Summary
Background
Esophageal candidiasis (EC) often occurs in human immunodeficiency virus (HIV)-infected patients, but is uncommon in non-HIV-infected patients. It is known that malignancy, diabetes mellitus, previous gastric surgery, and medications (antibiotics, proton pump inhibitors, and steroids) are risk factors for esophageal candidiasis in non-HIV-infected patients. However, the relationship between liver cirrhosis and esophageal candidiasis was unclear. This study aimed to elucidate the role of liver cirrhosis in esophageal candidiasis.
Methods
A retrospective chart review study was conducted on non-HIV-infected patients with esophageal candidiasis who presented to Tri-Service General Hospital from January 2009 to December 2012. The diagnosis of EC was primarily based on endoscopic findings. The incidence of EC in cirrhotic and noncirrhotic patients was compared. Furthermore, differences in baseline characteristics, clinical variables, and mortality after antifungal treatment between the two groups were analyzed.
Results
In this study, 43,217 non-HIV-infected patients were enrolled, 3017 of whom had liver cirrhosis. The incidence of EC in cirrhotic patients was higher than that in noncirrhotic patients (0.8% vs. 0.36%; relative risk = 2.2; p < 0.001). Multivariate logistic regression analysis identified liver cirrhosis as an independent risk factor for EC (odds ratio, 1.74; 95% confidence interval, 1.06–2.87; p = 0.029). Moreover, cirrhotic patients tended to be asymptomatic compared with noncirrhotic patients (45.8% vs. 9%; p < 0.01). The most common coexisting endoscopic finding was reflux esophagitis (83.9%). However, antifungal treatment did not decrease the mortality of patients with EC during hospitalization.
Conclusion
Liver cirrhosis is an independent risk factor for EC. EC may be asymptomatic in cirrhotic patients. Although antifungal treatment did not improve the outcome in this study, a prospective study is still required to investigate this issue.
Keywords
Cirrhosis ; Esophageal candidiasis ; Esophagogastroduodenoscopy ; Risk factor
Introduction
Esophageal candidiasis (EC) is a common opportunistic infection that occurs most often in immunocompromised patients who have either AIDS caused by human immunodeficiency virus (HIV) infection, or iatrogenic immunosuppression caused by cancer treatment and prevention of transplanted-organ rejection [1] ; [2] ; [3] ; [4] ; [5] ; [6] . In non-HIV-infected patients, the prevalence of EC is approximately 0.3% [7] ; [8] ; [9] . However, prior to the introduction of highly active antiretroviral therapy, 42% of HIV-infected patients had been diagnosed with EC [10] . Under current highly active antiretroviral therapy regimens, 17% of HIV-infected patients experience EC [10] , suggesting that the incidence of EC has substantially increased in HIV-infected patients. Similarly, renal allograft recipients who received three immunosuppressive drugs had a higher incidence of EC compared with recipients who took two immunosuppressive drugs (28.6% vs. 10.4%) [6] . These examples demonstrate that the incidence of EC is related to immune status.
Candida albicans is the most common pathogen causing EC. It has been isolated from 90% of EC patients [11] ; [12] . The common presenting symptoms are odynophagia, dysphagia, and retrosternal pain [2] , but some patients are asymptomatic [13] ; [14] ; [15] ; [16] . Esophagogastroduodenoscopy (EGD) plays an important role in the diagnosis of EC. EGD reveals the gross appearance of esophageal mucosa and provides an opportunity to perform biopsy [1] ; [16] ; [17] ; [18] . The typical endoscopic appearance is multiple raised small or thick white plaques on the esophageal mucosa [1] ; [10] ; [16] . Histological examination of the biopsy specimens using a potassium hydroxide smear reveals yeast cells and pseudohyphae [19] ; [20] . Sensitivity and specificity of EGD for the diagnosis of EC are 100% and 83.3%, respectively; positive and negative predictive values are 88.5% and 100%, respectively [17] . Therefore, EGD is a reliable method for the diagnosis of EC.
