Highlights
- Ovariectomy accelerates doxycycline-induced acute liver injury.
- The expression levels of IL-6, IL-10, c-fos, cox-2 and HO-1 genes were strongly upregulated in ovx mice.
- Apocynin, totally improved DOXY-induced liver injury in both sham and ovx mice.
- NADPH oxidase is responsible for the development of drug-induced acute liver injury
Abstract
To determine the physiological role of estrogen in the development of liver injury, we examined the sensitivities of sham and ovariectomy (ovx) mice against doxycycline (DOXY)-induced acute liver injury. Ovx or sham operation was performed in C57BL/6J wild-type female mice of eight weeks of age. Sham mice and ovx mice were treated with DOXY (240 mg/kg ip) 8 weeks after the operation, 30 min after apocynin (5 mg/kg) or saline administration. Blood and liver samples were obtained at 3 and 6 h after DOXY administration. Liver dysfunction occurred soon after DOXY administration and became more severe in ovx mice than in sham mice. At early phase after DOXY injection, TNF-α and iNOS inductions upregulated almost the same levels in sham and ovx mice. On the other hand, expression levels of IL-6, IL-10, c-fos, cox-2 and HO-1, downstream genes of TNF-α, were significantly increased in ovx mice compared to those in sham mice, correlated with liver dysfunction. In addition, apocynin, a NADPH oxidase (Nox) inhibitor, totally improved DOXY-induced liver injury in both sham and ovx mice, indicating that reactive oxygen species generated through Nox activation by DOXY are responsible for development of acute liver injury.
Abbreviations
Ovx , ovariectomy ; DOXY , doxycycline ; ALF , acute liver failure ; ARF , acute renal failure ; IL-6 , interleukin-6 ; TNF-α , tumor necrosis factor-α ; STAT3 , signal transducers and activators of transcription-3 ; SOD , superoxide dismutase ; Nox , NADPH oxidase ; ROS , reactive oxygen species ; ALT , alanine aminotransferase ; iNOS , inducible nitric oxide synthase ; cox-2 , cyclooxygenase-2 ; HO-1 , heme oxygenase-1
Keywords
Ovariectmized ; Doxycycline-induced liver injury ; Apocynin ; NADPH oxidase
1. Introduction
Liver tissue has great potential for inducing local and/or systemic inflammatory responses [2] and appears to be a preferred source for and target of cytokine signaling. In fact, hepatic failure might represent the collapse of hepatic homeostasis as a result of an imbalance between damaging and protective signals that are very tightly regulated under physiological conditions [16] and [30] . Estrogen and its derivatives are powerful endogenous antioxidant agents that are able to reduce lipid peroxide levels in the liver and blood [22] . Most of the evidence of estrogen’s role in liver metabolism and inflammation has been obtained by measurements of enzyme expression in ovariectomized (ovx) or aromatase-deficient animals. Ovx mice have been used as estrogen-deficient models that reflect pathologic changes in peri- or post-menopausal women [20] and [23] . Doxycycline (DOXY), one of the tetracycline-derived compounds, is known to induce acute liver failure (ALF) [25] and [14] . Thus, we established a DOXY-induced ALF model in sham- and ovx-operated mice to evaluate the role of estrogen during the development of liver injury.
Interleukin-6 (IL-6) is a cytokine that provokes a broad range of cellular and physiological responses, including immune response, inflammation, hematopoiesis, and oncogenesis, by regulating cell growth, gene activation, proliferation, survival, and differentiation. IL-6 activates the phosphorylation of JAK kinases, signal transducers and activators of transcription-3 (STAT3) and Src homology-2 [9] and [10] . We previously reported that IL-6 induction was observed during the development of cisplatin-induced acute renal failure (ARF) in wild-type (WT) mice and that predominant progression of renal dysfunction in IL-6 knockout (IL-6−/− ) mice was also observed at the early stage, indicating that IL-6 plays a protective role in the progress of ARF [17] , [18] and [19] . We also reported that IL-6 deficiency results in an increase of oxidative stress caused by a decrease of superoxide dismutase (SOD; an anti-oxidative enzyme) activity and over-expressed 4-hydroxy-2-nonenal protein (an oxidative stress marker) in proximal tubular epithelial cells during cisplatin-induced ARF, indicating that IL-6 modulates the generation of oxidative stress and plays a protective role against oxidative stress evoked by cisplatin administration [18] .
