Abstract
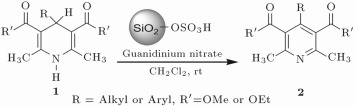
In this paper, a new and convenient method is introduced for the oxidation of a variety of Hantzsch 1, 4-dihydropyridine derivatives to their corresponding pyridine compounds using guanidinium nitrate and silica sulfuric acid. The reactions were carried out in dichloromethane at room temperature and the products were isolated at high to excellent yields.
Keywords
Guanidium nitrate ; Silica sulfuric acid ; 1, 4-dihydropyridines ; Oxidation ; Aromatization
1. Introduction
Since more than 100 years ago, when aryldihydropyridines were synthesized, great effort has been made to introduce a new reagent or reagent systems for the oxidation and synthesis of dihydropyridine derivatives. The oxidation of DHPs to the corresponding pyridine derivatives is the principal metabolic route in biological systems [1] and [2] . In recent years, it was found that drugs, such as nifedipine and niguldipine, undergo redox processes, due to the catalysis of cytochrome P-450 in the liver during their metabolism [3] . Therefore, the oxidation of 1, 4-dihydropyridines has attracted considerable attention. Numerous reagents and catalysts, such as SeO2 [4] , lead(IV) tetraacetate [5] , FeCl3 /KMnO4 [6] , ceric ammonium nitrate (CAN) [7] , bentonite clay-supported manganese(IV) oxide [8] , solid supported pyridinium chlorochromate (PCC) [9] , silica gel supported ferric nitrate [10] , nitricoxide, 6MnO2 [11] , BaMnO4 [12] , catalytic aerobic oxidation by using RuCl3 [13] , Pd/C [14] , activated carbon [15] , Fe(ClO4 )3 [16] , iron(III) and copper(II) nitrates [17] , Zr(NO3 )4 [18] , H2 O2 /Co(OAc)2 [19] , CrO3 [20] , Co(II)-catalyzed auto oxidation [21] , Al(NO3 )3 ⋅9H2 O and/or Fe(NO3 )3 ⋅9H2 O/SiO2 –OSO3 H [22] , supported nitric acid on silica gel and poly vinyl pyrrolidone [23] and KBrO3 /SnCl4 ⋅5H2 O [24] , have been used for this purpose. Even though we can find many articles regarding oxidation of these compounds, many efforts are still being made to introduce a new procedure for better oxidation of DHPs.
2. Results and discussion
In order to participate in these great challenges, I decided to develop a new convenient method for the oxidation of 1, 4-dihydropyridines to the corresponding pyridine derivatives using guanidiniun nitrate and silica sulfuric acid in a proper solvent under mild and heterogeneous conditions.
The reaction was initially tested in different solvents. From the solvent screening (Table 1 ), dichloromethane emerged as the most convenient (Entry 7), due to the fact that the product was isolated in an almost quantitative yield in the shortest reaction time. The other tested solvents required prolonged stirring to reach satisfactory conversion (Entries 1–3), or reaction was not completed until after 24 h (Entries 4–6).
| Entry | Solvent | Time | Yield (%)b |
|---|---|---|---|
| 1 | CH2 CN | 17.5 h | 97 |
| 2 | CH3 Cl | 2 h | 98 |
| 3 | 3.5 h | 72 | |
| 4 | CH3 COCH3 | 24 h | –c |
| 5 | CH3 CH2 OH | 24 h | –c |
| 6 | CH3 COOEt | 24 h | –c |
| 7 | CH2 Cl2 | 65 min | 99 |
a. Substrate/guanidinium nitrate/silica sulfuric acid: 1 mmol/3 mmol/0.45 g.
b. Isolated yield.
c. Reaction not completed.
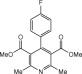
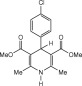
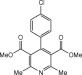
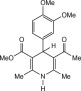
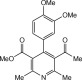
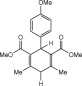
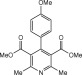
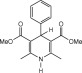
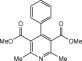
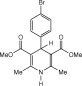
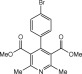
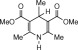
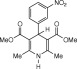
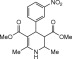
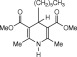
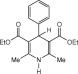
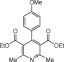
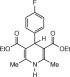
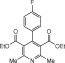
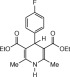
Eventually, with optimal conditions at hand, a variety of 1, 4-dihydropyridines (Scheme 1 ) were transformed into corresponding pyridines smoothly via reaction with a combination of guanidinium nitrate in the presence of silica sulfuric acid in dichloromethane at room temperature ( Scheme 1 ). The results of this transformation are summarized in Table 2 .
