Abstract
The need for sustainable biofuels has initiated a global search for innovative technologies that can sustainably convert nonfood bioresources to liquid transportation fuels. While 2nd generation cellulosic ethanol has begun to address this challenge, other resources including yellow and brown grease are rapidly evolving commercial opportunities that are addressing regional biodiesel needs. This review examines the technical and environmental factors driving the collection of trap FOG (Fats, Oils, and Greases), its chemical composition and technologies currently available and future developments that facilitate the conversion of FOG into biodiesel.
Introduction
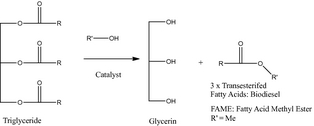
Over the last decade the sustainable conversion of bioresources to biofuels has become a global pursuit that is being tailored to regional resources and local needs [1, 2]. Todays biofuel successes are often contingent on using abundant and productive starch- and sucrose-based crops that has been challenged on “food-or-fuel” concerns which will only increase in the future as the global population grows [3, 4]. Key commercial breakthroughs in replacing significant amounts of petroleum-based fuels with renewable, nonfood bioresources, will come from translational research directed at reducing the recalcitrance of lignocellulosics for sustainable 2nd and 3rd generation biofuels [5] and overcoming the barriers to algae-derived biofuels [6] with positive life-cycle assessment (LCA) performance parameters. While these efforts are continuing to accelerate with technical issues being addressed, smaller successes also continue to evolve. For example, the recovery of yellow grease from commercial cooking facilities is a well-documented success in which spent fryer oil, containing a mixture of plant and animal fats, is stored, collected, and then transported to a central process site in which it is purified and transesterified to biodiesel (see Fig. 1) [7].
|
|
|
Figure 1. Biodiesel transesterification reaction. |
After purification, FAME is sold as biodiesel most often as B2, B5, or B10 fuel blends. The excess alcohol in FAME generation is recovered and recycled, whereas the glycerin is recovered and sold into established commercial markets. Given the volume of glycerin generated, a growing research effort is now being directed at using this chemical as a feedstock for alternative chemicals and polymers [8].
In many countries, companies will not only pay for used restaurant cooking oils but also from food processing plants, animal processing centers, supermarkets – almost any business that produces cooking oil waste or used fryer oil. It is now indeed difficult to envisage that these fats, oils, and greases (FOG) were once considered a waste product. Key to this conversion is a low level of free fatty acids (FFA) which are usually cited to be 15% or lower in yellow grease. The presence of FFA complicates the transesterification reaction as this is most often accomplished using an alkaline system (i.e., frequently NaOCH3) and FFA neutralize the base and lead to soap formation. A commonly cited practice with yellow grease with a moderate FFA content is to blend this feedstock with fats thereby lowering the overall FFA content to 1–3%. Literature reports indicate that a FFA content of <2.5% does not yield significant processing difficulties [9], although Mittlebach reported the level of FFA should be no more than ~1% for the alkaline-catalyzed transesterification reactions [10]. Alternative approaches to controlling FFA content include steam stripping [11], caustic stripping, solvent extraction [12], glycerolysis, or acid esterification, with the latter approach being highlighted by several biodiesel companies [13-17].
As the demand for biodiesel increases and viable yellow grease sources have been secured, entrepreneurs have begun to pursue the conversion of select brown grease resources (i.e., FFA content >15%) to biodiesel which is the focus of this review. The attractive features of commercially recovered brown grease are fourfold [18]:
- Lower feedstock cost.
- Large volume of resource available.
- Governmental mandates requiring collection and processing of select brown greases.
- Avoidance of “food versus fuel” concerns while contributing to the development of renewable fuels.
FOG Collection
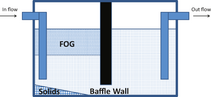
One of the biggest sources of brown grease is the material trapped and recovered in grease inceptors/traps that many commercial food processing centers are mandated to have. Grease abatement plumbing devices are usually nonmechanical gravity separation flow-through devices that facilitate the recovery of grease and food solids from aqueous waste streams. Depending on the size of food processing operations, modern building/business codes often require the installation of grease traps (~50 L) or interceptors (~3780–7570 L) [19, 20]. Grease interceptors are multicompartment chamber devices where the aqueous grease containing flow is retained long enough so that grease and some solids can rise to the water surface and most of the solids settle to the bottom. The clarified water is then eventually discharged to a sanitary sewer system (see Fig. 2).
|
|
|
Figure 2. Simplified illustration of fats, oils, and greases abatement device. |
Given their size, grease interceptors are usually located below ground and outside the food preparation area. The American Society of Mechanical Engineers (ASME) standard A112.14.3 requires that grease interceptors remove a minimum of 90% of the incoming FOG [21]. Grease traps are designed to retain a small amount of grease, usually servicing from 1 to 4 plumbing fixtures. A recent study has suggested that FOG removal may be less than these target values, as a pilot system suggested that retention-based grease interceptors achieved ~80% FOG removal and flow-based grease interceptors removed <50% FOG [22]. A review of the literature indicates that grease interceptors should be pumped out by permitted transporters, at a minimum every 90 days, whereas shorter maintenance cycles (i.e., 30 or 60 days) have been reported depending on facilitys service volume [23]. Alternatively, some municipalities have implemented a “25% rule” that requires the grease abatement unit be emptied when they reach 25% of the design capacity [24].
