Abstract
Objectives
There is limited data on how well 2D-QCA and OCT agree with each other for measurement of coronary artery lumen dimensions. We aimed to assess the agreement between the two modalities.
Methods
Patients undergoing OCT for assessment of coronary artery lesions were reviewed. Minimum luminal diameter (MLD), proximal reference diameter and distal reference diameter were measured for each lesion prior to stenting.
Results
OCT was performed in 64 patients and 40 lesions were suitable for analysis. There was a good correlation for proximal and distal reference diameters (r = 0.86, p < 0.0001 and r = 0.92, p < 0.0001 respectively). There was good agreement on Bland–Altman analysis; the proximal and distal reference diameters measured by QCA were on average 0.09 mm (95% CI, − 0.52 to 0.53 mm) and 0.1 mm (95% CI, − 0.59 to 0.6 mm) smaller than OCT respectively. There was a satisfactory correlation (r = 0.63, p = < 0.0001) between QCA and OCT for MLD. However, the MLD by QCA was 0.49 mm (95% CI, − 1.57 to 0.59 mm) smaller than OCT, suggesting a poor agreement for MLD.
Conclusions
There is a good correlation and agreement between QCA and OCT for measurement of proximal and distal reference diameters. However, the MLD was underestimated by QCA.
Keywords
Quantitative coronary angiography;Optical coherence tomography;Coronary artery lesion
1. Introduction
Suboptimal stent deployment has been shown to be associated with increased incidence of target vessel revascularization [1]; [2] ; [3]. An accurate assessment of intracoronary lumen dimensions is essential in the diagnosis and treatment of coronary artery disease. Coronary lumen dimensions can be assessed by a visual estimate by an experienced operator, by quantitative coronary angiography (QCA) or by more invasive techniques like intravascular ultrasound (IVUS) or optical coherence tomography (OCT).
Intracoronary lumen dimensions assessed by OCT correlate well with IVUS, however OCT measurements are usually smaller than IVUS [4]; [5] ; [6]. Previous studies comparing OCT and 2D/3D-QCA show a satisfactory to good correlation; however QCA measurements are usually smaller than OCT [5]; [6]; [7] ; [8]. Our aim was to assess the correlation between 2D-QCA and OCT for coronary lumen dimensions prior to stent implantation and to quantify the agreement between the two modalities by Bland–Altman analysis. We hypothesized that if the QCA measurements are within 0.5 mm of the OCT measurement, then it would be considered as an acceptable agreement between the two modalities.
2. Methods
2.1. OCT acquisition
Patients undergoing OCT for various clinical indications at our institution were reviewed. The OCT acquisition was performed using the C7 Dragonfly™ intracoronary imaging catheter and the ILUMIEN™ PCI Optimization System (St. Jude Medical). All images were acquired using a non-occlusive technique with injection of isosmolar iodixonoal (Visipaque™ by GE healthcare) contrast using automatic injection to clear the vessel of blood [9]. The lesion was crossed using a routine angioplasty wire. The imaging catheter was then advanced over the wire. Once the catheter was positioned distal to the lesion it was pull backed using an automated motor at a speed of 15 mm/s.
2.2. 2D-QCA
2D-QCA was performed offline using automated software. Measurements were performed on image sequences adequately filled with contrast and when the vessel was not foreshortened. Calibration was performed on the contrast filled segment of the guiding catheter. Measurements were performed on the lesion of interest using the automated software. Proximal and distal reference diameters were performed manually to measure the diameter of the normal segment of the vessel.
2.3. Measurements
Minimum luminal diameter (MLD), proximal reference diameter (PRD) and distal reference diameter (DRD) were measured for each lesion. PRD was defined as the diameter of the normal vessel proximally but within 10 mm of the lesion and DRD as the diameter of the normal vessel distally but within 10 mm of the lesion.
2.4. Lesions excluded
Lesions that were not suitable for analysis were excluded. OCT images with poor clearance of blood or inadequate short runs were excluded. Lesions that were dilated prior to performing OCT were excluded. Lesions with poor angiographic images not suitable for QCA were also excluded, e.g. foreshortened images or images with overlapping vessels.