TNF-α is a potent proinflammatory cytokine that is primarily secreted from monocytes and macrophases in response to inflammation, infection, and other environmental stresses.
Recent studies have shown that levels of oxidative stress due to reactive oxygen species (ROS) were increased and that levels of enzymatic antioxidants were decreased in ovx mice. NADPH oxidase (Nox) is an enzyme catalyzing the univalent reduction of oxygen to produce the superoxide anion radical, which in turn can be converted into other ROS [4] . Apocynin (4-hydroxy-3-methoxy-acetophenone) is an inhibitor of the intracellular translocation of two critical cytosolic components of the Nox complex present in the cell membrane, which results in the inhibition of peroxynitrite (ONOO− ) formation. Apocynin has also been reported to interrupt activation of redox-sensitive transcription factors [12] . Therefore, we hypothesized that apocynin attenuates DOXY-induced ALF.
The aim of the present study was to elucidate the role of estrogen in and the effect of apocynin on the development of DOXY-induced ALF by determining various physiological parameters and expression of genes related to inflammation and oxidative stress in sham and ovx mice.
2. Materials and methods
2.1. Animals and reagents
Eight-week-old female wild-type C57BL/6J mice (CLEA Japan, Inc., Japan) weighing 15–20 g were used for all of the mouse studies. All animal experiments in this study were approved by the Ethics Committee of Animal Experiments in accordance with the Guidelines for Animal Experiments of Takasaki University of Health and Welfare and the Japanese Government Animal Protection and Management Law. Efforts were made to minimize suffering and to reduce the number of animals used. Mice were maintained on a standard diet and water was freely available. Mice were housed 2–5 per cage under a 12-h light and dark schedule for at least 1 week before surgery. Doxycycline (DOXY, MP Biomedicals, LLC, USA) was freshly prepared in sterile saline adjusted to pH 6 at a concentration of 24 mg/ml. Apocynin (Sigma–Aldrich, Japan) was also freshly prepared in sterile saline at a concentration of 0.5 mg/ml. An ovariectomy (ovx) or sham operation was performed in C57BL/6J female mice at 8 weeks of age and maintained on a standard diet. Eight weeks after surgery, mice were given either apocynin (5 mg/kg) or saline ip 30 min before DOXY (240 mg/kg) or saline ip administration. This single dose of DOXY produced severe liver injury and different survival rates in sham and ovx mice. Under deep anesthesia with Nembutal (Dainippon Sumitomo Pharma, Japan), blood samples were collected via cardiac puncture and kidneys were removed 3 and 6 h after DOXY administration (n = 4–17 per time point).
2.2. Determination of blood alanine aminotransferase and IL-6
Liver function was assessed by determination of blood alanine aminotransferase (ALT) using ALT Test Wako (Wako Pure Chemical Industries, Ltd., Japan). Blood IL-6 levels were determined using an ELISA kit (Invitrogen Corp., USA) according to the manufacturer’s instructions. All samples were measured as triplicates and the mean was calculated for data analysis. A calibration curve and a negative control (a blank well without plasma) were run for each test plate.
2.3. Histology
Liver samples were fixed in 10% phosphate-buffered saline and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin for histopathological analysis. Histopathological evaluation was performed with a quantitative method. Assessment of tissue alterations in 5 different fields for each section examined under a Lyca DFC280 light microscope by Leica Q Win and Image Analysis System (Leica Micros Imaging Solutions Ltd., Cambridge, U.K.). The area consist of normal hepatocytes were calculated in each samples. Representative images from 2–4 mice/group were selected.
2.4. Real-time RT-PCR
Total RNA was obtained from kidney homogenate using TRIZOL Reagent (Invitrogen Corp., USA). RNA extract was treated with DNase using a DNase treatment kit (Takara Bio, Japan) prior to RT-PCR amplification. The expression levels of selected genes were quantified by real-time RT-PCR using an MX-3000P (Agilent Inc., USA). Briefly, total RNA was reverse-transcribed with SuperScript III reverse transcriptase and oligo-dT primers (Invitrogen Corp., USA). SYBR Premix Ex Taq II (Takara Bio. Japan) was used for real-time PCR analysis. The forward and reverse primer sequences for selected genes previously reported [17] , [18] and [19] are listed in Table 1 . The relative quantity (RQ) value (2−ΔΔCt , where Ct is the threshold cycle) normalized to GAPDH amplified was calculated for each sample. RQ values were calculated as fold change in gene expression relative to the control group.
