krauss. 2010. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr.91:535–546.
- Randel, R. D., C. C. Chase, and S. J. Wyse. 1992. Effects of gossypol and cottonseed products on reproduction of mammals. J. Anim. Sci.70:1628–1638.
- Berg, I. A., D. Kockelkorn, W. H. Ramos-Vera, R. F. Say, J. Zarzycki, M. Hügler, et al. 2010. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol.8:447–460.
- The Global Institute of Sustainable Forestry at Yale University. 2009. Available at http://environment.yale.edu/gisf/programs/landscape-management/global-and-regional-forest-conditions/ (accessed 2009).
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?