Among HIV-negative patients, those with cancer have a higher incidence of EC compared with those without cancer [4] ; [7] ; [8] ; [21] . A double-blind study showed that oropharyngeal candidiasis was detected in 54% of cancer patients who received placebo and in 3% of cancer patients who received antifungal prophylaxis during hospitalization [22] . Another double-blind study also revealed that 78.6% of patients with acute leukemia undergoing chemotherapy developed oropharyngeal candidiasis, but only 7.1% of acute leukemia patients undergoing both chemotherapy and antifungal prophylaxis had oropharyngeal candidiasis [5] . Furthermore, oropharyngeal candidiasis is a marker for EC in cancer patients [4] , suggesting that the risk for EC is increased in cancer patients. Similarly, other risk factors have been proven to increase the incidence of EC, such as diabetes mellitus (DM), previous gastric surgery, reflux esophagitis, and use of antibiotics, steroids, and proton pump inhibitors (PPIs) [6] ; [7] ; [8] ; [9] ; [16] ; [23] ; [24] ; [25] . In addition, the number of CD4+ T cells is crucial for determining the immune status, and it was decreased in cirrhotic patients [26] . The decrease in CD4+ T-cell count plays an important role in the disease progression of HIV-infected patients [27] ; [28] . Therefore, liver cirrhosis, similar to HIV infection, may also increase the incidence of EC. However, the relationship between cirrhosis and EC remains unclear.
In this study, our aim was to elucidate the role of liver cirrhosis in non-HIV-infected patients with EC. The clinical characteristics and symptoms of our patients with EC were first examined, and subsequently coexisting endoscopic findings were investigated. Finally, we analyzed the putative predisposing factors, and determined whether liver cirrhosis was an independent risk factor for EC.
Materials and methods
Patients
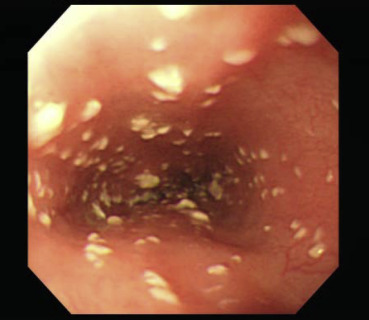
In this retrospective study, medical records of patients who underwent EGD at Tri-Service General Hospital, Taipei, Taiwan from January 2009 to December 2012 were analyzed. Age, sex, symptoms, medical history (such as liver cirrhosis, DM, malignancy, or end-stage renal disease), medications (such as PPIs, antibiotics, corticosteroids, immunosuppressive drugs, or chemotherapy), and endoscopic findings were investigated. HIV-infected patients were excluded from the study. The diagnosis of EC was based on the findings of endoscopy (Fig. 1 ) or biopsy. To verify the diagnosis of EC, endoscopic images were reviewed by two endoscopists.
|
|
|
Figure 1. Endoscopic images of esophageal candidiasis showed that whitish plaques were scattered over esophageal mucosa. |
Patients with EC were divided into two groups. The first group (Group 1) was associated with liver cirrhosis and the second group (Group 2) was without cirrhosis. The diagnosis of cirrhosis was based on the findings of coarsening parenchymal echo pattern, irregular surface, enlargement of caudate lobe, vascular irregularities, accompanied with splenomegaly, or ascites in abdominal sonography. The incidence rate of EC in the two groups was calculated. To confirm whether liver cirrhosis is an independent risk factor of EC, different putative risk factors were compared between these two groups. The mortality after antifungal treatment between the two groups was also analyzed. The study was approved by the Institutional Review Board of Tri-Service General Hospital.
Statistical analysis
All data are presented as the mean ± standard deviation for continuous variables, or the number (percentage) for categorical variables. Statistical analysis was performed using PASW (Predictive Analytics SoftWare) statistics software, version 18 (IBM Co., Somers, NY, USA). Continuous variables were compared using Student t test, and categorical variables were compared using Chi-square or Fisher exact tests. A logistic regression mode was used to control known risk factors and analyze the association of liver cirrhosis and EC. All reported p -values were two-tailed, and p < 0.05 was considered significant.