Scheme 1.
Aromatization of 1, 4-dihydropyridines.
| Entry | Substrate | Product | Substrate/Reagentsa | Time | Yield (%)b | |
|---|---|---|---|---|---|---|
| I | II | |||||
| 1 | 3 | 0.45 | 235 min | 99 | ||
| 2 | 3 | 0.45 | 15 min | 90 | ||
| 3 | 3 | 0.45 | 30 min | 94 | ||
| 4 | 3 | 0.45 | 90 min | 98 | ||
| 5 | 3 | 0.45 | 60 min | 93 | ||
| 6 | 3 | 0.45 | 55 min | 99 | ||
| 7 | 3 | 0.45 | 450 min | 98 | ||
| 8 | 3 | 0.45 | 445 min | 97 | ||
| 9 | 3 | 0.45 | 25 min | 92 | ||
| 10 | 3 | 0.45 | 440 min | 96 | ||
| 11 | 3 | 0.45 | 20 min | 99 | ||
| 12 | 3 | 0.45 | 560 min | 99 | ||
| 13 | 3 | 0.45 | 65 min | 99 | ||
| 14 | 3 | 0.45 | 125 min | 98 | ||
| 15 | 3 | 0.45 | 20 min | 96 | ||
| 16 | 3 | 0.45 | 20 min | 94 | ||
| 17 | 3 | – | 24 h | No reaction | ||
a. I refers to mmol of guanidinium nitrate and II refers to grams of silica sulfuric acid.
b. Isolated yield; all products were identified by comparison with authentic samples (mp, IR, 1 H and 13 C NMR).
All oxidation reactions have been performed heterogeneously. The pure product was easily obtained by simple filtration, washing by dichloromethane and evaporation of the solvent. In order to approve the role of acid in the described transformation, the effect of silica sulfuric acid on the oxidation process was investigated. It was found that the reaction did not proceed after 24 h without silica sulfuric acid (Table 2 , Entry 17). Also, it is interesting to note that due to mild reaction conditions, no side reaction, such as nitration, was observed, even for activated aromatic rings, such as Entries 4, 5, 14 and 15 from Table 2 .
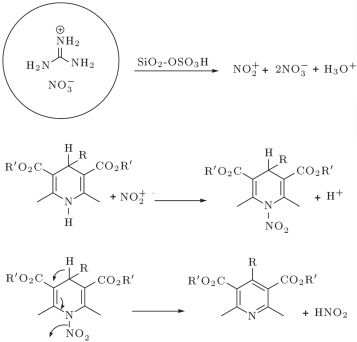
A suggested mechanism for this transformation has been outlined in Scheme 2 .
Scheme 2.
Mechanism of aromatization of 1, 4-dihydropyridines into pyridines.
3. Conclusion
This procedure has the following advantages:
- Short reaction time.
- High selectivity.
- Non-toxic conditions.
- Cost effective reagents.
- Clean and easy work up of products.
- Metal free conditions.
4. Experimental
Chemicals were purchased from Fluka, Merck and Aldrich chemical companies. The oxidation products were characterized by comparison of their spectral (IR, 1 H NMR, and 13 C NMR) and physical data with authentic samples. Silica sulfuric acid was prepared via the reported procedure by Zolfigol [25] .
4.1. General procedure for the oxidation of 1, 4-dihydropyridine to pyridine with guanidinium nitrate and silica sulfuric acid
The reaction was carried out at room temperature in a 25 mL flask equipped with a magnetic stirring bar. Guanidinium nitrate (0.367 g, 1 mmol) and silica sulfuric acid (0.45 g) were added to the 1, 4-dihyropyridine (1 mmol) in CH2 Cl2 (5 mL). The progress of the reactions was monitored by TLC on silica gel GF254 plates with -hexane and acetone (8:2 v/v) as eluent. After the reaction was completed, the residue was washed with CH2 Cl2 (20 mL). Anhydrous Na2 SO4 (1 g) was added to the filtrate and filtered off after 20 min. Finally, CH2 Cl2 was removed and the corresponding pure pyridine was obtained.