The need for active management and control of trap FOG is due to the detrimental impact on sewer systems. The U.S.A. EPA has estimated that 23,000 to 75,000 sanitary sewer overflows (SSO) per year occurred in the United States in the 2001–2003 time period of which 48% of the SSOs were caused by blockages and 47% of these blockages were grease related, leading to ~5000 to 17,000 FOG-caused SSOs nationwide annually [25-27]. Furthermore, it has been proposed that FOG contributes to sewer plant blockages and pump station failures [28]. These overall concerns for trap FOG in sewer systems have also been reported for a variety of other major metropolitan cities globally. For example:
- City of Dublin “Drainage maintenance records indicate that FOG is a serious problem in areas where there are concentrations of Food Service Establishments (FSE) such as pubs, restaurants, hotels, takeaways, convenience stores, etc.” [29].
- 2012, Metro Vancouver approved a bylaw that mandates new requirements for grease interceptors or grease traps [30].
- To reduce sewer blockages, South East Water in Australia implemented a three-phase grease reduction program which involved the use of grease interceptor and mandated pump-outs [31].
The grease trap waste has been reported to vary substantially depending on the source but a broad description of these abatement collection systems would include three phases: a top floatable layer rich in FOG; a middle aqueous layer that is organic rich; and bottom sludge containing food particles and other settleable solids. The trap material is about 95% water, 3% solids, and 2% FOG. Sato et al. surveyed 27 different restaurant grease samples and reported that the average chemical oxygen demand (COD) levels for the floatable layer was 478,000 mg/L, 66,200 mg/L for the middle layer and 1,007,061 mg/L for the sludge phase [32]. Historically, this material was collected and landfilled, although other options included land application, compositing, rendering for lubricants/soaps, or incineration. Direct disposal is becoming more challenging due to legislative regulations and overall decreased access to inexpensive landfill options. The two most attractive future applications for grease trap waste are anaerobic codigestion of FOG to methane/biogas or biodiesel production. The former pathway was extensively reviewed by Long et al. [33] in 2012 and this review examines opportunities in the biodiesel field. The latter route could be especially attractive to those regions of the globe where natural gas prices are low and biodiesel prices are high. In addition, for select developing nations, it was been reported that the market demand for FOG is very low and despite legislative efforts this material (i.e., gutter oil) has been reintroduced into the food services industry [34]. Clearly, there is a need to develop practical market-driven solutions that will provide viable market-driven outlets for trap FOG which the biodiesel route could readily provide.
FOG From Grease Traps/Interceptors
A recent National Renewable Energy Laboratory report estimated that in the United States, FOG is generated at a rate of 7.1 L FOG/person/year [35]. But Long et al. [33] has argued this may overstate the recoverable amounts of FOG as this value includes FOG in the sewer system hence the amount of annually recoverable FOG generated in the United States should be ~2.2 billion L/year. On a more regionally basis, literature reports indicate that substantial amounts of trap FOG are generated in most major metropolitan cities as highlighted in Table 1 [36].
| City/Sate | Annual trap grease generation (m. tons) |
|---|---|
| Sacramento/CA | 7530 |
| Denver/CO | 7200 |
| Boston/MA | 15,200 |
| Washington/DC | 22,700 |
| Memphis/TN | 8400 |
| Macon/GA | 2700 |
Given the fact this material must be collected, is relatively low cost, and present in large volumes in major metropolitan regions, it opens the opportunity for trap FOG to biodiesel processing centers. The chemical components that contribute to grease trap/interceptor FOG is highly variable, as to be expected, given the numerous sources that can contribute to this bioresource. Nonetheless, there are several published articles that highlight the components present in this resource as summarized in Table 2.