Results
The incidence of EC increased in cirrhotic patients
During 2009–2012, 43,217 patients underwent EGD at our hospital and 168 patients were diagnosed with EC (156 by endoscopic finding and 12 by biopsy). The incidence rate of EC over all cases of EGD was 0.39%. The characteristics and clinical symptoms of 168 patients are shown in Table 1 . Group 1 (with liver cirrhosis) comprised 24 patients and Group 2 (without liver cirrhosis) comprised 144 cases. Although the mean age was older in Group 1 (63.38 ± 10.7 years) than in Group 2 (62.99 ± 14.87 years), the difference did not reach statistical significance. The males were predominant in both groups, and there was no difference in the sex proportion between these two groups. Among the patients, 3017 had liver cirrhosis. Therefore, the incidence rate of EC in cirrhotic patients was 0.8% and in noncirrhotic patients was 0.36%, suggesting that the risk of EC was increased in cirrhotic patients compared with noncirrhotic patients (relative risk = 2.2, p < 0.001). In cirrhotic patients, 1398 were Child–Pugh A and eight had EC (0.57%); 1087 were Child–Pugh B and seven had EC (0.64%); 532 were Child–Pugh C and nine had EC (1.69%). The incidence of EC was higher in cirrhotic patients with Child–Pugh C compared with the others (p = 0.037).
| Group 1 (with cirrhosis) | Group 2 (without cirrhosis) | ||
|---|---|---|---|
| Number | 24 | 144 | |
| Age (y) | Mean ± SD | 63.38 ± 10.7 | 62.99 ± 14.87 |
| Range | 48–93 | 19–90 | |
| Sex (male/female) | 17/7 | 86/58 | |
| Symptoms (%) | Asymptomatic** | 11 (45.8) | 13 (9) |
| Acid regurgitation* | 1 (4.2) | 39 (27.1) | |
| Dysphagia | 1 (4.2) | 6 (4.2) | |
| Odynophagia | 0 (0) | 5 (3.5) | |
| Retrosternal pain | 0 (0) | 7 (4.9) | |
| Epigastric pain** | 5 (20.8) | 76 (52.8) | |
| Hematemesis/melena | 5 (20.8) | 20 (13.9) | |
| Nausea/vomiting | 2 (8.3) | 6 (4.2) | |
| Dyspepsia | 0 (0) | 5 (3.5) | |
| Other GI symptoms | 1 (4.2) | 4 (2.8) | |
- p < 0.05.
- p < 0.01.
GI = gastrointestinal; SD = standard deviation.
Cirrhotic patients with EC may be asymptomatic
None of the cases in either group underwent EGD due to suspected EC. Most of them underwent EGD due to suspected reflux esophagitis or peptic ulcer disease, suspected upper gastrointestinal tract bleeding, follow-up of reflux esophagitis or peptic ulcer disease, suspected esophageal varices, or anemia with unknown cause. The common clinical symptoms among all cases of EC were epigastric pain (48.2%), acid regurgitation (23.8%), and hematemesis/melena (14.9%). However, there were fewer patients with epigastric pain or acid regurgitation in Group 1 than in Group 2 (p < 0.05). Apart from epigastric pain and acid regurgitation, there were no statistically significant differences in the other presenting symptoms. By contrast, almost half of the cases in Group 1 were asymptomatic, which was significantly more than in Group 2 (45.8% vs. 9%, p < 0.01), suggesting that cirrhotic patients may not present specific gastrointestinal symptoms when they contract EC.
Endoscopic findings coexisting with EC
The endoscopic findings coexisting with EC among all cases of EC were reflux esophagitis (83.9%), gastritis (65.5%), duodenitis (35.7), gastric ulcer (24.4%), duodenal ulcer (13.1%), esophageal varices (7.1%), gastric cancer (1.8%), and esophageal cancer (0.6%; Table 2 ). No differences were observed in the endoscopic findings between Groups 1 and 2, except that esophageal varices were prominent in Group 1 (p < 0.001). The most common location of EC was the lower third of the esophagus (43.5%). Similarly, there was no difference in the distribution of EC between these two groups.
| Findings (%) | Group 1 | Group 2 |
|---|---|---|
| Negative except esophageal candidiasis | 1 (4.2) | 3 (2.1) |
| Reflux esophagitis | 18 (75) | 123 (85.4) |
| Esophageal cancer | 0 (0) | 1 (0.7) |
| Esophageal varices | 12 (50) | 0 (0) |
| Gastric ulcer | 9 (37.5) | 32 (22.2) |
| Gastric cancer | 0 (0) | 3 (2.1) |
| Gastritis | 16 (66.7) | 94 (65.3) |
| Duodenal ulcer | 1 (4.2) | 21 (14.6) |
| Duodenitis | 13 (54.2) | 47 (32.6) |
| Location | ||
| Whole | 4 (16.7) | 21 (14.6) |
| U/3 | 0 (0) | 3 (2.1) |
| U/3–M/3 | 5 (20.8) | 14 (9.7) |
| M/3 | 0 (0) | 16 (11.1) |
| M/3–L/3 | 4 (16.7) | 28 (19.4) |
| L/3 | 11 (45.8) | 62 (43.1) |
L/3 = lower third; M/3 = middle third; U/3 = upper third.
