Abstract
Backgrounds
It remains unclear whether a persistently high exhaled nitric oxide fraction (FeNO) in patients with controlled asthma is associated with the progressive loss of lung function.
Methods
This was a 3-year prospective study. We examined the changes in pre- and post-bronchodilator forced expiratory volume in 1 s (FEV1) and FeNO in 140 patients with controlled asthma. We initially determined the FeNO cut-off point for identifying patients with a rapid decline in FEV1 (>40 mL/yr). Next, a total of 122 patients who maintained high or non-high FeNO were selected, and the associations between the FeNO trend and changes in FEV1 and bronchodilator response (BDR) were investigated.
Results
A FeNO level >40.3 ppb yielded 43% sensitivity and 86% specificity for identifying patients with a rapid decline in FEV1. Patients with persistently high FeNO had higher rates of decline in FEV1 (42.7 ± 37.5 mL/yr) than patients with non-high FeNO (16.7 ± 31.5 mL/yr) (p < 0.0005). The changes in BDR from baseline to the end of the study, in patients who had high or non-high levels of FeNO were −0.8% and 0.1%, respectively (p < 0.01). In a multivariate analysis adjusted by age, body mass index, asthma control, blood eosinophil numbers, and FEV1% of predicted, a FeNO level of ≥40 ppb was independently associated with an accelerated decline in FEV1 (p < 0.05).
Conclusions
This study suggests that FeNO is potentially valuable tool for identifying individuals who are at risk of a progressive loss of lung function among patients with controlled asthma.
Graphical abstract
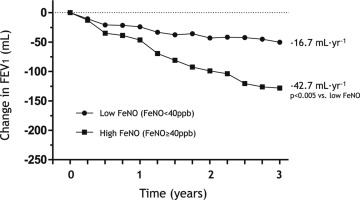
Mean changes in post-bronchodilator FEV1 from baseline to each time point according to the FeNO levels in patients with well-controlled asthma at baseline.
Keywords
Adult asthma; Airflow limitation; Airway inflammation; Exhaled nitric oxide; Remodeling
List of abbreviations used
ACT, Asthma Control Test; BDR, bronchodilator response; FeNO, exhaled nitric oxide fraction; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NO, nitric oxide; NOS, nitric oxide synthase; RNSs, reactive nitrogen species
Introduction
Nitric oxide (NO), a gaseous signaling molecule generated by NO synthase (NOS), is enhanced by inflammatory stimuli.1 The exhaled nitric oxide fraction (FeNO) has been proposed as a marker of airway inflammation and a guide for anti-inflammatory therapy in asthma.1, 2 and 3 However, a persistently high FeNO is occasionally observed despite steroid therapy in some patients.4 and 5 Excessive NO synthesis is well documented in severe asthma6 and 7 and a European multicenter study suggested that the FeNO levels of patients with severe asthma, who are refractory to conventional treatments may not be suppressed by corticosteroids.8 Importantly, this large study showed that the mean FeNO levels of patients with severe asthma are similar to those of patients with non-severe asthma.8 Also, the analysis of data from a Severe Asthma Research Program demonstrated that 40% of the study subjects had FeNO levels of >35 ppb regardless of the severity of asthma.4 The grouping of asthma by FeNO has now been proposed to provide an independent classification of the asthma severity.4
Recent evidence suggests that airway inflammation may play an important role in the progression of airflow limitation in asthma.9, 10, 11, 12 and 13 Cross-sectional studies that targeted patients with severe asthma have shown associations between persistent airflow limitation and eosinophilia in blood,9 sputum,10 and bronchial tissues.11 Also, some longitudinal studies reported that the baseline sputum eosinophil numbers and FeNO could predict a decline in forced expiratory volume in 1 s (FEV1) in asthmatic patients with fixed airflow limitation.12 and 13 To identify patients who are at risk of a rapid loss of lung function at an early stage of asthma is important. However, it remains unclear whether a persistently high FeNO in patients with controlled asthma is associated with the progressive loss of lung function.
In a 3-year prospective cohort study, we recently reported that the annual rate of change in FEV1 among patients with well-controlled asthma was highly variable and the rapid decliners were more likely to have higher levels of FeNO at baseline.14 In this subsequent analysis, we first determined the FeNO cut-off point for identifying patients with an accelerated decline in FEV1. Next, the patients who maintained high or non-high FeNO were selected, and the associations between the FeNO trend and changes in FEV1 and bronchodilator response (BDR) were investigated.