| Gene | Forward | Reverse | References |
|---|---|---|---|
| TNFα | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | [21] |
| iNOS | GGCAGCCTGTGAGACCTTTG | GCATTGGAAGTGAAGCGTTTC | NM0109027 |
| c-fos | CAACGCCGACTACGAGGCGTCAT | GTGGAGATGGCTGTCACCG | [7] |
| IL-6 | TCCAGTTGCCTTCTTGGGAC | GTGTAATTAAGCCTCCGACTTG | [11] |
| IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT | NM0105482 |
| Cox-2 | TTTGTTGAGTCATTCACCAGACAGAT | AGGATGTGTAAGGTTTCAGGGAGAAG | [8] |
| SOD1 | CCTGGGCAATGTGACTGCTG | CAATCACTCCACAGGCCAAG | [5] |
| SOD2 | AACTCAGGTCGCTCTTCAGC | GAACCTTGGACTCCCACAGA | [15] |
| HO-1 | CCTCACTGGCAGGAAATCATC | CCTCGTGGAGACGCTTTACATA | [19] |
| GAPDH | TTCACCACCATGGAGAAGGC | GGCATGGACTGTGGTCATGA | [8] |
2.5. Statistical analyses
Survival data were compared using the logrank test. Comparisons between sham and ovx groups were performed using analysis of variance (ANOVA) followed by the Dunn-Bonferroni test. Significance was defined as p < 0.05.
3. Results
3.1. DOXY-induced acute liver injury was accelerated in ovx mice and apocynin pretreatment prevented liver dysfunction
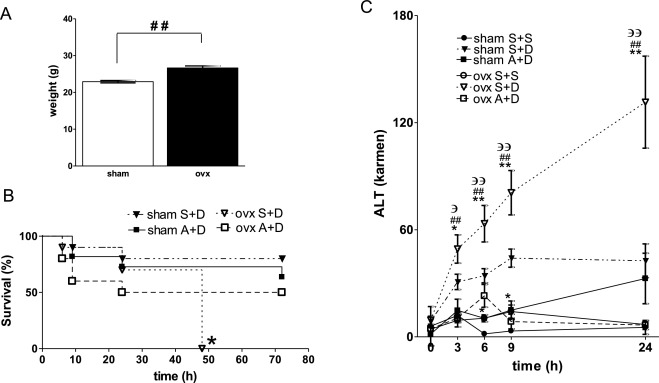
To determine the physiological role of estrogen in the development of liver injury, we examined the sensitivities of sham and ovx mice to DOXY-induced ALF. Eight weeks after surgery, ovx mice gained significantly greater body weight compared with age-matched sham mice (Fig. 1 A). There were no differences in initial body weights among mice before operation. Fig. 1 B shows survival plots of sham and ovx mice after DOXY injection. The survival rate of sham mice was 80% at 72 h after DOXY injection. On the other hand, all ovx mice died within 48 h after DOXY injection. The survival rate of sham mice was better than that of ovx mice. Lack of estrogen accelerated systemic injury caused by DOXY administration. Survival rates of sham apocynin + DOXY (A + D) mice and ovx A + D mice were 72% and 50% at 72 h after DOXY injection, respectively.