Acknowledgment
The financial support for this work from Ilam University, Ilam, Iran is gratefully acknowledged.
References
- [1] S.P. Chavan, S.W. Dantale, U.R. Kalkote, V.S. Jyothirmai, R.K. Kharul; An efficient oxidation of 1, 4-dihydropyridines using aqueous tert-butylhydroperoxide; Synth. Commun., 28 (1998), pp. 2782–2792
- [2] B. Wang, Y. Hu, H. Hu; The essential role of Saccharomyces cerevisiae CDC6 nucleotide-binding site in cell growth, DNA synthesis, and Orc1 association; Synth. Commun., 29 (1999), p. 4193
- [3] (a) Y. Ishii, S. Sakaguchi, T. Iwahama; Innovation of hydrocarbon oxidation with molecular oxygen and related reactions; For a review, see Adv. Synth. Catal., 343 (2001), pp. 393–427(b) Y. Ishii, T. Iwahama, S. Sakaguchi, K. Nakayama, Y. Nishiyama Alkane oxidation with molecular oxygen using a new efficient catalytic system: N-hydroxyphthalimide (NHPI) combined with ; J. Org. Chem., 61 (1996), pp. 4520–4526(c) T. Iwahama, S. Sakaguchi, Y. Ishii Production of hydrogen peroxide via aerobic oxidation of alcohols catalyzed by N-hydroxyphthalimide; Org. Process Res. Dev., 4 (2000), pp. 94–97(d) S. Sakaguchi, Y. Nishiwaki, T. Kitamura, Y. Ishii Efficient catalytic alkane nitration with NO2 under air assisted by N-hydroxyphthalimide ; Angew. Chem. Int. Ed., 40 (2001), pp. 222–224(e) T. Hirabayashi, S. Sakaguchi, Y. Ishii A new route to lactam precursors from cycloalkanes: direct production of nitrosocycloalkanes or cycloalkanone oximes by using tert-butyl nitrite and N-hydroxyphthalimide; Angew. Chem. Int. Ed., 43 (2004), pp. 1120–1123
- [4] X. Cai, H. Yang, G. Zhang; Aromatization of 1, 4-dihydropyridines with selenium dioxide; Can. J. Chem., 83 (2005), pp. 273–275
- [5] M. Litvic, I. Cepanec, M. Filipan, K. Kos, A. Bartolincic, V. Druskovic, M.M. Tibi, V. Vinkovic; Mild, selective, and high-yield oxidation of Hantzsch 1, 4-dihydropyridines with lead(IV) acetate; Heterocycles, 65 (2005), pp. 23–35
- [6] J.J. Xia, G.W. Wang; One-pot synthesis and aromatization of 1, 4-dihydropyridines in refluxing water; Synthesis (2005), pp. 2379–2383
- [7] J.R. Pfister; Rapid, high-yield oxidation of hantzsch-type 1, 4-dihydropyridines with ceric ammonium-nitrate; Synthesis (1990), pp. 689–690
- [8] F. Delgado, C. Alvarez, O. Garcia, G. Penieres, C. Marques; Unusual oxidative dealkylation of certain 4-alkyl-1, 4-dihydropyridines with MNO2 /bentonite using microwave irradiation, in the absence of solvent (II) ; Synth. Commun., 21 (1991), pp. 2137–2141
- [9] J.J. Vandeneynde, A. Mayence, A. Maquestiau; A novel application of the oxidizing properties of pyridinium chlorochromate-aromatization of Hantzsch 1, 4-dihydropyridines; Tetrahedron, 48 (1992), pp. 463–468
- [10] B. Khadikar, S. Brokar; Topological studies on X-ray absorption discontinuity: estimation of effective nuclear charge (Z(eff)) from Wiener index; Synth. Commun., 28 (1998), pp. 207–211