Methods
Study design and patients
This was a stratified analysis of a prospective cohort study. The study subjects were followed with Asthma Control Test (ACT), spirometry, and FeNO every 3 month over a 3-year period. The use of asthma controllers within 24 h before an examination was prohibited for all study visits. Blood eosinophil numbers and serum specific immunoglobulin E (IgE) for common inhaled allergens (house dust mite, cedar, ragweed, cocksfoot, dog, and cat) were examined. Positive specific IgE to at least one allergen was assumed to confirm the presence of atopy. The study was approved by the Ethics Committee of Wakayama Medical University (IRB #526) and registered with the University Hospital Medical Information Network (UMIN 000012105).
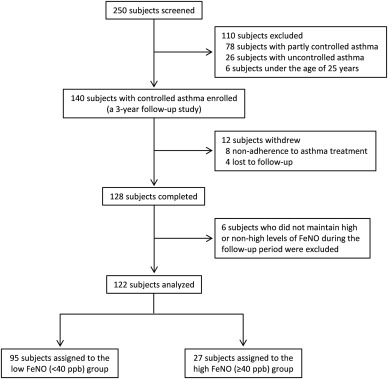
250 Adults with stable asthma following treatment with inhaled corticosteroids (ICS) with or without long-acting β2-agonist, leukotriene modifier, or theophylline for more than 4 years were recruited from the outpatient clinic of Wakayama Medical University Hospital. Current smokers and patients with >10 pack-year smoking history were excluded from the study. Also, patients with poor adherence to therapy (defined as <80% adherence calculated by dividing the number of days supplied for a medication by the number of days between the visits) or with other pulmonary diseases such as COPD were not included. All patients had a history of episodic dyspnea, wheezing, and documented significant airway reversibility and/or airway hyperresponsiveness. The changes in FEV1 before and 15 min after inhalation of 400 μg of salbutamol were measured to assess BDR. Airway reversibility is regarded as significant when FEV1 is increased by ≥12% and ≥200 mL of the absolute volume. Airway responsiveness to methacholine was measured using a device (Astograph; Chest, Tokyo, Japan) that displays respiratory resistance measured via the forced oscillation method. Airway hyperresponsiveness was defined as the cumulative provocative dose of methacholine causing a 100% increase in the baseline respiratory resistance of less than 25 mg/mL. In this analysis, 140 patients with controlled asthma aged over 25 years old were selected based on the GINA guidelines (twice or less/week daytime symptoms, no nocturnal symptoms, no limitation of daily activities, twice or less/week need for reliever, normal lung function (≥70% FEV1/FVC ratio and ≥80% FEV1% of predicted), no exacerbation in the previous one year).1 The flow diagram of the study is shown in Figure 1. Informed written consent was obtained from each participant.
|
|
|
Fig. 1. Disposition of the study patient population. |
Study assessments
The FVC and FEV1 values were measured using a dry rolling seal spirometer. The post-bronchodilator FEV1 was selected to reflect loss of lung function as in other studies following lung function in patients with asthma.14 and 15 The mean annual rates of changes in FEV1 and BDR over a 3-year period were estimated for each subject by fitting a least-square regression line. The random slope was based on time of FEV1 assessment. In total, 3168 FEV1 measurements were analyzed excluding lung function data during periods of worsening asthma (4 weeks before and 4 weeks after the start of a severe exacerbation, defined as worsening asthma requiring at least 3 days treatment with systemic corticosteroids or as a hospitalization due to asthma).16 Based on the magnitude of change in FEV1 over a 3-year period, we labeled those of less than the 25th percentile as rapid decliners. All rapid decliners had an estimated rate of decline in FEV1 of more than 40 mL/yr. The FeNO was measured by an online NO analyzer (NIOX MINO; Aerocrine, Solna, Sweden). Repeated exhalations were performed to obtain two acceptable measurements that agreed within 10% deviation, and the average of these two values was registered.1 and 17
Statistical analyses
We dichotomized the subjects into two groups based on the FeNO levels. The receiver operating characteristic (ROC) analysis was used to find a cut-off value for FeNO that would identify patients with a rapid decline in FEV1. The clinical characteristics were compared using the Chi-squared test for categorical variables, and unpaired t-tests or Mann–Whitney U tests as appropriate for continuous variables. Chi-squared test and multivariate logistic regression analysis were used to estimate odds ratios (ORs) with 95% confidence intervals (CIs) for rapid loss of FEV1. The variables with p values <0.20 in the univariate analysis were included in the multivariate model. All data were expressed as mean values ± standard deviation for continuous variables. For categorical variables, the numbers of observations were given in each category. A p value of <0.05 was considered statistically significant.