2.5. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 for windows. Continuous variables are expressed as means. Means were compared using Students t test. A p value of < 0.05 was considered to be significant. The association between the measurements by QCA and OCT was tested by plotting a scatter plot and measuring the correlation coefficient ‘r’ by Pearsons method. Bland–Altman plots were constructed to test the agreement between the two methods by plotting the average of the QCA and OCT measurements on x axis and the difference between the QCA and OCT measurements on y axis.
3. Results
64 patients underwent OCT between November 2010 and August 2012. 40 lesions in 38 patients were suitable for analysis by QCA and OCT. Mean age was 68 years with 28 males and 10 females. Majority of the cases were done by radial approach (87%). Clinical presentations were stable angina (10), unstable angina (6), non-ST elevation myocardial infarction (14) and ST elevation myocardial infarction (8). Vessels assessed were left anterior descending artery (17), right coronary artery (8), circumflex artery (5), obtuse marginal (1) and saphenous vein graft (7).
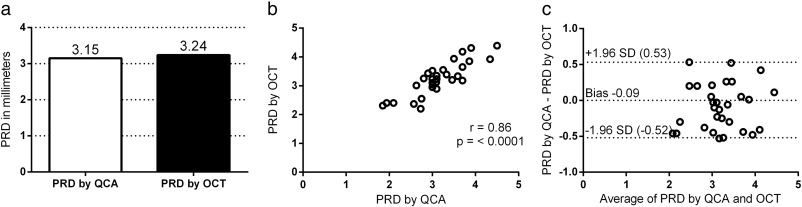
3.1. Proximal reference diameter
30 lesions were suitable for measurement of PRD by QCA and OCT. There was no significant difference between the mean PRD measured by QCA and OCT (3.15 vs. 3.24 mm, p = 0.56), see Fig. 1a. There was a good correlation (r = 0.86, 95% confidence interval 0.72 to 0.93, p = < 0.0001) between QCA and OCT (Fig. 1b). Bland–Altman plot showed a bias of − 0.09 mm with 95% confidence interval from − 0.52 to 0.53 mm, suggesting that on average the PRD by QCA was 0.09 mm smaller than that by OCT (Fig. 1c).
|
|
|
Fig. 1. Fig. 1a shows the mean PRD by QCA and OCT, p = 0.65. Fig. 1b shows a scatter plot of the correlation between QCA and OCT for PRD. Fig. 1c shows the Bland–Altman plot for PRD. PRD = proximal reference diameter, QCA = quantitative coronary angiography, OCT = optical coherence tomography. |
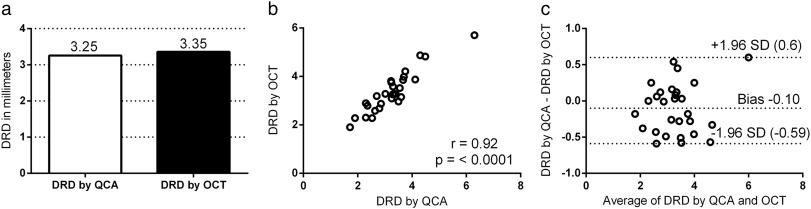
3.2. Distal reference diameter
28 lesions were suitable for measurement of DRD by QCA and OCT. There was no significant difference between the mean DRD measured by QCA and OCT (3.25 vs. 3.35 mm, p = 0.66), see Fig. 2a. There was a good correlation (r = 0.92, 95% confidence interval 0.84 to 0.96, p = < 0.0001) between QCA and OCT (Fig. 2b). Bland–Altman plot showed a bias of − 0.1 mm with 95% confidence interval from − 0.59 to 0.6 mm, suggesting that on average the DRD by QCA was 0.1 mm smaller than that by OCT (Fig. 2c).
