Abstract
Canine non-infectious, inflammatory meningoencephalomyelitis is termed meningoencephalomyelitis of unknown etiology (MUE) and may affect dogs of any age, breed or gender. Treatment with immunosuppressive medication has been widely reported, however no prospective clinical trials with a standard glucocorticoid monotherapy are available. The objectives were to compare the cerebrospinal fluid (CSF) analysis at diagnosis and after treatment with a standard glucocorticoid (GC) dose and to determine the survival time in dogs with MUE. We hypothesized that abnormal CSF findings would normalize in dogs with MUE, and survival time would be longer than previously reported for glucocortocoid therapy alone. Inclusion criteria were: (1) normal minimum database, (2) no GC use within 5 days, (3) magnetic resonance imaging performed, (4) negative infectious disease titres, and (5) abnormal CSF analysis. All dogs received GC therapy at 1 mg/kg per os q 12 h. Responders had normal CSF analysis at 1 month. Sixteen dogs met the inclusion criteria. Median total nucleated cell count (TNCC) and protein concentration at time of diagnosis were 39 cells/μL (0–1400 cells/μL), and 49 mg/dL (25–293 mg/dL), respectively. Median TNCC and protein concentration at 1 month were 1 cell/μL (0–120 cells/μL), and 24 mg/dL (13–175 mg/dL), respectively. Seven of 16 dogs (44%) were responders. There was no significant difference in survival between the CSF responders and CSF non-responders (P = 0.85). Overall median survival was 602 days (45–654 days). This study supports using GC therapy in dogs with MUE.
Introduction
Canine meningoencephalomyelitis is a heterogeneous collection of inflammatory and infectious diseases of the central nervous system (CNS). In the majority of dogs, meningoencephalomyelitis has an inflammatory non-infectious etiology. There are several non-infectious inflammatory forms of meningoencephalomyelitis, including granulomatous meningoencephalitis (GME), necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE) and eosinophilic meningoencephalitis (EME). Due to the distinctive high eosinophil presence in CSF and on histopathology, EME can be readily distinguished from GME, NME, and NLE. Dogs with GME, NME, and NLE may be any age, breed, or sex. Many dogs diagnosed with GME, NME, or NLE are small-breed dogs, but the presence of the disease is not restricted to this population. (Granger et al. 2010) Diagnostic imaging for GME, NME, or NLE often involves magnetic resonance imaging (MRI) and may be normal or show focal or multifocal changes. Mononuclear pleocytosis is often present but this varies widely, and cerebrospinal (CSF) analysis may be normal in 12–22% of cases. (Granger et al. 2010) In the absence of histopathologic examination, GME, NME, and NLE are indistinguishable on the basis of clinical presentation, breed, sex, age, advanced imaging, and CSF analysis. When histopathologic examination is not available for a given dog, the term meningoencephalomyelitis of unknown etiology (MUE) is used to describe suspected GME, NME, or NLE. (Zarfoss et al. 2006; Granger et al. 2010; Talarico & Schatzberg 2010)
Following a diagnosis of MUE, initial therapy consists of glucocorticoid (GC) drugs, with or without the addition of other immunosuppressant medications. (Adamo et al. 2007; Coates et al. 2007; Menaut et al. 2008; Pakozdy et al. 2009; Talarico & Schatzberg 2010; Wong et al. 2010; Flegel et al. 2011; Lowrie et al. 2013) Glucocorticoid monotherapy has been considered the standard therapy (Zarfoss et al. 2006; Adamo et al. 2007; Jung et al. 2007; Pakozdy et al. 2009; Talarico & Schatzberg 2010; Wong et al. 2010; Flegel et al. 2011); however, its efficacy without adjunct immunosuppression in the treatment of MUE has been evaluated in only a limited number of studies. (Munana & Luttgen 1998; Jung et al. 2007; Pakozdy et al. 2009; Flegel et al. 2011) Use of GC drugs, with variable dosing within each study, has been reported in 59 dogs. The doses have ranged from 5–30 mg/kg per day (Pakozdy et al. 2009), 0.17–2.5 mg/kg per day (Flegel et al. 2011), 1 mg/kg per day (Jung et al. 2007), and 0.5–4 mg/kg per day. (Munana & Luttgen 1998) Therefore, the aims of the current study were compare the CSF analysis at diagnosis and after treatment with a standard GC dose in dogs with MUE. The second aim was to determine the survival time in dogs with MUE treated with GC monotherapy. We hypothesized that abnormal CSF findings would normalize in dogs with MUE, and survival time would be longer than in previous studies utilizing variable GC protocols.