Results
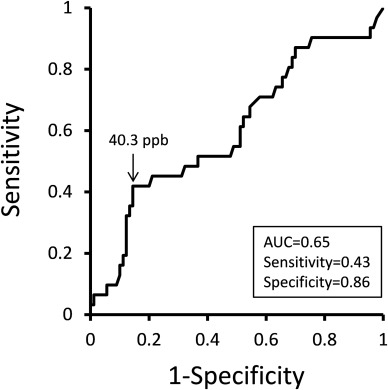
The 3-year follow-up study was completed in 128 patients (Fig. 1). A FeNO level >40.3 ppb yielded 43% sensitivity and 86% specificity for identifying patients with a rapid decline in FEV1 (AUC = 0.65) (Fig. 2). We labeled those with less than 40 ppb as the non-high FeNO group, and those with greater than 40 ppb as the high FeNO group. During the study period, 4 subjects in the high FeNO group and 2 subjects in the non-high FeNO group were switched over to the other group. The subsequent analyses were performed on 122 patients who maintained high (n = 27) or non-high (n = 95) levels of FeNO throughout the study period (Fig. 1).
|
|
|
Fig. 2. Receiver operating characteristics (ROC) curve to estimate the FeNO cut-off point for identifying patients with a rapid decline in FEV1 (>40 mL yr−1). Data labels represent cut-off point of FeNO (arrow), area under the curve (AUC), sensitivity, and specificity. |
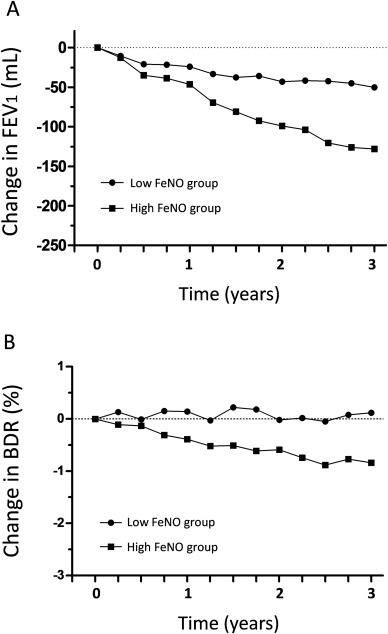
The baseline patient characteristics subdivided by FeNO levels are presented in Table 1. The age, gender, BMI, smoking history, and asthma treatment were not different between the two groups, although the high FeNO group had lower FEV1/FVC ratio (p < 0.005), and higher blood eosinophils and FeNO (all p < 0.0001) at baseline. Table 2 shows the characteristics during the follow-up period and at the end of the study. Neither the dose of ICS nor other controller use showed any difference between the two groups. The FeNO levels were not associated with the rates of severe exacerbations. At the end of the study, patients with high FeNO showed greater impairments in their ACT scores (p < 0.01) and had lower FEV1 and FEV1/FVC ratio (all p < 0.005). As shown in Figure 3A, patients with persistently high FeNO had higher rates of decline in FEV1 than patients with non-high FeNO (non-high FeNO, 16.7 ± 31.5 mL/yr; high FeNO, 42.7 ± 37.5 mL/yr; p < 0.0005). Proportion of patients who had a rate of FEV1 decline of more than 40 mL/yr, in patients who had high or non-high levels of FeNO were 48% and 19%, respectively (p < 0.005). Patients with high FeNO had a significantly higher risk of a rapid decline in FEV1 compared to those with non-high FeNO (OR, 2.73; 95% CI, 1.44–5.15; p < 0.01). The changes in BDR from baseline to the end of the study, in patients who had high or non-high levels of FeNO were −0.8% and 0.1%, respectively (p < 0.01) ( Table 2 and Fig. 3B). As shown in Table 2 and 5 of 95 subjects (5.2%) in non-high FeNO group and 5 of 27 subjects (18.5%) in high FeNO group had FEV1/FVC ratio of <70% at the end of the study. Patients with high FeNO had a significantly higher risk of a fixed airway obstruction (FEV1/FVC < 70%) compared to those with non-high FeNO (OR, 4.09; 95% CI, 1.09–15.38; p < 0.05).