Liver cirrhosis is an independent risk factor for EC
Underlying disorders (such as HIV infection, malignancy, and DM), previous gastric surgery and medications (such as antibiotics, steroids, and PPIs) are known risk factors for EC [6] ; [7] ; [8] ; [16] ; [23] . The presentation of these risk factors was analyzed in patients with EC (Table 3 ). In this study, HIV-positive patients were excluded. Malignancy, DM, previous gastric surgery, and medications (antibiotics, steroids, and PPIs) were related to EC. To test whether liver cirrhosis is an independent risk factor for EC, a multivariate logistic regression was used to analyze these risk factors (Table 4 ), and it revealed that liver cirrhosis was significantly associated with EC [odds ratio (OR), 1.74; 95% confidence interval (CI), 1.06–2.87; p = 0.029]. However, the association of DM (OR, 0.78; 95% CI, 0.52–1.19; p = 0.255) and antibiotics (OR, 0.91; 95% CI, 0.57–1.42; p = 0.664) with EC did not reach statistical significance. These results suggest that liver cirrhosis is an independent risk factor for EC, similar to malignancy, previous gastric surgery, and medications (steroids and PPIs).
| Risk factors | No. (%) of patients with esophageal candidiasis | No. (%) of patients without esophageal candidiasis | p |
|---|---|---|---|
| Malignancy | 39 (23.2)∗ | 5430 (12.6) | < 0.001 |
| DM | 40 (23.8) | 7536 (17.5) | 0.041 |
| Antibiotics | 27 (16.1) | 4729 (11.0) | 0.048 |
| Steroids | 10 (6.0) | 1169 (2.7) | 0.019 |
| PPIs | 25 (14.9) | 3549 (8.2) | 0.003 |
| Gastric surgery | 8 (4.8) | 842 (2.0) | 0.019 |
| Liver cirrhosis | 24 (14.3) | 2993 (7.0) | < 0.001 |
DM = diabetes mellitus; PPIs = proton pump inhibitors.
∗. Hepatocellular carcinoma (n = 14), lung cancer (n = 5), colon cancer (n = 5), nasopharyngeal cancer (n = 4), gastric cancer (n = 3), bladder cancer (n = 3), breast cancer (n = 1), cholangiocarcinoma (n = 1), esophageal cancer (n = 1), lymphoma (n = 1), thyroid cancer (n = 1).
| Risk factors | Odds ratio (95% CI) | p |
|---|---|---|
| Malignancy | 8.43 (5.74–12.39) | < 0.001 |
| DM | 0.78 (0.52–1.19) | 0.255 |
| Antibiotics | 0.91 (0.57–1.42) | 0.664 |
| Steroids | 2.26 (1.1–4.68) | 0.027 |
| PPIs | 8.86 (5.61–13.97) | < 0.001 |
| Gastric surgery | 6.81 (3.36–13.79) | < 0.001 |
| Liver cirrhosis | 1.74 (1.06–2.87) | 0.029 |
CI = confidence interval; DM = diabetes mellitus; PPIs = proton pump inhibitors.