Materials and methods
Study population and inclusion criteria
In this prospective study, client-owned dogs were accrued continuously between September 2006 and May 2009. All clients signed informed consent forms prior to enrollment. Complete physical and neurologic examinations were performed by 1 of the authors. Dogs were included in the study if the following inclusion criteria were met: (1) normal minimum database; (2) GC was not administered within 5 days prior to presentation; (3) MRI consistent with meningoencephalitis/myelitis; (4) negative infectious disease titres, including, tick-borne agents (Borrelia bergdorferi, Rickettsia rickettsii, and Ehrlichia canis), protozoa (Neospora caninum), fungi (Histoplasma, Blastomyces, Aspergillus, Coccidioidomyces, and Cryptococcus) and paired serum and CSF distemper virus titers; and (5) abnormal CSF findings without evidence of neoplastic or infectious etiology. A minimum database was defined as a complete blood count, serum biochemical panel, urinalysis, and three-view thoracic radiographs. Large breed dogs with cervical hyperpathia only were excluded from the study.
Diagnostic testing
Magnetic resonance imaging (GE Horizon 1.0 Tesla magnet, Pittsburg, PA, USA) was performed under general anesthesia in all dogs based on lesion localization from the neurologic examination. Dogs were pre-medicated with hydromorphone (Baxter Pharmaceutical, Deerfield, IL, USA) at 0.1 mg/kg IV and diazepam (Valium; Hospira, Lake Forest, IL, USA) at 0.25 mg/kg IV and induced with propofol (PropoFlo; Abbott Animal Health, Abbott Park, IL, USA) at 4 mg/kg IV to effect. Anesthesia was maintained with isoflurane (IsoFlo®; Abbott Animal Health). The standard MRI protocol for this study included T1- and T2- weighted images in sagittal and transverse planes, fluid attenuated inversion recovery images in the transverse planes, and T1-weighted images after administration of Gadolinium (MultiHance; Bracco Diagnostics, Monroe Township, NJ, USA) in transverse and sagittal planes. Magnetic resonance imaging findings were classified as normal, focal or multifocal for this study.
Cerebrospinal fluid (CSF) was collected from the cerebellomedullary or lumbar cisterns during the same anesthetic episode as neuroimaging. Normal CSF analysis was defined as nucleated cell count (TNCC) <5 cells/μL and protein concentration <25 mg/dL for cerebellomedullary cistern puncture and <40 mg/dL for lumbar puncture. (Platt & Olby 2004) After collection, the CSF sample was divided into two aliquots for transportation; in 1 sample, a 1:10 dilution of CSF to autologous serum was made to improve cell stabilization for transportation, and the other sample was CSF only. (Cellio 2001) All samples were transported in additive-free sterile glass tubes. Analysis was performed by an affiliated laboratory (Antech Diagnostic Laboratory, Oakbrook, IL, USA) within 12 h of collection and analysed according to standard procedure. Correction factors were not applied to the data, and all reported values were uncorrected. (Summers et al. 1995) Approximately 1 month after initial CSF analysis, a second CSF sample was collected from the same cistern, using the same anesthetic protocol, and analysed as detailed above.