| Characteristics | Non-high FeNO (n = 95) | High FeNO (n = 27) | p value |
|---|---|---|---|
| Age (yrs) | 42.4 ± 13.4 | 47.0 ± 14.6 | 0.13 |
| Gender (female/male) | 67/28 | 16/11 | 0.27 |
| Body mass index (kg/m2) | 21.8 ± 4.0 | 22.9 ± 4.2 | 0.18 |
| Smoking status (Never/Ex), n | 71/24 | 19/8 | 0.65 |
| Atopy, n (%) | 74 (77.9) | 23 (85.2) | 0.41 |
| Duration of asthma (yrs) | 10.3 ± 3.4 | 10.6 ± 3.2 | 0.68 |
| Dose of inhaled corticosteroids (μg/day)† | 322 ± 116 | 332 ± 110 | 0.67 |
| Use of long-acting β2-agonist, n (%) | 45 (47.4) | 13 (48.1) | 0.94 |
| Use of leukotriene modifier, n (%) | 10 (10.5) | 5 (18.5) | 0.27 |
| Use of theophylline, n (%) | 6 (6.3) | 3 (11.1) | 0.40 |
| Asthma Control Test (points) | 23.3 ± 1.1 | 22.9 ± 0.9 | 0.08 |
| FVC % of predicted (%) | 103.5 ± 9.3 | 102.9 ± 10.0 | 0.77 |
| FEV1/FVC ratio (%) | 82.2 ± 6.9 | 77.2 ± 6.1 | <0.005 |
| FEV1% of predicted (%) | 97.6 ± 7.4 | 95.2 ± 9.0 | 0.22 |
| FEV1 reversibility (%) | 9.2 ± 4.1 | 10.0 ± 5.4 | 0.38 |
| Blood eosinophil numbers (cells/μL) | 173 ± 151 | 469 ± 241 | <0.0001 |
| Exhaled nitric oxide fraction (ppb) | 20.2 ± 7.4 | 56.5 ± 11.2 | <0.0001 |
Data are presented as means ± SD unless otherwise stated. p Values compared between the groups. High FeNO defined as ≥40 ppb, and non-high FeNO defined as <40 ppb.
†. Inhaled corticosteroids, expressed as fluticasone propionate equivalent.
| Characteristics | Non-high FeNO (n = 95) | High FeNO (n = 27) | p Value |
|---|---|---|---|
| Follow-up period | |||
| Use of inhaled corticosteroids (%)† | 95 (100.0) | 27 (100.0) | 1.00 |
| Dose of inhaled corticosteroids (μg/day)‡§ | 330 ± 110 | 359 ± 113 | 0.20 |
| Use of long-acting β2-agonist, n (%)† | 45 (47.4) | 15 (55.6) | 0.27 |
| Use of leukotriene modifier, n (%)† | 9 (9.5) | 5 (18.5) | 0.19 |
| Use of theophylline, n (%)† | 6 (6.3) | 3 (11.1) | 0.40 |
| Number of patients with a severe exacerbation, n (%) | 19 (20.0) | 8 (29.6) | 0.29 |
| At the end of the study | |||
| Asthma Control Test (points) | 23.3 ± 1.7 | 22.3 ± 1.8 | <0.01 |
| Exhaled nitric oxide fraction (ppb) | 18.0 ± 6.9 | 57.1 ± 15.6 | <0.0001 |
| FVC % of predicted (%) | 101.7 ± 10.7 | 98.3 ± 9.7 | 0.14 |
| FEV1/FVC ratio (%) | 82.1 ± 7.3 | 76.4 ± 7.0 | <0.005 |
| FEV1% of predicted (%) | 95.1 ± 8.7 | 88.7 ± 10.8 | <0.005 |
| FEV1 reversibility (%) | 9.2 ± 3.8 | 9.2 ± 4.9 | 0.98 |
| Annual rates of decline in FEV1 (mL yr−1) | 16.7 ± 31.5 | 42.7 ± 37.5 | <0.0005 |
| Number of patients with FEV1 decline >40 mL yr−1, n (%) | 18 (18.9) | 13 (48.2) | <0.005 |
| Number of patients with FEV1/FVC ratio <70%, n (%) | 5 (5.2%) | 5 (18.5%) | <0.05 |
| Annual rates of change in FEV1 reversibility (%·yr−1)‡ | 0.03 ± 0.50 | −0.27 ± 0.47 | <0.0005 |
Data are presented as means ± SD unless otherwise stated. p Values compared between the groups.