|
|
|
Fig. 2. Fig. 2a shows the mean DRD by QCA and OCT, p = 0.66. Fig. 2b shows a scatter plot of the correlation between QCA and OCT for PRD. Fig. 2c shows the Bland–Altman plot for PRD. DRD = distal reference diameter, QCA = quantitative coronary angiography, OCT = optical coherence tomography. |
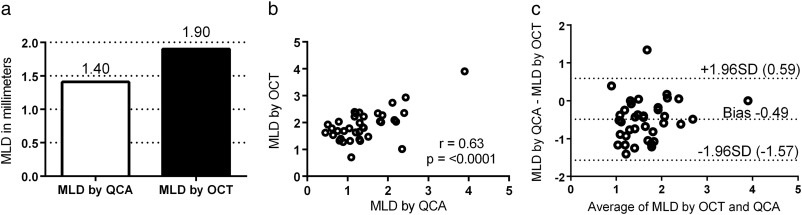
3.3. Minimum luminal diameter
37 lesions were suitable for measurement of MLD by QCA and OCT. There was a satisfactory correlation (r = 0.63, 95% confidence interval 0.38 to 0.79, p = < 0.0001) between QCA and OCT, however the mean MLD measured by QCA was significantly lower compared to OCT (1.40 vs. 1.90 mm, p = 0.001), see Fig. 3a and b. Bland–Altman plot showed a bias of − 0.49 mm with 95% confidence interval from − 1.57 to 0.59 mm, suggesting that on average the MLD by QCA was 0.49 mm smaller than that by OCT (Fig. 3c). Hence there was a poor agreement for estimation of MLD.
|
|
|
Fig. 3. Fig. 3a shows the mean MLD by QCA and OCT, p = 0.001. Fig. 3b shows a scatter plot of the correlation between QCA and OCT for MLD. Fig. 3c shows the Bland–Altman plot for MLD. MLD = minimum luminal diameter, QCA = quantitative coronary angiography, OCT = optical coherence tomography. |
4. Discussion
Visual estimation is commonly used to quantify the severity of a coronary artery lesion in the catheterization laboratory but is limited by significant inter-observer variability [10]. QCA is more precise than visual estimation and has less inter-observer variability [11] ; [12]. It has been in use as a clinical and research tool since the 1980s [12]. It uses automated edge detection software to detect the contrast filled lumen of the coronary artery [12].
OCT and IVUS are the two main intra-coronary imaging modalities in clinical use. Unlike IVUS which uses ultrasound waves, OCT creates an image by directing an optical beam of infrared light onto the tissue and measuring the reflected intensity of light [13]. It has a resolution of 10–20 μm which is 10 times higher than IVUS [13]. In an ex-vivo study assessing coronary lumen dimensions, IVUS and OCT measurements were larger than histologic specimens, but this may have been due to tissue shrinkage when preparing specimens for histology [4]. In clinical studies, OCT measurements are usually smaller than IVUS and QCA measurements even smaller than OCT [4]; [5] ; [6]. In a study using a phantom model of known dimension, OCT measured the lumen area accurately while IVUS overestimated the lumen area [14]. This may be due to poor border detection by IVUS, whereas, OCT has the advantage of higher resolution with a clear lumen to vessel interface [15] (15). Hence, OCT is emerging as the new gold standard for measuring coronary lumen dimensions. It also has less inter-observer variability than IVUS in measuring lumen dimensions [14] ; [16].
Proximal and distal reference diameters are crucial dimensions needed for selecting the appropriate stent size during coronary angioplasty. This is even more important when selecting bioresorbable vascular scaffolds (BVS), due to their limited distensibility [17]. Previous studies comparing OCT and 2D-QCA show a good correlation, but how well the two agree with each other has not been well studied [5] ; [8]. This study shows a good correlation and agreement between 2D-QCA and OCT for estimating PRD and DRD. There was no significant difference in the mean PRD and DRD in both groups. On average, the PRD by 2D-QCA was 0.09 mm smaller and the DRD by 2D-QCA was 0.1 mm smaller than the OCT measurements. Importantly, most of the reference diameter measurements by 2D-QCA were within 0.5 mm of the OCT measurements. Although OCT is the gold standard in measuring coronary lumen dimensions, this study shows that in the absence or non-availability of OCT, 2D-QCA can be used to estimate reference vessel diameters for sizing of stent prior to angioplasty. This is also supported by the ABSORB EXTEND study in which mandatory use of QCA prior to implantation of BVS showed a reduction in the undersizing of BVS [17].
QCA has the advantage of being non-invasive, inexpensive and less time consuming. The main technical challenge with QCA is selection of an appropriate image sequence. The accuracy of QCA is improved by selecting an image with minimal foreshortening and minimal overlap with other vessels or other radio-opaque structures [12].