Treatment protocol
Immediately upon recovery from anesthesia all dogs received intravenous methylprednisolone (Solu-Medrol; Pfizer, New York, NY, USA) at 30 mg/kg IV followed by 15 mg/kg IV 3 h after the initial dose and 10 mg/kg IV 3 h after the second dose was administered. Following treatment with methylprednisone, prednisone (West-Ward Pharmaceutical, Eatontown, NJ, USA) or prednisolone (Lloyd, Inc., Shenandoah, IA, USA) at 1 mg/kg PO q12 h, sulfadimathoxine/ormetoprim (Primor® tablets; Pfizer) at 15 mg/kg PO q12 h, doxycycline (Doxycycline tablets; West Ward Pharmaceutical) at 5–10 mg/kg PO q24 h, and famotidine (Famotidine tablets; Wockhardt, Ltd, Morton Grove, IL, USA) at 1 mg/kg PO q24 h were initiated. After obtaining negative results for infectious disease testing, sulfadimathoxine/ormetoprim and doxycycline were discontinued.
If the second CSF results were within reference intervals, the dog was classified as a CSF responder and a gradually decreasing dose of prednisone or prednisolone was administered as follows: 1 mg/kg per os q24 h × 30 days, then 1 mg/kg per os q48 h × 30 days and then discontinued. If the second CSF results were abnormal, the dog was classified as a CSF non-responder and the same dose of prednisone or prednisolone was continued for an additional 30 days. If dogs did not improve at 60 days or greater following the above treatment, or worsened during treatment, additional immunosuppressive agents were recommended.
Referring veterinarians were contacted by telephone within 48 months after diagnosis of MUE and questioned about the survival of each dog. If the dog had died, the reason for the death was noted. Referring veterinarians were asked if clinical signs had recurred for CSF responders. Relapse was defined as a reoccurrence of clinical signs in CSF responders, after discontinuing GC therapy.
Statistical analysis
Descriptive statistics including medians, minimums and maximums were recorded for continuous data. A Kaplan–Meier survival curve was created for survival data. Overall survival time and survival time of CSF responders and CSF non-responders was evaluated with the Mantel-Cox Log-Rank test comparison.
Results
Sixteen dogs met the inclusion criteria. Breeds included Lhasa apso (n = 2), Dachshund (n = 2), and 1 each of the following breeds: Golden Retriever, Retriever mix, Pug, Welsh Springer Spaniel, Boston Terrier, Pug-Beagle mix, Beagle, Jack Russell Terrier, Rottweiler, Collie, Maltese terrier and mixed breed. The median age was 4.5 years (2–11 years) and the median weight was 12.0 kg (5.2–38.4 kg). Neuroanatomical lesion localization, based on the neurologic examination, was prosencephalon (5/16 dogs), brainstem (3/16 dogs), T3-L3 myelopathy (2/16 dogs), L6-S2 radiculopathy (1/16 dogs) and peripheral neuropathy (1/16 dogs) involving the vestibulocochlear nerves. Four of 16 dogs were diagnosed with multifocal lesion localization. Magnetic resonance imaging findings were normal in 2 dogs, focal in 3 dogs and multifocal in 11 dogs. Abnormalities on MRI corresponded with the neuroanatomic lesion localization in all dogs. The median prednisone or prednisolone dose was 1 mg/kg (0.8–1.2 mg/kg) PO q12 h. Sulfadimethoxine/ormetoprim and doxycycline were discontinued by day 7 of treatment in all dogs.
Initial CSF analysis
Initial CSF samples were collected from the cerebellomedullary cistern in 13 dogs and the lumbar cistern in 3 dogs. See Table 1 for median CSF TNCC and protein concentration. Results indicated mixed mononuclear or lymphocytic pleocytosis (n = 15) and neutrophilic pleocytosis (n = 1); in 3 dogs the TNCC was within the reference interval, but the protein concentration was increased.