| Compound | Average of 27 restaurant grease samples % [32] | Grease trap influent for Korea O Barbecue restaurant % [38] | Cafeteria and restaurant, Thailand % [39] | Polish meat processing plant % [40] | Canteen National Singapore University % [41] | Trap grease provided by Guangzhou E.P.A. of China [42] |
|---|---|---|---|---|---|---|
| Caprylic Acid – C8:0 | 0.9 | <0.01 | ||||
| Capric Acid – C9:0 | 0.04 | |||||
| Decanoic Acid – C10:0 | 0.9 | 0.1 | <0.01 | |||
| Undecylic Acid – C11:0 | ND | |||||
| Lauric Acid – C12:0 | 1.3 | 0.58 | 0.03 | |||
| Myristic Acid – C14:0 | 8.4 | 0.56 | 0.02 | 0.01 | 1.3 | 1.16 |
| Pentadecylic – C15:0 | 0.02 | |||||
| Palmitic Acid – C16:0 | 23.1 | 25.8 | 28.83 | 38.3 | 30.38 | |
| Palmitoleic Acid – C16:1 | 0.03 | 1.2 | 1.42 | |||
| Margaric Acid – C17:0 | 1.06 | |||||
| Stearic Acid – C18:0 | 9.8 | 4.71 | 0.06 | 16.31 | 7.2 | 6.02 |
| Nonadecylic Acid – C19:0 | ND | |||||
| Arachidic Acid – C20:0 | 2.1 | 0.17 | ||||
| Palmitoleic Acid – C16:1ω7c | 0.73 | |||||
| Cis-9-heptadecenoic Acid –C17:1ω8c | 3.12 | |||||
| Linoleic Acid – C18:2 | 15.3 | 19.9 | 15.1 | 18.83 | ||
| Alpha linoleic acid – C18:2ω6.9c | 20.92 | |||||
| Gamma linoleic acid – C18:3ω6,9,12c | 3.29 | |||||
| Linolenic Acid – C18:3 | – | 1.31 | ||||
| Palmitoleic Acid – C16:1 | – | <0.01 | ||||
| Oleic Acid – C18:1ω9c | 36.1 | 22.87 | 39.6 | 53.37 | 36.9 | 38.39 |
| Cis-13-nonadeconic Acid –C19:1ω6c | 2.56 | |||||
| Gondoic Acid – C20: 1ω9c | 0.28 | |||||
| Arachidic Acid – C20:0 | <0.01 | <0.01 | ||||
| Behenic Acid – C22:0 | <0.01 | |||||
| Lignoceric Acid: C24:0 | <0.01 | |||||
| 60–80 minor free fatty acids | 31.40 | 2.49 |
As summarized in Table 2 and other reports [43], often the predominant saturated fat is palmitic, primary unsaturated fat is oleic, and the major polyunsaturated fat is linoleic acid. A report by Robbins et al. [44] indicates that the free fatty acid content of trap greases can vary from 35% to 100%. Other reports have provided lower FFA values such 26.2% from trap grease FOG isolated from restaurants and cafeterias in Thailand [39]. Although the trap FOG collected in grease abatement plumbing devices may have started as a yellow grease, the high and varied levels of FFA is not surprising given the assorted cuisines, collection systems/operating environments, time of collection, comingled containments, presence of food particles, salts, alkaline cleaning products, detergents, and biological agents. For example, the presence of detergents and sanitizers has been reported to enhance the hydrolysis of triglycerides yielding FFA [20].
Given the variability of potential nonfood inputs into grease traps (i.e., cleaning solutions, municipal water trace containments, pesticides, etc.), it is well appreciated that this resource could contain a variety of other materials and the open literature does provide some guidance to these elements as summarized in Tables 3, 4.
| Reported range for 17 samples (μg/L) | |
|---|---|
| Acetone | 157.0–713.0 |
| Benzene | 3.8–10.5 |
| 2-Butane | 92.6–1040.0 |
| Carbon disulfide | 1.6–172.0 |
| Chloroethane | 11.2–62.0 |
| Chloroform | 2.9–655.0 |
| Chloromethane | 2.6–27.0 |
| 1,2-Dichloroethene | 28.2–102.0 |
| Ethylbenzene | 11.5–98.8 |
| 2-Hexanone | 12.0 |
| Methylene chloride | 2.0–288.0 |
| Styrene | 4.9–17.3 |
| Tetrachloroethene | 31.5–8510.0 |
| Toluene | 13.9–1370 |
| Trichloroethene | 146.0–600.0 |
| Xylene | 16.6–687.0 |
| Reported range for samples testeda | |
|---|---|
| |
| Calcium (mg/L) | 240–37,359 |
| Copper (mg/L) | 1.8–1092 |
| Iron (mg/L) | 70–577 |
| Lead (mg/L) | 1.1–81.7 |
| Magnesium (mg/L) | 30–3575 |
| Phosphorous (mg/L) | 6.5–326 |
| Potassium (mg/L) | 14–11,968 |
| Sodium (mg/L) | 34–54,348 |
| Zinc (mg/L) | 21–467 |
| Nitrogen (mg/L) | 0.2–6.3 |
| Chloride (mg/L) | 0–4275 |
| % Sulfur | 0.1–0.3 |
The reported chlorinated compounds were attributed to reactions of dissolved organics in water chlorination programs and/or biological routes.