|
|
|
Fig. 1. (A) body weight of sham and ovx mice 8 weeks after operation. The average weights of both group before operation were almost the same (20 ± 0.5 g). (B) Survival plots of sham and ovx mice after DOXY injection. Survival rates of sham and ovx mice treated with saline + DOXY (S + D) or apocynin + DOXY (A + D) are presented. *p < 0.05. All ovx mice (n = 10) died within 48 h after saline and DOXY (240 mg/kg) injection. Survival rate of sham mice (n = 10) at 72 h was better than that of ovx mice. (C) Changes in ALT levels in sham or ovx mice treated with saline + saline (S + S), saline + DOXY (S + D) or apocynin + DOXY (A + D) at 0, 3, 6, 9 and 24 h after DOXY injection. *p < 0.05, **p < 0.01 vs sham S + D mice. #p < 0.05, ##p < 0.01 vs ovx A + D mice. Эp < 0.05, ЭЭp < 0.01 vs ovx S + S mice. Data are average ± SE. sham S + S (n = 2), sham S + D (n = 13), sham A + D (n = 8), ovx S + S (n = 2), ovx S + D (n = 13), ovx A + D (n = 7). |
Next, we examined changes in liver function in sham and ovx mice after DOXY injection and/or pretreatment with apocynin. Fig. 1 C shows changes in ALT levels in control, DOXY- and apocynin + DOXY-treated sham and ovx mice. ALT levels in DOXY-treated sham mice tended to be increased compared to those in control mice at 3, 6, 9 and 24 h. ALT levels in DOXY-treated ovx mice were significantly increased compared to those in control ovx mice and in DOXY-treated sham mice at 3, 6, 9 and 24 h. ALT levels in A + D treated sham mice were significantly decreased compared to those in DOXY-treated sham mice at 6 and 9 h. ALT levels in A + D-treated ovx mice were significantly decreased compared to those in DOXY-treated ovx mice at 3, 6, 9 and 24 h. Apocynin improved the survival rate and reduced ALT levels after DOXY injection in both sham and ovx mice. ALT levels in apocynin + saline-treated sham and ovx mice were not changed compared to those in control mice at each time point (data not shown).
3.2. DOXY denatured hepatocytes and apocynin pretreatment prevented morphological damage in the liver
No notable morphological change was observed in livers obtained from control or apocynin-treated sham and ovx mice (Fig. 2 A, B, E and F). Livers obtained from both sham and ovx mice showed denatured hepatocytes around the central vein at 3 h after DOXY injection (Fig. 2 C and G). Livers obtained from ovx mice showed more severe and extensive injury than that in livers from sham mice. Morphological damage in hepatocytes was improved in livers obtained from both apocynin-pretreated sham and ovx mice compared to that in livers from DOXY-treated groups (Fig. 2 D and H). Fig. 2 I summarizes the histological changes in sham and ovx mice.
|
|
|
Fig. 2. Hematoxylin and eosin tissue staining at 3 h after DOXY injection with/without apocynin pretreatment. Sham groups; saline + saline (S + S) (A), apocynin + saline (A + S) (B), saline + DOXY (S + D) (C), apocynin + DOXY (A + D) (D). Ovx groups; saline + saline (S + S) (E), apocynin + saline (A + S) (F), saline + DOXY (S + D) (G), apocynin + DOXY (A + D) (H). Arrows indicate denatured hepatocytes. Normal hepatocyte areas were quantified (I). *p < 0.05, **p < 0.01 vs S + S mice. |
3.3. Apocynin lowered upregulated liver Nox activity after DOXY injection in ovx mice
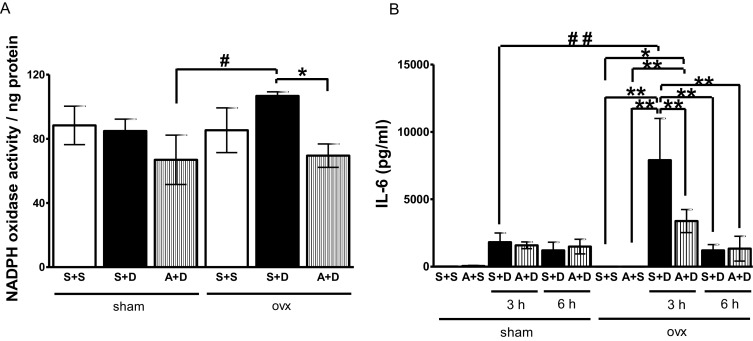
Liver Nox activities were determined by fluorescence analysis [6] . Nox activities in ovx mice were tended to increase compared to those in sham mice at 3 h after DOXY injection (Fig. 3 A). Apocynin significantly lowered upregulated liver Nox activity after DOXY injection in ovx mice.