The MLD measured by QCA was significantly smaller compared to OCT. On average the MLD by QCA was 0.49 mm smaller than the OCT measurements. The possible reasons for this difference may be poor border detection by QCA or foreshortening of the image or incomplete filling of the vessel with contrast.
4.1. Limitations
Although the reference diameters on the two modalities were matched as closely as possible, the measurements were not done at exactly the same point in the vessel, as it was not possible to make an exact match. However, the measurements were done as it would be in routine clinical practice to measure the reference diameters for a lesion. Lesion lengths were not measured as it was difficult to correlate exactly the start and end of a lesion on the two modalities. However, fusion technology is now available that can match QCA with IVUS or OCT and allows exact correlation of plaque seen on angiography with IVUS or OCT [18] ; [19].
5. Conclusion
There is a good correlation and agreement between QCA and OCT for measurement of proximal and distal reference diameters of a lesion. There was no significant difference in the mean proximal and distal reference diameters in both groups. The MLD was underestimated by QCA.
Conflict of interest
None.
Acknowledgement
We would like to acknowledge the radiographers at our department, especially chief radiographer Nadeem Mughal for maintaining the imaging database.
References
- [1] M.A. Costa, D.J. Angiolillo, M. Tannenbaum, M. Driesman, A. Chu, J. Patterson, et al.; Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial); Am J Cardiol, 101 (12) (Jun 15 2008), pp. 1704–1711 [PubMed PMID: 18549844. Epub 2008/06/14. eng]
- [2] P.A. Lemos, F. Saia, J.M. Ligthart, C.A. Arampatzis, G. Sianos, K. Tanabe, et al.; Coronary restenosis after sirolimus-eluting stent implantation: morphological description and mechanistic analysis from a consecutive series of cases; Circulation, 108 (3) (Jul 22 2003), pp. 257–260 [PubMed PMID: 12860901. Epub 2003/07/16. eng]
- [3] A. Aminian, T. Kabir, E. Eeckhout; Treatment of drug-eluting stent restenosis: an emerging challenge; Catheter Cardiovasc Interv, 74 (1) (Jul 1 2009), pp. 108–116 [PubMed PMID: 19199365. Epub 2009/02/10. eng]
- [4] N. Gonzalo, P.W. Serruys, H.M. Garcia-Garcia, G. van Soest, T. Okamura, J. Ligthart, et al.; Quantitative ex vivo and in vivo comparison of lumen dimensions measured by optical coherence tomography and intravascular ultrasound in human coronary arteries; Rev Esp Cardiol, 62 (6) (Jun 2009), pp. 615–624 [PubMed PMID: 19480757. Epub 2009/06/02. Eng spa]
- [5] T. Okamura, Y. Onuma, H.M. Garcia-Garcia, R.J. van Geuns, J.J. Wykrzykowska, C. Schultz, et al.; First-in-man evaluation of intravascular optical frequency domain imaging (OFDI) of Terumo: a comparison with intravascular ultrasound and quantitative coronary angiography; EuroIntervention, 6 (9) (Apr 2011), pp. 1037–1045 [PubMed PMID: 21518674. Epub 2011/04/27. eng]
- [6] R. Puri, A.J. Nelson, G.Y. Liew, S.J. Nicholls, A. Carbone, D.T. Wong, et al.; Variations in coronary lumen dimensions measured in vivo; JACC Cardiovasc. Imaging, 5 (1) (Jan 2012), pp. 123–124 [PubMed PMID: 22239902. Epub 2012/01/14. eng]
- [7] S. Tu, L. Xu, J. Ligthart, B. Xu, K. Witberg, Z. Sun, et al.; In vivo comparison of arterial lumen dimensions assessed by co-registered three-dimensional (3D) quantitative coronary angiography, intravascular ultrasound and optical coherence tomography; Int J Cardiovasc Imaging, 28 (6) (Aug 2012), pp. 1315–1327 [PubMed PMID: 22261998. Pubmed Central PMCID: PMC3463784. English]
