Abstract
A study was carried out to assess levels of contamination of aflatoxins and fumonisins (B1 + B2 ) in maize produced, stored and consumed in rural households in Malawi. A total of 9 districts were selected across the country representing 3 districts from each of the Northern, Central and Southern regions respectively. Households were selected at random in each district where 10 maize samples were collected for laboratory analysis. Aflatoxins and fumonisins were analyzed using a single step lateral flow immunochromatographic assay based on a competitive immunoassay format. The detection limit for aflatoxins was 2 μg/kg with a quantitation range of 2–150 μg/kg and that for fumonisins was 1 mg/kg with a quantitation range of 1–7 mg/kg. It was found that samples in the Southern region were highly contaminated, with the Chikhwawa district having high levels of both aflatoxins and fumonisins in maize. The Northern region had the least contamination. The maximum detected amount of aflatoxins was 140 μg/kg. The maximum detected amounts of fumonisins was 7 mg/kg. About 20% of maize samples exceeded the tolerable maximum limit for aflatoxins in Malawi. Aflatoxins and fumonisins were found to co-occur with contamination levels exceeding 100 μg/kg for both aflatoxins and fumonisins.
Keywords
Mycotoxins ; Aflatoxin ; Fumonisins ; Malawi
1. Introduction
Mycotoxicosis in sub-Saharan Africa is due mainly to aflatoxin contamination. About 250,000 hepatocellular carcinoma-related deaths occur annually in parts of sub-Saharan Africa due to aflatoxin ingestion alone [30] . Up until the mid-1990’s reports of acute aflatoxin poisonings, approximately 25% of which result in deaths has been reported [30] .
No serious mycotoxicosis outbreak has been reported so far in Malawi, but the climatic conditions, outbreaks of mycotoxicosis in neighboring countries and knowledge of pre- and post-harvest practices strongly suggest that Malawians are consuming mycotoxin-contaminated foods. This research, therefore, was aimed at assessing the extent of mycotoxin contamination in maize consumed in Malawi.
Malawi is a country in southern Africa with a population of about 17 million people. The backbone of Malawi’s economy is agriculture, which employs about 90% of the population. Agriculture contributes more than 35% of the countrys gross domestic product (GDP) and accounts for almost 85% of the export earnings [18] . Maize in Malawi is the most important staple food crop; it is grown by 97% of farming households and accounts for 60% of total food consumption. It is cultivated on more than 70% of the total arable land and contributes significantly to diets of more than 80% of the population, with per capita consumption of 182 kg per year [16] .
Over half of Malawi’s farming households operate below subsistence. Because of low productivity and small farm size, only 20% of maize farmers produce surplus and sell their product [6] . This means that about 80% of these farmers are not able to produce enough maize for their own home consumption. Most farmers will sell the best quality maize that they have. As a result what is left as food for family consumption is frequently grain of poor quality, some of which may be contaminated by mycotoxins, leaving this population at a health risk.
Food safety with its relationship to food quality in the developing countries of Africa is an issue which frequently must be balanced by issues of food security with an emphasis on sufficiency of supply [25] .
The lack of an effective regulatory and enforcement framework coupled with a lack of consumer awareness and understanding of the effect of molds and mycotoxins on human health combine to increase risk to human health. Currently in Malawi there are insufficient data on the extent of fumonisin prevalence and there is only limited data regarding aflatoxin contamination in maize. Aflatoxins are regulated in Malawi, but this only applies to maize or any farm produce meant for export or for those products meant for super markets. Fumonisins on the other hand are not regulated currently in Malawi; however, there is as much risk associated with consuming maize contaminated with fumonisin as is the effect of consuming maize contaminated with aflatoxins [20] and [32] . It has been shown in Tanzania that aflatoxins coexist with fumonisins in maize [14] , and there is also evidence suggesting that aflatoxins act synergistically with fumonisins [20] putting consumers at more risk from their combined effects. When animals or humans consume foods contaminated with aflatoxins, AFB1 is metabolized in the liver leading to formation of highly reactive chemical intermediates. The binding of these intermediates to DNA results in the disruption of transcription and in abnormal cell proliferation, leading to mutagenesis and carcinogenesis [10] , [12] and [24] . Consumption of moldy maize containing fumonisin B1 has been associated with an outbreak of abdominal pain and diarrhea in India [2] . Pathogenic effects due to fumonisin ingestion in animals include leukoencephalomalacia, pulmonary edema, hepatotoxicity, hepatocarcinogenicity and nephrotoxicity [23] . Consumption of contaminated maize has been associated with an elevated risk of human esophageal cancer in the Transkei region in South Africa and China [32] . It has been shown that culture material of Fusarium verticillioides was hepatocarcinogenic in rats, exhibiting both initiating and promoting effects. FB1 was subsequently shown to be a liver cancer promoter in a diethyl nitrosamine-initiated rat model. Fumonisins, in particularly FB1 are prototypic inhibitors of cellular sphingosine (sphinganine) N -acetyltransferase. Inhibition of this enzyme is followed by an accumulation of sphinganine and sometimes also sphingosine and a depletion of complex sphingolipids in eukaryotic cells. The beginning and progression of diseases associated with FB1 have a close relationship to the disruption of sphingolipid metabolism [17] , [21] and [29] . This leads to impairment of cell cycle regulation and cellular differentiation. It also results in oxidative stress as well as apoptosis and necrosis [11] . Altered apoptosis and mitosis is thought to contribute to carcinogenesis through an altered balance of cell death and replication [31] .