| Median CSF TNCC (cell/μL) | Median CSF protein (mg/dL) | |||
|---|---|---|---|---|
| Initial | 1 month | Initial | 1 month | |
| Responder | 8.5 (0–1160) | 0 (0–1) | 40.5 (25–251) | 19 (13–24) |
| Non-responder | 59 (0–1400) | 15 (0–120) | 68.5 (28–293) | 38 (17–175) |
| Combined | 39 (0–1400) | 49 (25–293) | 1 (0–120) | 24 (13–175) |
| CSF, cerebrospinal fluid; TNCC, total nucleated cell count; MUE, meningoencephalomyelitis of unknown etiology. Responders achieved normal CSF results at 1 month; non-responders did not achieve normal CSF results at 1 month. Combined values refer to CSF responders and CSF non-responders together. | ||||
Second evaluation and CSF analysis
In seven of 16 dogs (44%), CSF analytes were within reference intervals and they were therefore considered CSF responders. One dog did not have sufficient sample to perform CSF protein concentration therefore this dog was considered a CSF responder based on the TNCC. Median TNCC and protein concentration for CSF responders and CSF non-responders are listed in Table 1.
The neurologic examination was normal in 10 of 16 dogs (63%) and abnormal in 6 dogs (38%). In 5 of 10 dogs (50%) with normal neurologic examinations, CSF analysis was also normal. In 2 of 6 dogs (33%) with abnormal neurologic examinations, CSF analysis was normal. All abnormalities on neurologic examination indicated persistent changes; no new abnormalities were detected. Three CSF non-responders started adjunct immunosuppression at a median of 68 days (62–75 days) from diagnosis due to a failure to improve on GC treatment. All 3 dogs received cytosine arabinoside (Generic; Hospira Inc, Lake Forrest, IL, USA) at 50 mg/m2subcutaneous q 12 h. for 48 h, repeated every 3 weeks as needed.
Survival time
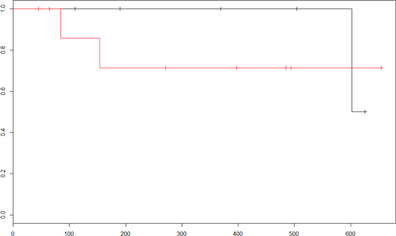
Follow-up information was obtained within 48 months (45–1100 days) of initial diagnosis in all dogs. One of seven CSF responders (14%) was reported to have clinical signs consistent with relapse and was euthanized without additional diagnostic testing or treatment. Two CSF non-responders were euthanized because of progressive disease; two CSF non-responders and one CSF responder were euthanized due to unrelated causes; and two CSF responders and one CSF non-responder were euthanized or died acutely of unknown causes. The remaining three CSF responders and four CSF non-responders dogs were alive at the time of writing (Median 485 days). Median survival for all dogs based on Kaplan–Meier survival analysis was 602 days (45–654 days). Median survival for CSF responders was 602 days and the median was not reached in the CSF non-responders. (Fig. 1) There was no significant difference in survival between the CSF responders and CSF non-responders (P = 0.85). Due to the small number of dogs that died due to disease, reporting of survival time between CSF responders and CSF non-responders was not accurate. Additional statistical analysis was not performed due to small sample size.
|
|
|
Figure 1. Kaplan-Meier survival curves for 7 responder dogs (black line) and 8 non-responder dogs (red line) with meningoencephalomyelitis of unknown etiology (MUE). Tick marks represent dogs censured for events other than progression of disease. |
Discussion
Treatment of dogs with MUE using standardized GC monotherapy resulted in normalization of the CSF analytes in 44% of dogs. None of the previous studies evaluating solo GC therapy has evaluated repeated CSF analysis as a marker for immunosuppression in canine MUE. However, repeated CSF analysis has been evaluated for other immunosuppressant protocols used in treating MUE (Lowrie et al. 2013), as well as other inflammatory CNS diseases. (Cizinauskas et al. 2000) One study suggested that serial monitoring of CSF TNCC and protein concentrations is a sensitive indicator of successful treatment of inflammatory disease; however, clinical relapse was not evaluated statistically. (Cizinauskas et al. 2000) Another study failed to find an association between normal CSF analysis and improved outcome, but did find an association between abnormal CSF analysis and relapse or poor outcome. (Lowrie et al. 2013) In the current study, only one CSF responder was reported to have relapsed, suggesting that CSF analysis may be a valid tool for monitoring success or failure of treatment in dogs diagnosed with MUE and treated with GC monotherapy. Larger studies are needed to evaluate this association more fully.