†. Usage rate of medications during the follow-up period.
‡. Mean values during the follow-up period.
§. Inhaled corticosteroids, expressed as fluticasone propionate equivalent.
|
|
|
Fig. 3. Mean changes in (A) post-bronchodilator FEV1 and (B) bronchodilator response (BDR) from baseline to each time point according to the FeNO levels. High FeNO defined as ≥40 ppb, and non-high FeNO defined as <40 ppb. |
To identify a predictor of an accelerated decline in FEV1, associations between the binary outcome (decline in FEV1 >40 mL/yr) and a set of covariates at baseline were also analyzed. According to the multivariate analysis adjusted by age, BMI, ACT score, blood eosinophil numbers, and FEV1% of predicted, a FeNO level of ≥40 ppb was independently associated with an accelerated decline in FEV1 (OR, 4.42; 95% CI, 1.34–14.66; p < 0.05) ( Table 3).
| Baseline characteristics | All asthma (n = 122) | |
|---|---|---|
| Odds ratio (95% confidence interval) | p Value | |
| Age, yrs | 1.03 (0.99–1.06) | 0.12 |
| Body mass index, kg/mm2 | 1.03 (0.92–1.14) | 0.64 |
| Asthma Control Test, points | 1.13 (0.74–1.72) | 0.58 |
| Blood eosinophil numbers, cells/μL | 1.00 (0.99–1.00) | 0.68 |
| High level of FeNO (≥40 ppb)† | 4.42 (1.34–14.66) | <0.05 |
| FEV1% of predicted, % | 1.02 (0.96–1.08) | 0.56 |
†. Independent predictor of FEV1 decline >40 mL yr−1.
Discussion
In this prospective cohort study of patients with controlled asthma at baseline, we found that a persistently high FeNO was associated with an accelerated decline in FEV1 and reduction in BDR over time. A FeNO >40.3 ppb yielded 43% sensitivity and 86% specificity for identifying patients with a rapid decline in FEV1. There was no association between decline in lung function and other potential predicting factors, such as aging, obesity, systemic eosinophilia, asthma control, and airflow limitation. This study shows that FeNO is a specific predictor of progressive loss of lung function in patients with well-controlled asthma.
The role of high levels of FeNO in the progression of airflow limitation in asthma is still uncertain. In inflammatory conditions, excessive NO derived from the inducible type of NO synthase (iNOS) is produced as well as superoxide anion from nicotinamide adenine dinucleotide oxidase or xanthine oxidase.18 and 19 In general, NO rapidly reacts with superoxide anion to produce highly reactive nitrogen species (RNSs) such as peroxynitrite.18 and 19 Excessive RNSs cause tissue injury, lipid peroxidation, and airway inflammation.19, 20 and 21 Nitro-oxidative stress could be one of the factors responsible for airway remodeling,6, 7 and 22 which may be associated with the development of persistent airflow limitation in asthma. The patients with severe adult-onset asthma mostly have persistent sputum eosinophilia and increased FeNO levels despite steroid therapy.23 Recent evidence shows that the up-regulation of iNOS in the airway epithelium via STAT-6 and Th2-cytokines interleukin (IL)-4 and IL-13, produces enhanced NO concentrations in exhaled air.24 It is known that these Th2-cytokines stimulate epithelial cells and fibroblasts to promote a profibrotic response.25 and 26 Persistently high FeNO have also been seen in association with elevations of concomitant Th1 cytokines such as interferon (IFN)-γ.7 and 27 IL-4 and IL-13 in combination with IFN-γ synergistically enhance iNOS expression.7 and 27 This combination may partly explain the observation that FeNO can be elevated in patients with both severe and non-severe asthma, and it may contribute to inducing a high degree of nitro-oxidative stress. Recent studies suggest that the characteristics associated with Th2 signatures such as blood eosinophils, periostin, and FeNO may differ by molecular background of asthma.28, 29 and 30 The mixed Th1/Th2 characteristics in patients with asthma, even in milder disease, may be involved in their relative steroid unresponsiveness.30, 31, 32 and 33 The IFN-γ-mediated induction of iNOS expression is virtually steroid-resistant, as evidenced both in vitro34 and in vivo. 35 Furthermore, a recent study suggests that osteopontin and periostin, both of which can bind to extracellular matrix proteins, might have affected the long-standing decline of lung function by their involvement in persistent eosinophilic inflammation and airway remodeling.36
The present study also identified that a persistently high FeNO is correlated with the decrease in BDR. A reduced BDR has been used as a surrogate marker for airway remodeling,37 and 38 although there are no prospective studies showing that the lack of BDR is evidence of structural changes. A positive relationship was observed between BDR and volume fraction of airway smooth muscle in endobronchial biopsies from children with asthma.38 Moreover, the BDR is impaired in steroid-resistant asthma and is associated with a shift in matrix metalloprotease 9/tissue inhibitor of metalloproteinases 1 ratio.37
An official ATS clinical practice guideline indicates that the FeNO values should be interpreted by considering asthma symptoms when monitoring airway inflammation.2 If a patient is asymptomatic but has a high FeNO (>50 ppb), monitoring the FeNO trend over time is recommended.2, 39 and 40 However, several recent studies reported that the association of high FeNO to uncontrolled asthma4 and 5 and future loss of lung function.12 and 13 Our study extends these data to real-life clinical care. Specifically, in patients with controlled asthma, a persistently high FeNO was associated with a more rapid decline in FEV1 and decrease in airway reversibility over time, with an optimum cut-off point of FeNO 40 ppb.