|
|
|
Fig. 3. (A) Changes in Nox activity in livers obtained from sham or ovx mice at 3 h after DOXY injection with/without apocynin pretreatment. Sham groups; saline + saline (S + S), saline + DOXY (S + D), apocynin + DOXY (A + D). Ovx groups; saline + saline (S + S), saline + DOXY (S + D), apocynin + DOXY (A + D). Data are average ± SE. *p < 0.05 vs ovx (S + D). #p < 0.05 vs sham (A + D). (B) Changes in IL-6 levels in plasma obtained from sham or ovx mice treated with saline (S + S), apocynin + saline (A + S), saline + DOXY (S + D) or apocynin + DOXY (A + D) at 3 and 6 h after DOXY injection. Data are average ± SE. *p < 0.05, **p < 0.01 vs ovx (S + S) or (S + A), #p < 0.05 vs sham (S + D). |
3.4. Serum IL-6 level after DOXY injection was elevated in ovx mice
Serum IL-6 levels were determined by ELISA. IL-6 levels were markedly increased in ovx mice at 3 h after DOXY injection. Apocynin significantly decreased serum IL-6 level in ovx mice at 3 h after DOXY injection (Fig. 3 B). No notable changes in serum IL-6 level were observed in sham mice after DOXY injection.
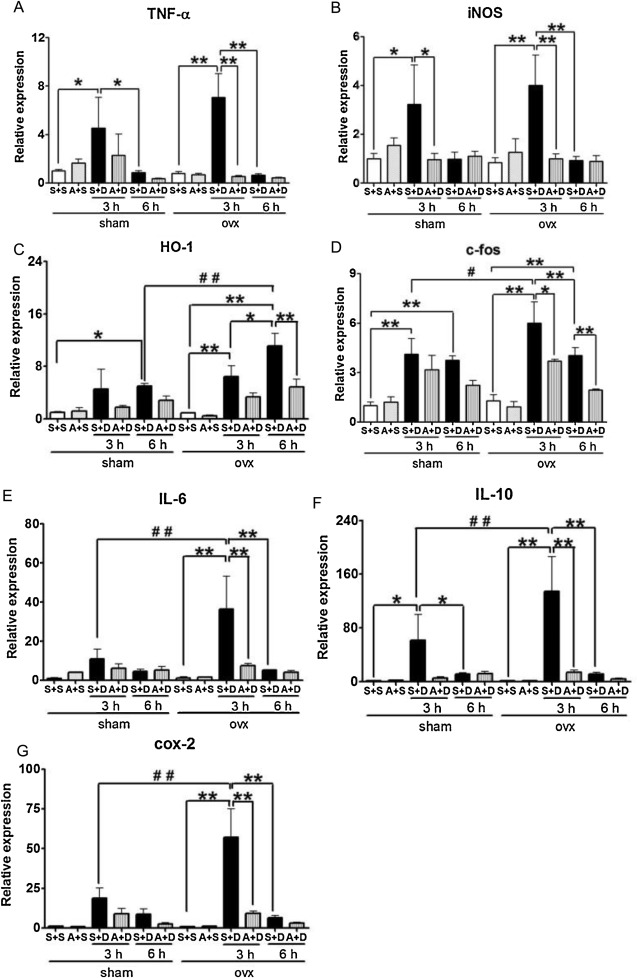
To determine the role of estrogen in development of inflammation in the liver caused by DOXY, inflammation-related gene expression levels were examined in sham and ovx mice after DOXY injection. Significant increases in TNF-α and inducible nitric oxide synthase (iNOS) gene expression levels were observed in both sham and ovx mice at 3 h after DOXY injection. Apocynin significantly reduced the expression levels of TNF-α and iNOS genes in both sham and ovx mice at 3 h after DOXY injection. The profiles of TNF-α and iNOS gene expression in the two groups were almost identical (Fig. 4 A and B). Interestingly, increases in heme oxygenase-1 (HO-1) gene expression levels started at 3 h after DOXY injection and continued until 6 h in both sham and ovx mice. Apocynin significantly decreased expression levels of the HO-1 gene in ovx mice at 6 h after DOXY injection (Fig. 4 C). IL-6 increases HO-1 expression via the Jak/STAT pathway [26] . The expression levels of HO-1 in ovx mice were higher than those in sham mice at 6 h after DOXY injection (Fig. 3 C). Increases in c-fos gene expression levels were observed in both sham and ovx mice at 3 and 6 h after DOXY injection. Apocynin reduced the levels of c-fos expression only in ovx mice at 3 and 6 h after DOXY injection. Expression levels of the c-fos gene in ovx mice were higher than those in sham mice at 3 h after DOXY injection (Fig. 4 D). IL-6, IL-10 and cyclooxygenase-2 (cox-2) gene expression levels were increased in both sham and ovx mice at 3 h after DOXY injection (Fig. 4 E–G). Apocynin significantly decreased the expression levels of the three genes in ovx mice at 3 h after DOXY injection. The expression profiles of IL-6, IL-10 and cox-2 genes in the two groups were almost identical. The expression levels of the three genes in ovx mice were higher than those in sham mice at 3 h after DOXY injection (Fig. 4 E–G).