Survival times for dogs in the current study were longer than those in all previous studies of dogs treated with GC monotherapy. Median survival times were reported to be 28 (Pakozdy et al. 2009), 91 (Lowrie et al. 2013), and 323 (Lowrie et al. 2013) days, compared with 602 days in this study. The difference in survival times among studies may reflect different immunosuppression protocols or the prospective nature of the current study. In the current study, we evaluated an immunosuppressive dose of GC that was standardized for all 16 dogs. Methylprednisolone was administered intravenous following the initial diagnosis in all dogs to try to achieve rapid immunosuppression. In studies of treatment protocols using GC in combination with other immunosuppressant agents, median survival times ranged from 26 to 1834 days (Adamo et al. 2007; Coates et al. 2007; Menaut et al. 2008; Pakozdy et al. 2009; Talarico & Schatzberg 2010; Wong et al. 2010; Flegel et al. 2011; Lowrie et al. 2013), with a combined median survival time of 375 days. Some of these studies attributed the survival times exclusively to the alternative immunosuppressant. Based on the current study, GC immunosuppression may be a contributing factor to survival times reported for studies, using combination treatments. Furthermore, the long median survival time in this study supports the use of GC monotherapy for dogs diagnosed with MUE.
Lack of a histopathologic diagnosis was a limitation of this study. Although antemortem diagnostic criteria have been established for MUE, inclusion of patients with acute phase infectious disease or neoplasia is an inherent risk in the diagnosis of MUE. (Granger et al. 2010) To partially address this possibility, extensive serologic testing was performed at the time of diagnosis for the most common infectious disease in the geographic area as well as paired CSF and serum titres for distemper virus. Serologic testing evaluates immune response to a specific pathogen, therefore it is possible that a dog with acute infectious disease may not yet have mounted an immune response and therefore had negative serology when initially tested. Death or euthanasia due to worsening signs would be expected for dogs with infectious diseases receiving immunosuppressive treatment without concurrent long-term antimicrobial treatment. All dogs included in the study survived for the first 30 days of treatment; therefore it is unlikely that dogs with infectious meningoencephalomyelitis were included. Although neoplastic cells were not identified in CSF, histopathologic examination of the CNS would be expected to be more sensitive than CSF analysis for detection of neoplasia. Brain biopsy was not performed in the dogs in this study due to the risk of complications. Additionally, necropsy was not required and was not obtained in any of the deceased dogs. Requiring diagnosis by either CNS biopsy or necropsy may have resulted in bias towards shorter survival. Therefore, although considered unlikely, occurrence of neoplastic diseases mistakenly diagnosed as meningoencephalomyelitis cannot be excluded. Other limitations of this study included the small size of the study population and limited follow-up time. Larger studies may be able to detect prognostic variables, such normalization differences in CSF protein or TNCC for dogs with MUE that were not evaluated in the current study.
Conclusions
In dogs with MUE, 44% had normal CSF analysis after 1 month of GC immunosuppression; further studies are needed to determine if a relationship exists between prognosis and results of CSF analysis. Dogs with normal CSF findings at 1 month had longer survival times than dogs with abnormal CSF findings at 1 month. Glucocorticoid therapy in this study, resulted in median survival time longer than previously reported times in studies evaluating combination immunosuppressant protocols; therefore a positive GC effect on survival time in those studies should be considered. Dogs may benefit from the administration of combination immunosuppression protocols if the side effects of GC warrant limited use of GC. In conclusion, this study supports the use of immunosuppression with GC monotherapy in dogs diagnosed with MUE.
Acknowledgement
This study was supported by VCA-Antech.
Source of Funding
VCA Antech Resident Support.
Conflicts of Interest
None.