The high level of FeNO was a specific but not a sensitive marker for predicting that patients would experience a rapid decline in FEV1. This is in agreement with previous studies describing that factors other than the FeNO level are associated with the excessive loss of lung function.41, 42 and 43 Importantly, we found no statistical interaction between the rapid decline in FEV1 and other risk factors indicating that there is potentiation of risk by the presence of high FeNO. However, in the absence of another prospective study examining the relationship between the FeNO trend and the changes in FEV1 over time, further confirmation of our finding is needed.
This study had several limitations. There are some possible explanations for the persistently high FeNO. Certain variables such as poorly inhaled drug delivery or persistent exposure to allergen could not be completely accounted for and verified. Also, no biopsy specimens were obtained to examine whether the changes in BDR were correlated with structural changes in the airways. Furthermore, a highly selection of the study population was necessary to examine the associations between the FeNO trend and changes in lung function. Thus, it can limit the generalizability of the current findings.
In summary, we showed that a persistently high FeNO in patients with controlled asthma is associated with the progression of airflow limitation and reduction in airway reversibility over time. FeNO is suggested to be a valuable tool for identifying individuals who are at risk of a rapid loss of lung function among patients with asthma. These findings warrant further study of the mechanisms of persistent FeNO elevation including patients molecular profile.
Conflict of interest
The authors have no conflict of interest to declare.
Authors' contributions
KM, conception, design research, performance of analysis and preparation of manuscript; KM, TH and AO, acquisition of data; KM, TH, AO, KI and NE, interpretation of results of analysis. All the authors approved final version of the manuscript.
Acknowledgments
This analysis was performed as a part of the Factors affecting the Long-term Asthma Therapy (FLOAT) study. No support was provided. The authors thank Mr. Brent Bell for reading the manuscript.
References
- 1 ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide; Am J Respir Crit Care Med, 171 (2005), pp. 912–930
- 2 An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications; Am J Respir Crit Care Med, 184 (2011), pp. 602–615
- 3 A.D. Smith, J.O. Cowan, K.P. Brassett, G.P. Herbison, D.R. Taylor; Use of exhaled nitric oxide measurements to guide treatment in chronic asthma; N Engl J Med, 352 (2005), pp. 2163–2173
- 4 R.A. Dweik, R.L. Sorkness, S. Wenzel, J. Hammel, D. Curran-Everett, S.A. Comhair, et al.; Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma; Am J Respir Crit Care Med, 181 (2010), pp. 1033–1041
- 5 K. Matsunaga, S. Yanagisawa, T. Hirano, T. Ichikawa, A. Koarai, K. Akamatsu, et al.; Associated demographics of persistent exhaled nitric oxide elevation in treated asthmatics; Clin Exp Allergy, 42 (2012), pp. 775–781
- 6 H. Sugiura, Y. Komaki, A. Koarai, M. Ichinose; Nitrative stress in refractory asthma; J Allergy Clin Immunol, 21 (2008), pp. 355–360
- 7 N. Voraphani, M.T. Gladwin, A.U. Contreras, N. Kaminski, J.R. Tedrow, J. Milosevic, et al.; An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma; Mucosal Immunol, 7 (2014), pp. 1175–1185