|
|
|
Fig. 4. Changes in expression levels of TNF-α (A), iNOS (B), HO-1 (C), c-fos (D), IL-6 (E), IL-10 (F) and cox-2 (G) genes in livers obtained from mice treated with saline + saline (S + S), apocynin + saline (A + S), saline + DOXY (S + D) or apocynin + DOXY (A + D) at 3 and 6 h after DOXY injection. Data are average ± SE. *p < 0.05, **p < 0.01 vs S + S mice, #p < 0.05, ##p < 0.01 vs sham mice. |
4. Discussion
To determine estrogen deficiency in the development of liver injury, we examined the sensitivities of sham and ovx mice to DOXY-induced ALF. The survival rate of ovx mice was worse than that of sham mice. ALT levels in DOXY-treated ovx mice were significantly increased compared to those in DOXY-treated sham mice. Morphological damage in hepatocytes in livers obtained from ovx mice showed more severe and extensive injury than that in sham mice. Ovariectomy accelerated liver injury and also systemic injury caused by DOXY administration. Apocynin, a Nox inhibitor, improved DOXY-induced liver injury in both sham and ovx mice. These results indicate that ROS generated through Nox activation by DOXY are responsible for the development of acute liver injury.
IL-6, which is a downstream gene of TNF-α/NF-κB, activates signal transducer and STAT-3 pathways [24] , [28] and [29] . IL-6 is a predominant mediator of the acute phase response triggered by inflammation and also mediates the proinflammatory response during tissue injury. Kireev et al. reported that expression of IL-6 protein was significantly increased in the liver during aging and after ovariectomy [13] . However, we observed no significant changes in IL-6 expressions in naive livers obtained from sham and ovx mice. In addition, no significant changes in IL-6 expression were observed in the serum and livers obtained from sham mice after DOXY injection. On the other hand, expression levels in IL-6 in both the serum and livers were markedly increased in ovx mice after DOXY injection. Ovariectomy enhances the IL-6 response to inflammatory stimuli.
Cox-2 is upregulated in response to various inflammatory stimuli including overproduction of PGE2. Peroxidase reaction converts PGG2 to PGH2 by removing oxygen, a source of oxygen radicals. Therefore, upregulation of cox-2 expression results in over-generation of oxygen radicals. TNF-α induces a dose- and time-dependent increase in cox-2 expression by way of transient NF-κB activation in the cox-2 promoter [3] . Expression levels of cox-2 and c-fos were increased in livers obtained from ovx mice compared to those in livers from sham mice. Ovariectomy enhanced cox-2 and c-fos expression after DOXY administration.
We also examined the expression levels of TNF-α, an upstream gene of IL-6, and iNOS, an inducer of IL-6 expression, to determine the trigger of inflammatory response during development of liver injury caused by DOXY injection. Induction of TNF-α and iNOS gene expression peaked at 3 h after DOXY injection. No significant differences in TNF-α and iNOS expression levels were observed between sham and ovx mice after DOXY administration. At the early stage after DOXY injection, initial responses such as induction of TNF-α and iNOS were almost the same in sham and ovx mice.
We also observed high activity of NADPH oxidase in DOXY-treated ovx mice, suggesting that ovariectomy increases oxidative stress and inflammatory response to DOXY stimulation. Apocynin reduced ALT level, morphological damage of hepatocytes and expression levels of proinflammatory genes in both sham and ovx mice. These results indicate that Nox is responsible for the progress of DOXY-induced acute liver injury. Apocynin may have the potential to downregulate intracellular levels of ROS and also suppress the production of inflammatory cytokines.