Antifungal treatment may not improve the outcome of EC
Eighty-two patients with EC underwent antifungal treatment, and 93.9% of these patients were alive when they were discharged from the hospital. By contrast, 86 patients with EC did not receive any antifungal drugs, and 91.9% of patients were alive when they were discharged from the hospital. There was no difference in the mortality during hospitalization between patients with and without antifungal treatment. Within Group 1, 15 patients had been treated with anti-fungal drugs and 12 were alive when they were discharged form hospital (2 died of hepatocellular carcinoma, and 1 died of disseminated candidiasis with septic shock); nine patients did not receive any antifungal drugs and six were alive when they were discharged form hospital (3 died of decompensated cirrhosis). Antifungal treatment did not decrease the mortality in cirrhotic patients with EC (20% vs. 33.3%, p = 0.46). Within Group 2, 67 patients had been treated with antifungal drugs and 65 were alive when they were discharged from the hospital (1 died of hepatocellular carcinoma, and 1 died of colon cancer); 77 patients did not receive antifungal drugs and 73 were alive when they were discharged from the hospital (2 died of gastric cancer, 1 died of hepatocellular carcinoma, and 1 died of lung cancer). Antifungal treatment did not decrease the mortality in the noncirrhotic patients with EC (3% vs. 5.2%, p = 0.51).
Discussion
EC is an unusual problem in the general population. In this study, the incidence of EC in HIV-negative patients was 0.39%, which was similar to the incidence rate found in previous reports [7] ; [8] ; [9] .
Candida colonization of the mucosal surface is common in the esophagus [29] . Once the hosts immunity is impaired, the colonizing candida may have the opportunity to invade the epithelium and develop EC [30] . It had been shown that the immune status of HIV-infected patients is associated with the CD4+ T-cell count and that a decrease in CD4+ T-cell count increases the incidence of opportunistic infections [31] ; [32] ; [33] . EC is one of the opportunistic infections that frequently develop in HIV-infected patients [1] ; [2] ; [3] . In addition, the CD4+ T-cell count depletion is found in cirrhotic patients and correlates with the severity of the disease [26] ; [34] . Therefore, the incidence of EC should be increased in cirrhotic patients, similar to HIV-infected patients. In this study, the incidence of EC in cirrhotic patients was twice as high as noncirrhotic patients. This result suggests that our hypothesis is correct.
Dysphagia, odynophagia, and retrosternal pain are common presenting symptoms of EC, but some patients with EC can be asymptomatic [2] . For example, 43% HIV-infected patients with EC were asymptomatic [14] . Another study showed that 21.4% of patients who took immunosuppressive drugs after renal transplantation were asymptomatic when they had been diagnosed with EC [6] . By contrast, only 5.9% of HIV-negative patients with EC did not present specific gastrointestinal symptoms [8] , suggesting that immunocompromised patients may tend to present without symptoms. In our study, there was no significant difference in the baseline characteristics and coexisting endoscopic findings between cirrhotic and noncirrhotic patients with EC. However, 45.8% and 9% of EC were asymptomatic in cirrhotic and noncirrhotic patients, respectively. Only 4.2% of cirrhotic patients presented with dysphagia and none presented with odynophagia or retrosternal pain. Therefore, similar to immunocompromised patients, cirrhotic patients may tend to present with asymptomatic EC.
Previous studies have shown that HIV infection, cancer, DM, gastric surgery, and medications (antibiotics, steroids, and PPIs) are risk factors for EC [6] ; [7] ; [8] ; [16] ; [23] . We analyzed these putative risk factors and found that there was an increased presentation of these known factors in patients with EC. A multivariate logistic regression revealed that liver cirrhosis is an independent risk factor for EC. However, the associations of DM and antibiotics with EC were not significant after adjustment with other risk factors. It was possible that DM and use of antibiotics were strongly correlated with other risk factors, so it did not show statistical significance after adjustment.
In this study, the mortality was lower in patients administered antifungal treatment than those without antifungal treatment, but the difference did not reach statistical significance, suggesting that antifungal treatment did not affect the outcome of EC. However, this was a retrospective study and the number of patients with EC was not large enough to draw a strong conclusion. Therefore, a double-blind randomized controlled study is needed to resolve this issue.