Approximately 25% of the world’s food crops are affected each year by mycotoxins [4] with aflatoxin and fumonisin contamination being of particular importance. Most African countries are lagging behind industrialized countries in pre- and post-harvest practices that would minimize mycotoxin consumption. Donating (“dumping”) mycotoxin contaminated food products and the introduction of contaminated commodities into the human food chain during acute and chronic food shortage due to drought, political and economic instability also contribute to the problem.
Aflatoxins are produced by the fungi Aspergillus parasiticus and Aspergillus flavus as secondary metabolites when the temperatures are between 24 °C and 35 °C. They form in many commodities in conditions of excess moisture during harvest and storage. Aflatoxins are considered by the United States Food and Drug administration (USFDA) to be unavoidable contaminants of foods.
Among the aflatoxins and their metabolites, only AFB1 , AFB2 , AFG1 and AFG2 have been found as natural contaminants in agricultural products. They cause mycotoxicosis in poultry and mammals. Acute aflatoxicoses have been reported in humans in Taiwan, Canada, Uganda, Germany, India and Kenya [3] , [27] and [8] .
AFB1 ingestion by humans is becoming increasingly important as new results from laboratory animal studies and epidemiological studies are reported. Chronic aflatoxicosis with high incidence of primary liver cancer has been reported in Uganda, Thailand, Kenya, Mozambique and China [3] and [27] . Aflatoxin ingestion impaired child growth in Benin and Togo [9] . Despite of nearly 50 years of research, the extent of the global exposure to this carcinogen is still poorly documented, hampering estimation of the associated disease burden. Currently, the World Health Organization does not recognize mycotoxins as a disease burden. Using aflatoxin biomarkers, it has been shown that aflatoxins cross the placental barrier as revealed by the presence of aflatoxin albumin adducts in cord blood samples [31] . In West Africa, this exposure has been shown to continue in infancy and once children are weaned, they have a similar high prevalence and level of exposure as observed in adults [31] . The regulatory limit for aflatoxins in Malawi is 3 μg/Kg but which is in the process of revision to match the limit in the Common Market for Eastern and Southern Africa (COMESA) harmonised standard.
Fumonisins, like aflatoxins, are a group of toxic metabolites produced by the molds Fusarium verticillioides, F. proliferatum and F . nygamai with Fusarium verticillioides being the predominant contaminant in food and feeds, (Michael and Wyatt, 1993). Fumonisins were first isolated in 1988 and consist of a long hydroxylated hydrocarbon chain with added tricarboxylic acid, methyl, and amino groups. They are polyols with a long chain (20 carbons) esterified in the C14 and C15 with two groups of tricarboxylic acids. Fumonisin B1 (FB1 ), Fumonisin B2 (FB2 ) and Fumonisin B3 (FB3 ) are the major naturally occurring fumonisins. However, Fumonisin A1 and A2 (FA1 & FA2 ) also occur naturally [23] . In 1993, the International Agency for Research in Carcinogenesis, (IARC) classified fumonisins as Group 2B compounds – “probably carcinogenic for humans” [13] . Fumonisin contamination of maize occurs in many parts of the world with reported levels greater than 100 mg/Kg in some regions. Fumonisin contamination of agricultural produce is dependent on geographical region, season and the conditions under which the particular grain is grown, harvested and stored. Grain grown in tropical and subtropical regions is more prone to fumonisin contamination due to the relatively long and warm growing season Michael and Wyatt, 1993. Contamination of corn with high levels of fumonisin has been reported in Tanzania, South Africa, United States and China [14] , [7] , [8] and [26] .