- 8 European Network for Understanding Mechanisms of Severe Asthma. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma; Eur Respir J, 22 (2003), pp. 470–477
- 9 D. Bumbacea, D. Campbell, L. Nguyen, D. Carr, P.J. Barnes, D. Robinson, et al.; Parameters associated with persistent airflow obstruction in chronic severe asthma; Eur Respir J, 24 (2004), pp. 122–128
- 10 A. Ten Brinke, A.H. Zwinderman, P.J. Sterk, K.F. Rabe, E.H. Bel; Factors associated with persistent airflow limitation in severe asthma; Am J Respir Crit Care Med, 164 (2001), pp. 744–748
- 11 C. Miranda, A. Busacker, S. Balzar, J. Trudeau, S.E. Wenzel; Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation; J Allergy Clin Immunol, 113 (2004), pp. 101–108
- 12 M. Contoli, S. Baraldo, B. Marku, P. Casolari, J.A. Marwick, G. Turato, et al.; Fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up; J Allergy Clin Immunol, 125 (2010), pp. 830–837
- 13 I.H. Van Veen, A. Ten Brinke, P.J. Sterk, J.K. Sont, S.A. Gauw, K.F. Rabe, et al.; Exhaled nitric oxide predicts lung function decline in difficult-to-treat asthma; Eur Respir J, 32 (2008), pp. 344–349
- 14 K. Matsunaga, T. Ichikawa, A. Oka, Y. Morishita, K. Kanai, M. Hiramatsu, et al.; Changes in forced expiratory volume in 1 second over time in patients with controlled asthma at baseline; Respir Med, 108 (2014), pp. 976–982
- 15 R.A. Covar, J.D. Spahn, J.R. Murphy, S.J. Szefler; Progression of asthma measured by lung function in the childhood asthma management program; Am J Respir Crit Care Med, 170 (2004), pp. 234–241
- 16 An official ATS/ERS statement: asthma control and exacerbations; Am J Respir Crit Care Med, 180 (2009), pp. 59–99
- 17 K. Matsunaga, T. Hirano, K. Akamatsu, A. Koarai, H. Sugiura, Y. Minakata, et al.; Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects; Allergol Int, 60 (2011), pp. 331–337
- 18 W. MacNee; Oxidative stress and lung inflammation in airways disease; Eur J Pharmacol, 429 (2000), pp. 195–207
- 19 F.L. Ricciardolo, A. Di Stefano, F. Sabatini, G. Folkerts; Reactive nitrogen species in the respiratory tract; Eur J Pharmacol, 533 (2006), pp. 240–252
- 20 J.S. Beckman, T.W. Beckman, J. Chen, P.A. Marshall, B.A. Freeman; Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide; Proc Natl Acad Sci U S A, 87 (1990), pp. 1620–1624
- 21 H. Sugiura, M. Ichinose, T. Oyake, Y. Mashito, Y. Ohuchi, N. Endoh, et al.; Role of peroxynitrite in airway microvascular hyperpermeability during late allergic phase in guinea pigs; Am J Respir Crit Care Med, 160 (1999), pp. 663–671
- 22 T. Ichikawa, H. Sugiura, A. Koarai, S. Yanagisawa, M. Kanda, A. Hayata, et al.; Peroxynitrite arguments fibroblast-mediated tissue remodeling via myofibroblast differentiation; Am J Physiol Lung Cell Mol Physiol, 295 (2008), pp. 800–808
- 23 M. Amelink, J.C. de Groot, S.B. de Nijs, R. Lutter, A.H. Zwinderman, P.J. Sterk, et al.; Severe adult-onset asthma: a distinct phenotype; J Allergy Clin Immunol, 132 (2013), pp. 336–341
- 24 K. Alving, A. Malinovschi; Basic aspects of exhaled nitric oxide; Eur Respir Mon, 49 (2010), pp. 1–31
- 25 X. Liu, T. Kohyama, H. Wang, Y.K. Zhu, F.Q. Wen, H.J. Kim, et al.; Th2 cytokine regulation of type 1 collagen gel contraction mediated by human lung mesenchymal cells; Am J Physiol, 282 (2002), pp. 1049–1056
- 26 F.Q. Wen, T. Kohyama, X. Liu, Y.K. Zhu, H. Wang, H.J. Kim, et al.; Interleukin-4- and interleukin-13-enhanced transforming growth factor-beta2 production in cultured human bronchial epithelial cells is attenuated by interferon-gamma; Am J Respir Cell Mol Biol, 26 (2002), pp. 484–490