HO-1 catalyzes the first and rate-limiting step in heme degradation to produce equimolar quantities of biliverdin, carbon monoxide and free iron. Bilirubin exhibits the highest endogenous antioxidant activity, and sequestration of free iron by ferritin lowers the prooxidant state of the cell. Therefore, the induction of HO-1 can provide cytoprotection against oxidative stress [27] . The expression profile of HO-1 was not identical to those of proinflammatory factors. The expression levels of HO-1 in ovx mice continuously increased until 6 h after DOXY administration. Nrf2 reportedly is the main mediator of cellular adaptation to redox stress [1] Since oxidative stress is easy to generate in ovx mice, it is possible that Nrf2 continues transcription of antioxidant proteins including HO-1.
In conclusion, ovariectomy accelelates the development of DOXY-induced acute liver injury and NADPH oxidase might be responsible for the development of acute liver failure.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Financial support
None.
Trial registration number
No. 1401 approved by the Ethics Committee of Animal Experiments in accordance with the Guidelines for Animal Experiments of Takasaki University of Health.
Transparency document
Transparency Document.
References
- [1] J.A. Araujo, M. Zhang, F. Yin; Heme oxygenase-1, oxidation, inflammation, and atherosclerosis; Front. Pharmacol., 3 (2012), p. 119
- [2] A. Ayala, M.M. Perrin, W. Ertel, I.H. Chaudry; Differential effects of hemorrhage on kupffer cells: decreased antigen presentation despite increased inflammatory cytokine (Il-1, Il-6 and Tnf) release; Cytokine, 4 (1992), pp. 66–75
- [3] P.A. Baeuerle; Ikappab-Nf-kappab structures: at the interface of inflammation control; Cell, 95 (1998), pp. 729–731
- [4] C. Brenner, L. Galluzzi, O. Kepp, G. Kroemer; Decoding cell death signals in liver inflammation; J. Hepatol., 59 (2013), pp. 583–594
- [5] V. Fedchenko, A. Globa, A. Kaloshin, I. Kapitsa, L. Nerobkova, E. Val’dman, O. Buneeva, et al.; The effect of short-term administration of (−)-deprenyl and isatin on the expressions of some genes in the mouse brain cortex; Med. Sci. Monit., 14 (2008), pp. BR269–BR273
- [6] K.K. Griendling, C.A. Minieri, J.D. Ollerenshaw, R.W. Alexander; Angiotensin Ii stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells; Circ. Res., 74 (1994), pp. 1141–1148
- [7] C. Grimm, A. Wenzel, F. Hafezi, C.E. Reme; Gene expression in the mouse retina: the effect of damaging light; Mol. Vis., 6 (2000), pp. 252–260
- [8] J.Y. Guo, H.R. Huo, B.S. Zhao, H.B. Liu, L.F. Li, Y.Y. Ma, S.Y. Guo, et al.; Cinnamaldehyde reduces Il-1beta-induced cyclooxygenase-2 activity in rat cerebral microvascular endothelial cells; Eur. J. Pharmacol., 537 (2006), pp. 174–180
- [9] P.C. Heinrich, I. Behrmann, G. Muller-Newen, F. Schaper, L. Graeve; Interleukin-6-type cytokine signalling through the Gp130/jak/stat pathway; Biochem. J., 334 (Pt. 2) (1998), pp. 297–314
- [10] T. Hirano, K. Ishihara, M. Hibi; Roles of stat3 in mediating the cell growth, differentiation and survival signals relayed through the il-6 family of cytokine receptors; Oncogene, 19 (2000), pp. 2548–2556
- [11] N. Iizuka, S. Hazama, K. Yoshimura, S. Yoshino, A. Tangoku, K. Miyamoto, K. Okita, et al.; Anticachectic effects of the natural herb coptidis rhizoma and berberine on mice bearing colon 26/clone 20 adenocarcinoma; Int. J. Cancer, 99 (2002), pp. 286–291
- [12] S.Y. Kim, K.A. Moon, H.Y. Jo, S. Jeong, S.H. Seon, E. Jung, Y.S. Cho, et al.; Anti-inflammatory effects of apocynin an inhibitor of NADPH oxidase, in airway inflammation; Immunol. Cell Biol., 90 (2012), pp. 441–448