Fumonisin B1 is considered the most prevalent and most toxic derivative within the group of fumonisins [31] . Contamination of cereals with the fungus Fusarium moniliforme , a common contaminant of corn throughout the world, has been associated with several human and animal diseases. Consumption of moldy maize containing fumonisin B1 has been associated with an outbreak of abdominal pain and diarrhea in India [2] . Pathogenic effects due to fumonisin ingestion in animals include leukoencephalomalacia, pulmonary edema, hepatotoxicity, hepatocarcinogenicity and nephrotoxicity [23] . Consumption of contaminated maize has been associated with an elevated risk of human esophageal cancer in the Transkei region in South Africa and China [32] . The regulatory limit for fumonisins in the United States is 2–4 mg/Kg (2 to 4 ppm) in foods meant for direct human consumption [28] and in the European Union it is 4 mg/Kg (4 ppm) in foods meant for further processing and 1 mg/Kg (1 ppm) in foods meant for direct human consumption, (European Union 1881/2006). The maximum tolerable daily intake limit as set by the Food and Agriculture Organization (FAO)/World Health Organization (WHO) is 2 μg/kg body weight/day for FB1 , FB2 and FB3 alone or combined.
The regulatory limit by Codex Alimentarius is 4 mg/kg (4 ppm) for fumonisins B1 + B2 in unprocessed corn [5] . No regulatory limits have been set in Malawi for fumonisins.
2. Materials and methods
2.1. Sampling plan for maize
Maize is grown and consumed throughout Malawi and therefore a country-wide sampling plan was established. Malawi is divided politically into Northern, Central, and Southern Regions. Each region is divided into districts and three districts from each region were randomly chosen for sampling. The districts selected are shown in Table 2 . In each district 10 households were randomly selected to provide samples. 1 kg of maize from the bag of maize currently being consumed was sampled from the selected households. The maize samples taken were those stored from the produce harvested during the current growing season from existing subsistence farms of the households. A total of 30 samples of maize were collected per region resulting in a total of 90 samples for the whole country (Table 1 and Fig. 1 ).
| Region | District | ||
|---|---|---|---|
| North | Karonga | Nkhata-Bay | Mzimba |
| Central | Kasungu | Lilongwe | Salima |
| South | Machinga | Mulanje | Chikhwawa |
|
|
|
Fig. 1. Map of Malawi showing districts sampled. |
2.2. Sample preparation and extraction
The aflatoxin and fumonisin content of maize samples was determined according to the manufacturer’s directions provided with Reveal Q+ kits (Neogen® Corporation, Lansing, MI, USA). Briefly, the 1 kg samples collected from rural households were thoroughly mixed and 500 g was ground with a blender (OMNIBLEND V-Heavy duty professional blender, TM-800A, JTC-China). The ground samples were stored in plastic bags in a cool, dry place until analyzed. 10 g of a ground sample was weighed into a 250 ml round bottomed flask using a top-loading pan balance (METTLER PJ 300, METTLER instrument AG, CH-8606 Greifensee-Zurich Switzerland). 50 ml of 65% ethanol was added to the flask and mycotoxins were extracted by shaking the mixture for 3 min. The mixture was filtered through fluted filter paper (Whatman No. 1, WHATMAN International Ltd., Mad stone, England) and both aflatoxin and fumonisin assays were performed on the 65% ethanol extract. The Reveal Q+ kits are for total aflatoxin and fumonisin quantitation, they are single-step lateral flow immunochromatographic assays, based on a competitive immunoassay format. Lateral flow strips coated with antibodies interact with antigen (mycotoxin) molecules in the sample extract. The developed strip was removed and inserted into a Reveal Accuscan III Reader System (AS 5130, Neogen® Corporation, Lansing, MI, USA) and the reader displayed the aflatoxin or fumonisin content of the sample. The Reveal Q+ assay for aflatoxin is quantitative for total aflatoxins. The linear range of detection is 2–150 μg/Kg. The Reveal Q+ assay for fumonisin is semi-quantitative for quantification of B1 plus B2 . The linear range of detection for B1 plus B2 is 1–7 mg/kg. Maize samples were analyzed in duplicate.
2.3. Statistical analyses
The differences in group means of the ranked scores for aflatoxin and fumonisin contamination in each district sampled were tested for significance (ρ ˂ 0.05) by using the Kruskal–Wallis non-parametric multiple comparison test for all pairwise differences between means. Means and standard deviations for each district were calculated individually using Microsoft Excel 2010.