- 27 F.H. Guo, K. Uetani, S.J. Haque, B.R. Williams, R.A. Dweik, F.B. Thunnissen, et al.; Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators; J Clin Investig, 100 (1997), pp. 829–838
- 28 P.G. Woodruff, B. Modrek, D.F. Choy, G. Jia, A.R. Abbas, A. Ellwanger, et al.; T-helper type 2-driven inflammation defines major subphenotypes of asthma; Am J Respir Crit Care Med, 180 (2009), pp. 388–395
- 29 K.W. McGrath, N. Icitovic, H.A. Boushey, S.C. Lazarus, E.R. Sutherland, V.M. Chinchilli, et al.; A large subgroup of mild-to-moderate asthma is persistently noneosinophilic; Am J Respir Crit Care Med, 185 (2012), pp. 612–619
- 30 A. Ray, T.B. Oriss, S.E. Wenzel; Emerging molecular phenotypes of asthma; Am J Physiol Lung Cell Mol Physiol, 308 (2015), pp. 130–140
- 31 M.C. Peters, Z.K. Mekonnen, S. Yuan, N.R. Bhakta, P.G. Woodruff, J.V. Fahy; Measures of gene expression in sputum cells can identify T2-high and T2-low subtypes of asthma; J Allergy Clin Immunol, 133 (2014), pp. 388–394
- 32 S.F. Seys, M. Grabowski, W. Adriaensen, A. Decraene, E. Dilissen, J.A. Vanoirbeek, et al.; Sputum cytokine mapping reveals an 'IL-5, IL-17A, IL-25-high' pattern associated with poorly controlled asthma; Clin Exp Allergy, 43 (2013), pp. 1009–1017
- 33 K. Matsunaga, T. Ichikawa, S. Yanagisawa, A. Koarai, T. Hirano, H. Sugiura, et al.; Clinical application of exhaled breath condensate analysis in asthma: prediction of FEV1 improvement by steroid therapy; Respiration, 78 (2009), pp. 393–398
- 34 L.E. Donnely, P.J. Barnes; Expression and regulation of inducible nitric oxide synthase from human primary airway epithelial cells; Am J Respir Cell Mol Biol, 26 (2002), pp. 144–151
- 35 J.O. Lundberg, E. Weitzberg, J. Rinder, A. Rudehill, O. Jansson, N.P. Wiklund, et al.; Calcium-independent and steroid-resistant nitric oxide synthase activity in human paranasal sinus mucosa; Eur Respir J, 9 (1996), pp. 1344–1347
- 36 Y. Kanemitsu, I. Ito, A. Niimi, K. Izuhara, S. Ohta, J. Ono, et al.; Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma; Am J Respir Crit Care Med, 190 (2014), pp. 472–474
- 37 E. Goleva, P.J. Hauk, J. Boguniewicz, R.J. Martin, D.Y. Leung; Airway remodeling and lack of bronchodilator response in steroid-resistant asthma; J Allergy Clin Immunol, 120 (2007), pp. 1065–1072
- 38 A. Gupta, A. Sjoukes, D. Richards, W. Banya, C. Hawrylowicz, A. Bush, et al.; Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma; Am J Respir Crit Care Med, 184 (2011), pp. 1342–1349
- 39 A.D. Smith, J.O. Cowan, K.P. Brassett, S. Filsell, C. McLachlan, G. Monti-Sheehan, et al.; Exhaled nitric oxide: a predictor of steroid response; Am J Respir Crit Care Med, 172 (2005), pp. 453–459
- 40 M.W. Pijnenburg, W. Hofhuis, W.C. Hop, J.C. De Jongste; Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission; Thorax, 60 (2005), pp. 215–218
- 41 A. Dijkstra, J.M. Vonk, H. Jongepier, G.H. Koppelman, J.P. Schouten, N.H. ten Hacken, et al.; Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex; Thorax, 61 (2006), pp. 105–110
- 42 J.H. Lee, T. Haselkorn, L. Borish, L. Rasouliyan, B.E. Chipps, S.E. Wenzel; Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR study; Chest, 132 (2007), pp. 1882–1889
- 43 K. Matsunaga, K. Akamatsu, A. Miyatake, M. Ichinose; Natural history and risk factors of obstructive changes over a 10-year period in severe asthma; Respir Med, 107 (2013), pp. 355–360
Document information
Published on 05/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?