Contributions
None.
References
- Adamo P.F., Rylander H. & Adams W.M. (2007) Ciclosporin use in multi-drug therapy for meningoencephalomyelitis of unknown aetiology in dogs. The Journal of Small Animal Practice48, 486–496.
- Cellio B.C. (2001) Collecting, processing, and preparing cerebrospinal fluid in dogs and cats. Compendium Continuing Education for Veterinarians23, 786–792.
- Cizinauskas S., Jaggy A. & Tipold A. (2000) Long-term treatment of dogs with steroid-responsive meningitis-arteritis: clinical, laboratory and therapeutic results. The Journal of Small Animal Practice41, 295–301.
- Coates J.R., Barone G., Dewey C.W., Vitale C.L., Holloway-Azene N.M., Sessions J.K. (2007) Procarbazine as adjunctive therapy for treatment of dogs with presumptive antemortem diagnosis of granulomatous meningoencephalomyelitis: 21 cases (1998–2004). Journal of Veterinary Internal Medicine2, 100–106.
- Flegel T., Boettcher I.C., Matiasek K., Oevermann A., Doherr M.G., Oechtering G., Henke D. (2011) Comparison of oral administration of lomustine and prednisolone or prednisolone alone as treatment for granulomatous meningoencephalomyelitis or necrotizing encephalitis in dogs. Journal of the American Veterinary Medical Association238, 337–345.
- Granger N., Smith P.M. & Jeffery N.D. (2010) Clinical findings and treatment of non-infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Veterinary Journal184, 290–297.
- Jung D.I., Kang B.T., Park C., Yoo J.H., Gu S.H., Jeon H.W., Kim J.W., Heo R.Y., et al. (2007) A comparison of combination therapy (cyclosporine plus prednisolone) with sole prednisolone therapy in 7 dogs with necrotizing meningoencephalitis. Journal of Veterinary Medical Science69, 1303–1306.
- Lowrie M., Smith P.M. & Garosi L. (2013) Meningoencephalitis of unknown origin: investigation of prognostic factors and outcome using a standard treatment protocol. The Veterinary Record172, 527–533.
- Menaut P., Landart J., Behr S., Lanore D., Trumel C. (2008) Treatment of 11 dogs with meningoencephalomyelitis of unknown origin with a combination of prednisolone and cytosine arabinoside. The Veterinary Record162, 241–245.
- Munana K.R. & Luttgen P.J. (1998) Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases. Journal of the American Veterinary Medical Association212, 1902–1906.
- Pakozdy A., Leschnik M., Kneissl S., Gumpenberger M., Gruber A., Tichy A., Thalhammer J.G. (2009) Improved survival time in dogs with suspected GME treated with cyclosporin. The Veterinary Record164, 89–91.
- Platt S.R. & Olby N.J. (2004) In BSAVA Manual of Canine and Feline Neurology, 35–53. British Small Animal Veterinary Association: Gloucester.
- Summers B.A., Cummings J.F., deLahunta A. (1995) Inflammatory diseases of the central nervous system. In: Veterinary Neuropathology, (This is the only edition of this book), pp. 95–162. Mosby: St. Louis.
- Talarico L.R. & Schatzberg S.J. (2010) Idiopathic granulomatous and necrotizing inflammatory disorders of the canine central nervous system: a review and future perspectives. The Journal of Small Animal Practice51, 138–149.
- Wong M.A., Hopkins A.L., Meeks J.C., Clarke J.D. (2010) Evaluation of treatment with a combination of azathioprine and prednisone in dogs with meningoencephalomyelitis of undetermined etiology: 40 cases (2000–2007). Journal of the American Veterinary Medical Association237, 929–935.
- Zarfoss M., Schatzberg S., Venator K., Cutter-Schatzberg K., Cuddon P., Pintar J., et al. (2006) Combined cytosine arabinoside and prednisone therapy for meningoencephalitis of unknown aetiology in 10 dogs. The Journal of Small Animal Practice47, 588–595.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?