3. Results and discussion
3.1. Aflatoxin contamination
All maize samples contained detectable concetrantions of aflatoxin. The maximum concentration of aflatoxin in maize was 140 μg/kg with an overall mean of 8.3 μg/kg ± 8.2 for maize collected from 90 households (Table 2 ).
| Parameter | Aflatoxin (μg/kg) |
|---|---|
| Overall mean±SD (μg/kg) | 8.3±8.2 |
| Range, all samples (μg/kg) | 0.7–140 |
| No. of districts | 9 |
| No. of samples | 90 |
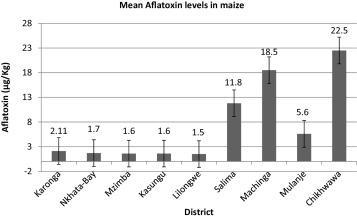
The highest aflatoxin concentrations were observed in the Southern region in the Chikhwawa district with mean of 22.5 followed by Machinga, 18.5 and then Salima with 11.8 μg/kg (Fig. 2 ). All three districts sampled in the Northern region and two in Central region had mean aflatoxin concentration below 3 μg/kg. About 20% of the households were consuming maize that exceeded the regulatory limit for aflatoxins in Malawi (3 μg/kg, Table 3 ).
|
|
|
Fig. 2. Average aflatoxin concentrations in maize in nine districts representing a cross section of Malawi. |
| Maize samples | |
|---|---|
| Maximum tolerable limits (μg/kg) for: Malawi (3) | 71 |
| European Union (3–4) | 3 |
| USA (15–20) | 2 |
| No. of samples containing >20μg/kg | 7 |
Chikhwawa and Machinga had more households with aflatoxin contamination greater than 3 μg/kg, the regulatory limit for aflatoxin in Malawi. Based on the Kruskal–Wallis test, there were significant differences in aflatoxin contamination between districts (p ≤ 0.05). Even though highest contamination was on average observed in Chikhwawa, Machinga and Salima, only a few households were observed to have the highest contamination, like 2, 1 and 3 households in Chikhwawa, Salima and Machinga, respectively. The differences in contamination may probably be attributable to poor pre and post harvest practices. These samples were taken from the bags of maize currently being consumed, and the differences in contamination is an indication of the differences in handling of the produce.
Only about 21% of maize, samples exceeded the tolerable maximum limit for Malawi. These results compare very well with those reported by Sangere-Tigori et al. [22] in Ivory Coast, Madbouly et al. [15] in Egypt, and Kimanya et al. [14] in Tanzania. In a similar study in Malawi, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) and National Association of Smallholder Farmers of Malawi (NASFAM) reported very high levels of aflatoxin in maize, 1335 μg/kg [19] . However, in the previous and current study, the southern region in general and the Chikhwawa district in particular had the highest aflatoxin contamination. In the previous study this was attributed to high literacy levels in the North as compared to farmers in the Central and Southern regions respectively.
Fig. 2 shows mean aflatoxin concentrations for all districts sampled. Samples obtained from the Chikhwawa district had the highest aflatoxin concentrations for maize, with a mean value of 22.5 μg/kg ± 46.51. Mean values above maximum tolerable limit for Malawi were observed in Chikhwawa, Mulanje, Machinga and Salima. Machinga and Chikhwawa had more households with aflatoxin concentrations greater than the maximum regulatory limit in the country (3 μg/kg).
3.2. Fumonisin contamination
Unlike aflatoxin almost all maize samples contained non-detectable concentrations of fumonisin. Seventy-six out of ninety samples (84%) of maize tested had fumonisin levels <1 mg/kg the maximum tolerable limit for fumonisin in the European Union in maize. The maximum concentration of fumonisin (B1 + B2 ) in maize was 7 mg/kg with an overall mean of 0.9 mg/kg ± 1.0 for the maize collected in all the 90 households (Table 4 ).
| District | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mulanje | Nkhata Bay | Kasungu | Lilongwe | Chikhwawa | Mzimba | Salima | Machinga | Karonga | |
| 47.9 | 1.3 | 1.6 | 1.3 | 69.8a | 1.6 | 1.5 | 10.9 | 2.5 | |
| 0.8 | 1.3 | 1.4 | 1.4 | 140a | 3.5 | 1.4 | 67.5a | 2 | |
| 0.9 | 1.1 | 1.5 | 1.7 | 0.7 | 1.6 | 120.7a | 1.1 | 1.4 | |
| 0.8 | 5.7 | 1.8 | 1.3 | 0.8 | 1.4 | 5.7 | 5.5 | 3.2 | |
| 0.8 | 1.6 | 1.6 | 1.6 | 1.4 | 1.6 | 1.8 | 0.9 | 1.9 | |
| 0.8 | 1.3 | 1.1 | 1.6 | 1.3 | 1.2 | 1.5 | 42.5a | 2 | |
| 1.2 | 1.4 | 1.5 | 1.5 | 5.6 | 1.6 | 1.8 | 10.2 | 2.2 | |
| 1.3 | 1.1 | 1.2 | 1.6 | 1.2 | 1.2 | 1.4 | 44.5a | 1.6 | |
| 0.9 | 1.6 | 1.2 | 1.4 | 2.7 | 1.3 | 1.9 | 1.2 | 2.2 | |
| 0.9 | 1.1 | 1.6 | 1.3 | 1.2 | 1.7 | 1.8 | 10.9 | 2.1 | |
| Ave (μg/kg) | 5.63 | 1.75 | 1.45 | 1.47 | 22.47 | 1.67 | 13.95 | 19.52 | 2.11 |
| SD | 14.85 | 1.40 | 0.22 | 0.15 | 46.51 | 0.67 | 37.53 | 23.35 | 0.49 |
| Households with aflatoxin>3μg/kg | 1 | 1 | 0 | 0 | 3 | 1 | 2 | 7 | 1 |
| ab | ab | abc | abcd | bcd | bcd | bcd | cd | d | |
a. Values are much greater than other households in same district leading to large standard deviations and skewed means.