In conclusion, liver cirrhosis is an independent risk factor for EC. Furthermore, clinical manifestation of EC may be asymptomatic or nonspecific in cirrhotic patients. Although we have demonstrated in cirrhotic patients that the antifungal treatment did not improve the outcome of EC, this issue needs to be addressed by further study.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] P.G. Bianchi, F. Parente, M. Cernuschi; The diagnosis of esophageal candidiasis in patients with acquired immune deficiency syndrome: is endoscopy always necessary?; Am J Gastroenterol, 84 (1989), pp. 143–146
- [2] R.O. Darouiche; Oropharyngeal and esophageal candidiasis in immunocompromised patients: treatment issues; Clin Infect Dis, 26 (1998), pp. 259–272
- [3] D.S. Krause, A.E. Simjee, C. van Rensburg, J. Viljoen, T.J. Walsh, B.P. Goldstein, et al.; A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis; Clin Infect Dis, 39 (2004), pp. 770–775
- [4] G. Samonis, P. Skordilis, S. Maraki, G. Datseris, P. Toloudis, I. Chatzinikolaou, et al.; Oropharyngeal candidiasis as a marker for esophageal candidiasis in patients with cancer; Clin Infect Dis, 27 (1998), pp. 283–286
- [5] J. Cuttner, K.M. Troy, L. Funaro, R. Brenden, E.J. Bottone; Clotrimazole treatment for prevention of oral candidiasis in patients with acute leukemia undergoing chemotherapy. Results of a double-blind study; Am J Med, 81 (1986), pp. 771–774
- [6] K.L. Gupta, A.K. Ghosh, R. Kochhar, V. Jha, A. Chakrabarti, V. Sakhuja; Esophageal candidiasis after renal transplantation: comparative study in patients on different immunosuppressive protocols; Am J Gastroenterol, 89 (1994), pp. 1062–1065
- [7] J.H. Choi, C.G. Lee, Y.J. Lim, H.W. Kang, C.Y. Lim, J.S. Choi; Prevalence and risk factors of esophageal candidiasis in healthy individuals: a single center experience in Korea; Yonsei Med J, 54 (2013), pp. 160–165
- [8] A. Chocarro Martinez, F. Galindo Tobal, G. Ruiz-Irastorza, A. González López, F. Alvarez Navia, C. Ochoa Sangrador, et al.; Risk factors for esophageal candidiasis; Eur J Clin Microbiol Infect Dis, 19 (2000), pp. 96–100
- [9] K.Y. Kim, J.Y. Jang, J.W. Kim, J.J. Shim, C.K. Lee, S.H. Dong, et al.; Acid suppression therapy as a risk factor for candida esophagitis; Dig Dis Sci, 58 (2013), pp. 1282–1286
- [10] M. Nkuize, W.S. De, V. Muls, M. Arvanitakis, M. Buset; Upper gastrointestinal endoscopic findings in the era of highly active antiretroviral therapy; HIV Med, 11 (2010), pp. 412–417
- [11] R. Ally, D. Schürmann, W. Kreisel, G. Carosi, K. Aguirrebengoa, B. Dupont, et al.; A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients; Clin Infect Dis, 33 (2001), pp. 1447–1454
- [12] G. Barbaro, G. Barbarini, W. Calderon, B. Grisorio, P. Alcini, G. Di Lorenzo; Fluconazole versus itraconazole for candida esophagitis in acquired immunodeficiency syndrome. Candida Esophagitis; Gastroenterology, 111 (1996), pp. 1169–1177
- [13] B. Clotet, M. Grifol, O. Parra, J. Boix, J. Junca, J. Tor, et al.; Asymptomatic esophageal candidiasis in the acquired-immunodeficiency-syndrome-related complex; Ann Intern Med, 105 (1986), p. 145
- [14] M. López-Dupla, S.P. Mora, V. Pintado García, E. Valencia Ortega, P.L. Uriol, M.A. Khamashta, et al.; Clinical, endoscopic, immunologic, and therapeutic aspects of oropharyngeal and esophageal candidiasis in HIV-infected patients: a survey of 114 cases; Am J Gastroenterol, 87 (1992), pp. 1771–1776
- [15] C.M. Wilcox, R.F. Straub, W.S. Clark; Prospective evaluation of oropharyngeal findings in human immunodeficiency virus-infected patients with esophageal ulceration; Am J Gastroenterol, 90 (1995), pp. 1938–1941