- [8] J.L. Gutierrez-Chico, P.W. Serruys, C. Girasis, S. Garg, Y. Onuma, S. Brugaletta, et al.; Quantitative multi-modality imaging analysis of a fully bioresorbable stent: a head-to-head comparison between QCA, IVUS and OCT; Int J Cardiovasc Imaging, 28 (3) (Mar 2012), pp. 467–478 [PubMed PMID: 21359517. Pubmed Central PMCID: PMC3326362. English]
- [9] F. Prati, M. Cera, V. Ramazzotti, F. Imola, R. Giudice, M. Albertucci; Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios; EuroIntervention, 3 (3) (Nov 2007), pp. 365–370 [PubMed PMID: 19737719. Epub 2007/11/01. eng]
- [10] C. Shub, R.E. Vlietstra, H.C. Smith, R.E. Fulton, L.R. Elveback; The unpredictable progression of symptomatic coronary artery disease: a serial clinical–angiographic analysis; Mayo Clin Proc, 56 (3) (Mar 1981), pp. 155–160 [PubMed PMID: 7206792. Epub 1981/03/01. eng]
- [11] R.K. Goldberg, N.S. Kleiman, S.T. Minor, J. Abukhalil, A.E. Raizner; Comparison of quantitative coronary angiography to visual estimates of lesion severity pre and post PTCA; Am Heart J, 119 (1) (Jan 1990), pp. 178–184 [PubMed PMID: 2404387. Epub 1990/01/01. eng]
- [12] P. Garrone, G. Biondi-Zoccai, I. Salvetti, N. Sina, I. Sheiban, P.R. Stella, et al.; Quantitative coronary angiography in the current era: principles and applications; J Interv Cardiol, 22 (6) (Dec 2009), pp. 527–536 [PubMed PMID: 19627430. English]
- [13] H.C. Lowe, J. Narula, J.G. Fujimoto, I.K. Jang; Intracoronary optical diagnostics current status, limitations, and potential; JACC Cardiovasc Interv, 4 (12) (Dec 2011), pp. 1257–1270 [PubMed PMID: 22192367. Epub 2011/12/24. eng]
- [14] T. Kubo, T. Akasaka, J. Shite, T. Suzuki, S. Uemura, B. Yu, et al.; OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study; JACC Cardiovasc. Imaging, 6 (10) (Oct 2013), pp. 1095–1104 [PubMed PMID: 24011777. Epub 2013/09/10. eng]
- [15] G. Guagliumi, R. Virmani; The race to achieve the gold standard in coronary imaging; Rev Esp Cardiol, 62 (6) (Jun 2009), pp. 599–602 [PubMed PMID: 19480754. Epub 2009/06/02. Eng spa]
- [16] F. Abnousi, K. Waseda, T. Kume, H. Otake, O. Kawarada, C.M. Yong, et al.; Variability in quantitative and qualitative analysis of intravascular ultrasound and frequency domain optical coherence tomography; Catheter Cardiovasc Interv, 82 (3) (Sep 1 2013), pp. E192–E199 [PubMed PMID: 23412754. Epub 2013/02/16. eng]
- [17] V. Farooq, J. Gomez-Lara, S. Brugaletta, B.D. Gogas, H.M. Garcia-Garcia, Y. Onuma, et al.; Proximal and distal maximal luminal diameters as a guide to appropriate deployment of the ABSORB everolimus-eluting bioresorbable vascular scaffold: a sub-study of the ABSORB Cohort B and the on-going ABSORB EXTEND Single Arm Study; Catheter Cardiovasc Interv, 79 (6) (May 1 2012), pp. 880–888 [PubMed PMID: 22514149. Epub 2012/04/20. eng]
- [18] J.C. Schuurbiers, N.G. Lopez, J. Ligthart, F.J. Gijsen, J. Dijkstra, P.W. Serruys, et al.; In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS); Catheter Cardiovasc Interv, 73 (5) (Apr 1 2009), pp. 620–626 [PubMed PMID: 19309696. Epub 2009/03/25. eng]
- [19] S. Tu, N.R. Holm, G. Koning, Z. Huang, J.H. Reiber; Fusion of 3D QCA and IVUS/OCT; Int J Cardiovasc Imaging, 27 (2) (Feb 2011), pp. 197–207 [PubMed PMID: 21264684. Pubmed Central PMCID: PMC3078305. English]
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?