b. Districts with similar letters beneath them have similar aflatoxin concentrations.
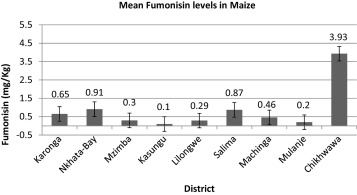
The highest fumonisin concentrations in maize were observed in the Southern region in the Chikhwawa district (Fig. 3 ). About 60% of maize samples (6 households out of 10) analyzed in the Chikhwawa district had fumonisin levels >4 mg/kg, the maximum regulatory limit in the United States (Table 5 ).
|
|
|
Fig. 3. Average fumonisin concentrations in maize in nine districts representing a cross section of Malawi. |
| Parameter | Fumonisin (mg/kg) |
|---|---|
| Overall mean±SD (mg/kg) | 0.9±1.0 |
| Range, all samples (mg/kg) | 0.1–7 |
| No. of districts | 9 |
| No. of samples | 90 |
There was a significant difference (p ≤ 0.05) in fumonisin contamination between the districts with Chikhwawa having the highest contamination with 60% of households exceeding the maximum tolerable limit of fumonisins in the European Union and United States and Kasungu the lowest with one household having high level of fumonisin contamination, (7 mg/kg). The Chikwawa district had the highest fumonisin contamination probably due to the existing long and warn moist conditions prevalent in the district that favour growth of the fusarium fungi. Despite of having more households with high fumonisin concentrations Chikhwawa was still not significantly different from districts; Nkhata Bay, Machinga, Karonga, Mulanje and Lilongwe, (Table 6 ). Based on the Krusakal–Wallis group rank mean scores, Kasungu had the lowest and Chikhwawa the greatest fumonisin (B1 + B2) contamination of maize (p ≤ 0.05) (Table 7 ).
| Maize samples | |
|---|---|
| Maximum tolerable limits (mg/kg) for: European Union (<1) | 76 |
| USA (1–4) | 5 |
| No. of samples containing >4mg/kg | 9 |
| District | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Kasungu | Mzimba | Salima | Lilongwe | Mulanje | Karonga | Machinga | Nkhata Bay | Chikhwawa | |
| 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.6 | 0.9 | 0.8 | 0.4 | |
| 0.1 | 1.9 | 0.1 | 0.1 | 0.1 | 0.2 | 0.9 | 1 | 7a | |
| 0.1 | 0.1 | 7a | 0.1 | 0.2 | 1.4 | 0.1 | 1.8 | 7a | |
| 0.1 | 0.1 | 0.7 | 0.2 | 0.1 | 0.8 | 0.2 | 0.2 | 7a | |
| 0.1 | 0.1 | 0.2 | 0.5 | 0.2 | 0.1 | 0.2 | 0.1 | 7a | |
| 0.1 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.4 | 0.2 | 0.1 | |
| 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 2.6 | 0.1 | 0.5 | 0.4 | |
| 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.1 | 0.4 | 0.1 | 7a | |
| 0.1 | 0 | 0.2 | 0.4 | 0.2 | 0.2 | 0.5 | 4.1 | 0.1 | |
| 0.1 | 0.1 | 0.1 | 1.2 | 0.1 | 0.2 | 0.9 | 0.3 | 7a | |
| Ave (mg/kg) | 0.1 | 0.3 | 0.87 | 0.29 | 0.18 | 0.65 | 0.46 | 0.91 | 3.93 |
| SD | 1.46 E-17 | 0.57 | 0.20 | 0.35 | 0.09 | 0.80 | 0.33 | 1.24 | 0.17 |
| Households with FB(1+2)>5mg/kgc | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 |
| ab | a | ab | ab | b | b | b | b | b | |
a. Values are much greater than other households in same district.
b. Districts with similar letters beneath them have similar FB (1 + 2) concentrations.
c. Codex proposed maximum allowed limit.