- [16] S. Nishimura, N. Nagata, T. Shimbo, N. Asayama, J. Akiyama, N. Ohmagari, et al.; Factors associated with esophageal candidiasis and its endoscopic severity in the era of antiretroviral therapy; PLoS One, 8 (2013), p. e58217
- [17] D. Redah, A.Y. Konutse, K. Agbo, E.H. Dogbey, G. Napo-Koura, S.T. Tchangai-Kao, et al.; Is endoscopic diagnosis of Candida albicans esophagitis reliable? Correlations with pathology and mycology ; Gastroenterol Clin Biol, 25 (2001), pp. 161–163
- [18] C.M. Wilcox, L.N. Alexander, W.S. Clark, S.E. Thompson 3rd; Fluconazole compared with endoscopy for human immunodeficiency virus-infected patients with esophageal symptoms; Gastroenterology, 110 (1996), pp. 1803–1809
- [19] E.G. Arathoon, E. Gotuzzo, L.M. Noriega, R.S. Berman, M.J. DiNubile, C.A. Sable; Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases; Antimicrob Agents Chemother, 46 (2002), pp. 451–457
- [20] S.L. Newman, T.P. Flanigan, A. Fisher, M.G. Rinaldi, M. Stein, K. Vigilante; Clinically significant mucosal candidiasis resistant to fluconazole treatment in patients with AIDS; Clin Infect Dis, 19 (1994), pp. 684–686
- [21] N. Weerasuriya, J. Snape; A study of candida esophagitis in elderly patients attending a district general hospital in the UK; Dis Esophagus, 19 (2006), pp. 189–192
- [22] G. Samonis, K. Rolston, C. Karl, P. Miller, G.P. Bodey; Prophylaxis of oropharyngeal candidiasis with fluconazole; Rev Infect Dis, 12 (1990), pp. S369–S373
- [23] K. Mimidis, V. Papadopoulos, V. Margaritis, K. Thomopoulos, A. Gatopoulou, V. Nikolopoulou, et al.; Predisposing factors and clinical symptoms in HIV-negative patients with candida oesophagitis: are they always present?; Int J Clin Pract, 59 (2005), pp. 210–213
- [24] N. Kanda, H. Yasuba, T. Takahashi, Y. Mizuhara, S. Yamazaki, Y. Imada, et al.; Prevalence of esophageal candidiasis among patients treated with inhaled fluticasone propionate; Am J Gastroenterol, 98 (2003), pp. 2146–2148
- [25] J.H. Sellin, E.B. Chang; Therapy Insight: gastrointestinal complications of diabetes—pathophysiology and management; Nat Clin Pract Gastroenterol Hepatol, 5 (2008), pp. 162–171
- [26] B.H. McGovern, Y. Golan, M. Lopez, D. Pratt, A. Lawton, G. Moore, et al.; The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients; Clin Infect Dis, 44 (2007), pp. 431–437
- [27] M. Connors, J.A. Kovacs, S. Krevat, J.C. Gea-Banacloche, M.C. Sneller, M. Flanigan, et al.; HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies; Nat Med, 3 (1997), pp. 533–540
- [28] E.S. Rosenberg, J.M. Billingsley, A.M. Caliendo, S.L. Boswell, P.E. Sax, S.A. Kalams, et al.; Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia; Science, 278 (1997), pp. 1447–1450
- [29] G.T. Cole, A.A. Halawa, E.J. Anaissie; The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside; Clin Infect Dis, 22 (Suppl. 2) (1996), pp. S73–S88
- [30] J.A. Underwood, J.W. Williams, R.F. Keate; Clinical findings and risk factors for candida esophagitis in outpatients; Dis Esophagus, 16 (2003), pp. 66–69
- [31] S.M. Crowe, J.B. Carlin, K.I. Stewart, C.R. Lucas, J.F. Hoy; Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons; J Acquir Immune Defic Syndr, 4 (1991), pp. 770–776
- [32] H. Nielsen, K.D. Bentsen, L. Højtved, E.H. Willemoes, F. Scheutz, M. Schiødt, et al.; Oral candidiasis and immune status of HIV-infected patients; J Oral Pathol Med, 23 (1994), pp. 140–143
- [33] M. Maiman, R.G. Fruchter, L. Guy, S. Cuthill, P. Levine, E. Serur; Human immunodeficiency virus infection and invasive cervical carcinoma; Cancer, 71 (1993), pp. 402–406
- [34] L. Lombardo, A. Capaldi, G. Poccardi, P. Vineis; Peripheral blood CD3 and CD4 T-lymphocyte reduction correlates with severity of liver cirrhosis; Int J Clin Lab Res, 25 (1995), pp. 153–156
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?