- [13] R.A. Kireev, A.C. Tresguerres, C. Garcia, C. Borras, C. Ariznavarreta, E. Vara, J. Vina, et al.; Hormonal regulation of pro-inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats; Biogerontology, 11 (2010), pp. 229–243
- [14] F. Lienart, M. Morissens, P. Jacobs, J. Ducobu; Doxycycline and hepatotoxicity; Acta Clin. Belg., 47 (1992), pp. 205–208
- [15] L. Llacuna, M. Mari, J.M. Lluis, C. Garcia-Ruiz, J.C. Fernandez-Checa, A. Morales; Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappab inactivation in prolonged ischemia/reperfusion; Am. J. Pathol., 174 (2009), pp. 1776–1785
- [16] T. Luedde, C. Trautwein; The role of oxidative stress and antioxidant treatment in liver surgery and transplantation; Liver Transpl., 12 (2006), pp. 1733–1735
- [17] S. Mitazaki, M. Hashimoto, Y. Matsuhashi, S. Honma, M. Suto, N. Kato, O. Nakagawasai, et al.; Interleukin-6 modulates oxidative stress produced during the development of cisplatin nephrotoxicity; Life Sci., 92 (2013), pp. 694–700
- [18] S. Mitazaki, S. Honma, M. Suto, N. Kato, K. Hiraiwa, M. Yoshida, S. Abe; Interleukin-6 plays a protective role in development of cisplatin-induced acute renal failure through upregulation of anti-oxidative stress factors; Life Sci., 88 (2011), pp. 1142–1148
- [19] S. Mitazaki, N. Kato, M. Suto, K. Hiraiwa, S. Abe; Interleukin-6 deficiency accelerates cisplatin-induced acute renal failure but not systemic injury; Toxicology, 265 (2009), pp. 115–121
- [20] H. Nakamuta; The ovariectomized animal model of postmenopausal bone loss; Nihon Rinsho, 62 (Suppl. 2) (2004), pp. 759–763
- [21] L. Overbergh, D. Valckx, M. Waer, C. Mathieu; Quantification of murine cytokine mrnas using real time quantitative reverse transcriptase Pcr; Cytokine, 11 (1999), pp. 305–312
- [22] I. Shimizu; Impact of oestrogens on the progression of liver disease; Liver Int., 23 (2003), pp. 63–69
- [23] Y.H. Sniekers, H. Weinans, S.M. Bierma-Zeinstra, J.P. Van Leeuwen, G.J. Van Osch; Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment—a systematic approach; Osteoarthr. Cartil., 16 (2008), pp. 533–541
- [24] K. Tanabe, R. Matsushima-Nishiwaki, S. Yamaguchi, H. Iida, S. Dohi, O. Kozawa; Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells; J. Neuroinflamm., 7 (2010), p. 16
- [25] D.M. Tang, C. Koh, W.S. Twaddell, E.C. Von Rosenvinge, H. Han; Acute hepatocellular drug-induced liver injury from bupropion and doxycycline; ACG Case Rep. J., 3 (2015), pp. 66–68
- [26] K. Tron, A. Samoylenko, G. Musikowski, F. Kobe, S. Immenschuh, F. Schaper, G. Ramadori, et al.; Regulation of rat heme oxygenase-1 expression by interleukin-6 via the jak/stat pathway in hepatocytes; J. Hepatol., 45 (2006), pp. 72–80
- [27] K. Ueda, T. Ueyama, K. Yoshida, H. Kimura, T. Ito, Y. Shimizu, M. Oka, et al.; Adaptive Hne-Nrf2-Ho-1 pathway against oxidative stress is associated with acute gastric mucosal lesions; Am. J. Physiol. Gastrointest. Liver Physiol., 295 (2008), pp. G460–G469
- [28] J.M. Wu, D. Bensen-Kennedy, Y. Miura, C.J. Thoburn, D. Armstrong, G.B. Vogelsang, A.D. Hess; The effects of interleukin 10 and interferon gamma cytokine gene polymorphisms on survival after autologous bone marrow transplantation for patients with breast cancer; Biol. Blood Marrow Transplant., 11 (2005), pp. 455–464
- [29] K.H. Wu, C.T. Peng, T.C. Li, L. Wan, C.H. Tsai, S.J. Lan, M.C. Chang, et al.; Interleukin 4, interleukin 6 and interleukin 10 polymorphisms in children with acute and chronic immune thrombocytopenic purpura; Br. J. Haematol., 128 (2005), pp. 849–852
- [30] Q.G. Zhang, L. Raz, R. Wang, D. Han, L. De Sevilla, F. Yang, R.K. Vadlamudi, et al.; Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation; J. Neurosci., 29 (2009), pp. 13823–13836
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?