Currently, fumonisins are not being regulated in Malawi and as such, levels of maximum contamination have not yet been set. Meanwhile, Codex Alimentarius has set the maximum limit for fumonisins in food at 4 mg/kg [5] . Only about 10% of maize, samples exceeded the maximum tolerable limit for the European Union and United States (Table 6 ). These results compare very well with those reported in other studies by Madbouly et al. [15] in Egypt and Kimanya et al. [14] in Tanzania. Similar results were also reported by Fandohan et al. [7] in Benin with total fumonisins ranging from 0.6–2.4 mg/kg in Zimbabwe and the Transkei region of South Africa [8] and [26] . In two of the samples from the Chikhwawa district and one from Salima, aflatoxins and fumonisins were present in high concentrations in maize. The levels were 140 μg/kg and 7 mg/kg for Chikwawa and 120 μg/kg and 7 mg/kg for Salima in maize for aflatoxins and fumonisins. This is in agreement with results from Tanzania where aflatoxins and fumonisins were found to coexist [14] .
Malawi is a maize-deficient country. The staple food shortage in the country was estimated at 700,000 t in 2002 [1] . The major causes of food shortage in Malawi include sporadic rainfall and frequent droughts, high fertilizer costs and lack of farm input loan facilities. Food safety in Malawi is subject to issues of food security, especially where food shortages are caused by natural phenomena such as drought. Many subsistence farmers in Malawi and in Africa in general [25] are reliant on the consumption of home-grown crops, irrespective of the quality considerations normally applied in the developed world. Even with adequate crops, poor traditional storage facilities often lead to deterioration of these crops. Given these harsh realities, it is not surprising that mycotoxin contamination, (aflatoxins in particular) of staple foods in this study was detected in most of the samples collected. Although only recognized during the previous century, human and animal mycotoxicoses resulting from fungal contamination have presumably existed for centuries in Africa [25] , none of which have been reported in Malawi either due to lack of proper documentation or due to ignorance at the local level, or as noted from the results most of the contamination is at low levels which may lead to chronic effects after a long term of exposure. Symptoms due to low, chronic intakes are difficult to associate with mycotoxin consumption.
Matumba et al. [16] reported that most of the traditional methods of processing maize reduce aflatoxins by an average of 40% with the best process reducing aflatoxin levels by 80%. Although most of the maize was contaminated, 82% of the aflatoxin contamination was less than 3 μg/kg. With a 40% reduction in contamination by processing, acute mycotoxicoses from aflatoxin and fumonisin is unlikely. Apart from outbreaks of acute aflatoxicosis, aflatoxin exposure is thought to substantially contribute to the disease burden of African communities due to chronic consumption of low levels of aflatoxins.
Studies on the correlation between the incidence of primary hepatocellular carcinoma and human exposure to aflatoxins in a number of African countries (Kenya, Mozambique, and Swaziland) helped demonstrate the role of aflatoxin as a human carcinogen [33] . The relationship between aflatoxins and the childhood disease of kwashiorkor is not clear. Although kwashiorkor is widely thought to be a form of protein energy malnutrition, there are hypotheses suggesting that some characteristic features of the disease are known to be among the pathological effects caused by aflatoxins in animals [33] and [32] . The prevalence and level of human exposure to mycotoxins in Malawi is neither well documented nor assessed. However, the prevalence and level of human exposure to aflatoxins on a global scale has been reviewed and the resulting conclusion was that approximately 4.5 billion persons living in developing countries, including Malawi, are chronically exposed to largely uncontrolled amounts of the toxin [33] . In locations where aflatoxin exposure has been studied, chronic low level intakes result in poor nutrition and immunity status [33] . It is also hypothesized that the aflatoxin exposure and the toxic effects of aflatoxins on immunity and nutrition combine to negatively affect health factors, including HIV and AIDS which is currently a major issue in Malawi and many other Sub Saharan African Countries.
This is the first time fumonisins have been studied in Malawi, and as shown in Table 6 and in Fig. 3 , levels greater than 6 mg/kg were detected. With these findings it means people in the affected areas are at risk of the negative effects of mycotoxin ingestion. The fact that fumonisins have been detected it is a clear indication that other Fusarium mycotoxins are likely present [22] putting people at even higher risk.
4. Study limitations
This study only looked at the prevalence of the mycotoxins in maize consumed by people in the rural Malawi but did not look at the actual exposure prevalence of the population to determine if the aflatoxin and exposure problem exists in the population. Also the method used could only determine total aflatoxins and fumonisins. There is need therefore to determine the actual prevalence of the individual toxins, AFB1, AFB2, AFG1 and AFG2 as well as the FB1 and FB2 with methods like the High Performance Liquid Chromatography (HPLC).