- [11] J.J. Vandeneynde, F. Delfosse, A. Mayence, Y. VanHaverbeke; Old reaction, new results-aromatization of hantzsch 1, 4-dihydropyridines with manganese-dioxide and 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone; Tetrahedron, 51 (1995), pp. 6511–6516
- [12] H.R. Memarian, M.M. Sadeghi, A.R. Momeni; Aromatization of Hantzsch 1, 4-dihydropyridines using barium manganate; Synth. Commun., 31 (2001), pp. 2241–2244
- [13] S.H. Mashraqui, M.A. Karnik; Catalytic oxidation of Hantzsch 1, 4-dihydropyridines by RuCl3 under oxygen atmosphere ; Tetrahedron Lett., 39 (1998), pp. 4895–4898
- [14] N. Nakamichi, Y. Kawashita, M. Hayashi; Oxidative aromatization of 1, 3, 5-trisubstituted pyrazolines and hantzsch 1, 4-dihydropyridines by Pd/C in acetic acid; Org. Lett., 4 (2002), pp. 3955–3957
- [15] N. Nakamichi, Y. Kawashita, M. Hayashi; Activated carbon-promoted oxidative aromatization of Hantzsch 1, 4-dihydropyridines and 1, 3, 5-trisubstituted pyrazolines using molecular oxygen; Synthesis (2004), pp. 1015–1020
- [16] M.M. Heravi, F.K. Behbahani, H.A. Oskooie, R.H. Shoar; Catalytic aromatization of Hantzsch 1, 4-dihydropyridines by ferric perchlorate in acetic acid; Tetrahedron Lett., 46 (2005), pp. 2775–2777
- [17] M. Balogh, I. Hermecz, Z. Meszaros, P. Laszlo; Clay-supported reagent-aromatization of 1, 4-dihydropyridines by clay-supported metal nitrate; Helv. Chim. Acta, 67 (1984), pp. 2270–2272
- [18] G. Sabitha, G.S.K.K. Ready, C.S. Reddy, C. Srinivas, N. Fatima, J.S. Yadav; Zr(NO3)(4): a versatile oxidizing agent for aromatization of Hantzsch 1, 4-dihydropyridines and 1, 3, 5-trisubstituted pyrazolines; Synthesis (2003), pp. 1267–1271
- [19] M.M. Hashemi, Y. Ahmadibeni, H. Ghafuri; Aromatization of Hantzsch 1, 4-dihydropyridines by hydrogen peroxide in the presence of cobalt(II) acetate; Monatsh. Chem., 134 (2003), pp. 107–110
- [20] A. Sausins, G. Duburs; Reaction of 1, 4-dihydropyridines; Heterocycles, 27 (1988), pp. 291–314
- [21] S.P. Chavan, R.K. Kharul, U.R. Kalkote, I. Shivakumar; An efficient Co(II) catalyzed auto oxidation of 1, 4-dihydropyridines; Synth. Commun., 33 (2003), pp. 1333–1340
- [22] A. Ghorbani-Choghamarani, J. Zeinivand; Aromatization of Hantzsch 1, 4-dihydropyridines with Al(NO3 )3 center dot 9H2 O and/or Fe(NO3 )3 center dot 9H2 O in the presence of silica sulfuric acid under mild and heterogeneous conditions ; Synth. Commun., 40 (2010), pp. 2457–2463
- [23] A. Ghorbani-Choghamarani, M. Nikoorazm, H. Goudarziafshar, L. Shiri, Z. Chenani; Oxidation of Hantzsch 1, 4-dihydropyridines using supported nitric acid on silica gel and poly vinyl pyrrolidone (PVP) under mild and heterogeneous conditions; Bull. Korean Chem. Soc., 30 (2009), pp. 972–974
- [24] B. Zeynizadeh, K.A. Dilmaghani, A. Roozijoy; Oxidative-aromatization of Hantzsch ester 1, 4-dihydropyridines by KBrO3 /SnCl4 center dot 5H(2)O under mild condition ; Synth. Commun., 35 (2005), pp. 557–562
- [25] M.A. Zolfigol; Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions ; Tetrahedron, 57 (2001), pp. 9509–9511
Document information
Published on 06/10/16
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?