5. Conclusion
This study confirms that aflatoxins and fumonisins are widespread contaminants of maize in food intended for human consumption in Malawi. It shows that populations in the rural areas of Malawi may be at a high risk of exposure to unacceptably high levels of aflatoxins and fumonisins especially in the Chikhwawa and Machinga districts in the Southern part of the country where relatively high levels of both aflatoxins and fumonisins were observed. The findings of this study should trigger further research that will generate data on the aflatoxin and fumonisin exposure among Malawians especially children.
Based on this study, there clearly is a need to assess the extent of exposure in the rural population using biomarkers. People in rural areas should also be made aware of the existence of these contaminants in their food crops and the risks associated with them.
Transparency document
References
- [1] F.K. Akinnifesi, W. Matumba, F.R. Kwesiga; Sustainable maize production using Gliricidia /maize intercropping in Southern Malawi ; Exp. Agric., 42 (2006), pp. 441–457
- [2] R.V. Bhat, P.H. Shetty, R.P. Amruth, R.V. Sudershan; A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins; Clin. Toxicol., 35 (1997), pp. 249–255
- [3] J.M. Casado, M. Theumer, D.T. Massih, S. Chulze, H.R. Rubinstein; Experimental sub chronic mycotoxicoses in mice: individual and combined effects of dietary exposure to fumonisins and aflatoxin B1; Food Chem. Toxicol., 39 (6) (2001), pp. 579–586
- [4] A.K. Choudhary, P. Kumari; Management of mycotoxin contamination in pre-harvest and postharvest crops: present status and future prospects; Pathology (Phila.), 2 (7) (2010), pp. 37–52
- [5] Codex Standard 193–1995. General Standard for Contaminants and Toxins in Food and Feed (2015).
- [6] G. Denning, P. Kabambe, P. Sanchez, A. Malik, R. Flor, R. Harawa, P. Nkhoma, C. Zamba, C. Banda, C. Magombo, et al.; Input subsidies to improve smallholder maize productivity in Malawi: towards an African green revolution; PLoS Biol., 7 (1) (2009), pp. 1–10
- [7] P. Fandohan, D. Zoumenou, D.J. Hounhouigan, W.F.O. Marasas, M.J. Wingfield, K. Hell; Fate of fumonisins during processing of maize into food products in Benin; Int. J. Food Microbiol., 98 (2005), pp. 249–259
- [8] R. Gamanya, L. Sibanda; Survey of Fusarium moniliforme (F. verticillioides) and production of fumonisin B1 in cereal grains and oilseeds in Zimbabwe ; Int. J. Food Microbiol., 71 (2001), pp. 145–149
- [9] Y.Y. Gong, K. Cardwell, A. Hounsa, S. Egal, P.C. Turner, A.J. Hall, C.P. Wild; Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study; Br. Med. J., 325 (2002), pp. 20–21
- [10] F.P. Guengerich; Forging the links between metabolism and carcinogenesis; Mutat. Res., 488 (2001), pp. 195–209
- [11] W.M. Haschek, G. Motelin, D.K. Ness, K.S. Harlin, W.F. Hall, R.F. Vesonder, R.E. Peterson, V.R. Beaskey; Characterization of fumonisin toxicity in orally and intravenously dosed swine; Mycopathologia, 117 (1992), pp. 83–96
- [12] S. Imaoka, S. Ikemoto, T. Shimada, Y. Funae; Mutagenic activation of aflatoxin B1 by pulmonary, renal, and hepatic cytochrome P450s from rats ; Mutat. Res., 269 (1992), pp. 231–236
- [13] International Agency for Research in Carcinogenesis (IARC); Toxins Derived from Fusarium moniliforme : Fumonisins B1 and B2 and Fusarium C. Monograph on the Evaluation of Carcinogenic Risks to Humans, 56, IARC Scientific Publications, Lyon (1993), p. 445
- [14] M. Kimanya, B. Demeulenaer, B. Tiisekwa, F. Devlieghere, S.M. Ndomondo, J. Van Camp, P. Kolsteren; Co-occurrence of aflatoxin and fumonisins in home stored maize for human consumption in rural villages of Tanzania; Food Addit. Contam. Part A—Chem. Anal. Control Expo. Risk Assess., 25 (11) (2008), pp. 1353–1364
- [15] A.K. Madbouly, M.I.M. Ibrahim, A.F. Sehab, M.A. Abdel-Wahab; Co-occurrence of mycoflora aflatoxins and fumonisins in maize and rice seeds from markets of different districts in Cairo,Egypt; Food Add. Contam. Part B, 5 (2) (2012), pp. 112–120
- [16] L. Matumba, M. Monjerezi, E. Chirwa, D. Lakudzala, P. Mumba; Natural occurrence of AFB1 in maize and effect of traditional maize flour production on AFB1 reduction in Malawi; Afr. J. Food Sci., 12 (2009), pp. 413–425
- [17] A.H. Merrill Jr., M.C. Sullards, E. Wang, K.A. Voss, R.T. Riley; Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins; Environ. Health Perspect., 109 (Suppl. 2) (2001), pp. 283–289
- [18] Mkumbila J., Cassava transformation in Southern Africa (CATISA) Project. Available from http://fsg.afre.msu.edu/cassava/malawi_country_report_on_Task2_cassava_productivity.pdf (2007).
- [19] Monyo S., Waliyar F., Osiru M., Siambi M., Chinyamanyamu B., Assessing occurrence and distribution of aflatoxins in Malawi. Available from http://community.eldis.org/.59ee3fb9/Aflatoxins.pdf (2009).
- [20] R.B. Orsi, C.A.F. Oliveira, P. Dilkin, J.G. Xavier, G.M. Direito, B. Correa; Effects of oral administration of aflatoxin B1 and fumonisin B1 in rabits (Oryctolagus cuniculus ) ; Chem. Biol. Interact., 170 (2007), pp. 201–208
- [21] R.T. Riley, E. Enongene, K.A. Voss, W.P. Norred, F.I. Meredith, R.P. Sharma, J. Spitsbergen, D.E. Williams, D.B. Carlson, A.H. Merrill Jr.; Sphingolipids perturbations as mechanisms for fumonisin carcinogenesis; Environ. Health Perspect., 109 (Suppl. 2) (2001), pp. 301–308
- [22] B. Sangere-Tigori, S. Moukha, H.J. Kouadio, A.M. Betbeder, D.S. Dano, E.D. Creppy; Co-occurrence of fumonisin B1, ochratoxin A and zearalenon in cereals and peanuts in Cote d’Ivoire; Food Addit. Contam., 23 (10) (2006), pp. 1000–1007
- [23] M. Segvic, S. Pepeljnjak; Fumonisins and their effects on animal health—a brief review; Vet. Arch., 71 (5) (2001), pp. 299–323
- [24] S. Sell, K.L. Xu, W.E. Huff, L.F. Kabena, R.B. Harvery, H.A. Dunsford; Aflatoxin exposure produces serum alpha-fetoprotein elevations and marked oval cell proliferation in young male Pekin ducklings; Pathology (Phila.), 30 (1998), pp. 34–39
- [25] G.S. Shephard; Aflatoxin and food safety: recent African perspectives; Toxicology, 22 (2&3) (2003), pp. 267–286
- [26] G.S. Shephard, W.F.O. Marasas, H.M. Burger, N.I.M. Somdyala, J.P. Rheeder, L.V. Westhuizen, P. Gatyeni, D.J.V. Schalkwyk; Exposure assessment for fumonisins in the former Transkei region of South Africa; Food Addit. Contam., 24 (6) (2007), pp. 621–629
- [27] W.G. Sorenson, J.P. Simpson, M.J. Peach III, T.D. Thedell, S.A. Olenchock; Aflatoxin in respirable corn dust particles; J. Toxicol. Environ. Health, 7 (3–4) (1981), pp. 669–672
- [28] United States Food and Drug Administration (USFDA) Guidance for industry-fumonisin levels in human foods and animal feeds. Available from http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/default.htm (2001).
- [29] K.A. Voss, R.T. Riley, W.P. Norred, C.W. Bacon, F.I. Meredith, P.C. Howard, R.D. Plattner, T.F. Collins, D.K. Hansen, J.K. Porter; An overview of rodent toxicities: liver and kidney effects of fumonisins and Fusarium moniliforme; Environ. Health Perspect., 109 (Suppl. 2) (2001), pp. 259–266
- [30] J.M. Wagacha, J.W. Muthomi; Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies; Int. J. Food Microbiol., 124 (2008), pp. 1–12
- [31] C.P. Wild, Y.Y. Gong; Mycotoxins and human disease: a largely ignored global health issue; Carcinogenesis, 31 (1) (2010), pp. 71–82
- [32] J.H. Williams, A.J. Grubb, J.W. Davis, V.S. Wang, P.E. Jolly, N.A. Ankrah, W.O. Ellis, E.A. Gyawu, N.M. Johnson, A.G. Robinson, T.D. Phillips; HIV and hepatocellular and esophageal carcinomas related to consumption of mycotoxin-prone foods in sab-Saharan Africa; Am. J. Clin. Nutr., 92 (2010), pp. 154–160
- [33] J.H. Williams, T.D. Phillips, P.E. Jolly, J.K. Stiles, C.M. Jolly, D. Aggarwal; Human aflatoxicosis in developing countries: a review of toxicology exposure, potential health consequences and interventions; Am. J. Clin. Nutr., 80 (2004), pp. 1106–1122
Document information
Published on 02/05/17
Accepted on 02/05/17
Submitted on 02/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?