To better understand how these containments need to be handled, the following section will examine the commonly followed procedure for the conversion of trap FOG to biodiesel, followed by a brief description of the purification steps employed.
Trap Grease FOG to Biodiesel
The chemistry of converting fatty acid triglycerides to biodiesel is well established from a commercial perspective and remains actively pursued as a research topic. Industrially, the most commonly practiced route is via a base-catalyzed transesterification methodology using methanol and sodium or potassium hydroxide due to the low cost and ease of processing [46, 47]. As discussed earlier, this approach requires a low level of FFA (i.e., <2%) as soap formation leads to a wasting of base and numerous process complications.
Conversion of FOG to FAME
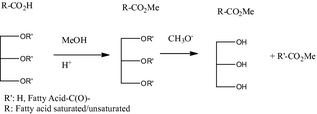
For trap FOG with free fatty acid contents >20%, the usually process “trick” used with yellow grease to lower the content of FFA (i.e., adding a high quality fat) is not operationally possible. To address this challenge, the most commonly applied conversion technology is to treat this material with an acidic methanol solution which converts the FFA to methyl esters followed by a conventional base-catalyzed transesterification step (see Fig. 3).
Several recent publications provide guidance as to optimal reaction conditions needed to convert FFA in trap FOG to their corresponding methyl ester as illustrated in Table 5.
| Methanol:Oil v/v | H2SO4 % v/v | Reaction Time/h | Reaction Temp./°C | % Residual acid content (mg KOH/g) |
|---|---|---|---|---|
| ||||
| Range studied | ||||
| 0.34–0.51 | 1.00–5.02 | 2.0–7.4 | 65 | 20.3–2.2 |
| Preferred conditions | ||||
| 0.42 | 2.50 | 4.0–7.4 | 65 | <2.6 |
|
|
|
Figure 3. Two-stage trap grease fats, oils, and greases conversion to biodiesel and glycerol. |
The presence of water in trap grease FOG is known to be detrimental to the overall acidic conversion of FFA to methyl esters. Indeed, several publications have noted the need for <1% water in the reaction mixture if the product is to achieve a preferred acid number of 2 mg KOH/g or less. A paper by Montefrio et al. [41] also reported high methyl ester conversion efficiencies for FFA in trap FOG as summarized in Table 6.
| % FFA content | Methanol:FFA molar | Final acid value mg KOH/g |
|---|---|---|
| ||
| 20 | 10.0 | 8.60 |
| 20.0 | 1.19 | |
| 50.0 | 0.26 | |
| 10 | 10.0 | 5.24 |
| 20.0 | 1.18 | |
| 50.0 | 0.26 | |
| 5 | 10.0 | 2.06 |
| 20.0 | 1.06 | |
| 50.0 | 0.13 | |
They also examined the role of FeSO4 as a catalyst instead of sulfuric acid for esterification of trap FOG FFA as the catalyst is relatively insoluble in a FOG/methanol solution which could facilitate post processing separations but the conversion efficiencies were not as high unless the reaction temperature was elevated to 95°C. Equally important, they examined the impact of stirring on conversion efficiencies which is important as most trap FOG is not soluble in acidic methanol. In the absence of mixing, FFA to methyl ester conversion efficiencies were reported to be in the low 60% range whereas orbital shaking in shake flasks at 350 rpm provided +90% conversion of FFA to FAME. Clearly, for industrial applications further studies will need to be done to optimize high-intensity mixing and one could envisage a future role for surfactant chemistry. A rich source of chemical information for the catalytic conversion of FFA to FAME in trap FOG is the numerous catalytic solid-acid esterification studies that have been accomplished on model compounds and yellow greases [48]. Although there are differences in the nature of the starting materials these prior studies should aid in future catalyst design. A recent notable example was reported by Kim et al. [49] which employed grease interceptor FOG from a Chinese restaurant located in Charlotte, NC. The authors examined the use of H2SO4, NiSO4/SiO2, zeolite, or SiO2 (0.75–17.5% charge) to convert the FFA in the trap FOG to the corresponding FAME in a methanolic solution. The use of sulfuric acid provided a 99.9% conversion to FAME and NiSO4/SiO2 a 85% conversion whereas the use of zeolite and SiO2 was 25% and 18%, respectively.
The second step of transesterifing the mixture of FAME and mono/di/triacylglycerides to biodiesel has not been as frequently reported, but Karnasuta et al. [39] has provided a detailed central composite design study which is summarized in Table 7.
| Methanol:Oil v/v | KOH concentration % w/v | Reaction Time/h | Free fatty acid methyl ester % yield |
|---|---|---|---|
| 0.22–0.30 | 0.50–1.00 | 0.45–1.55 | 84.3–96.1 |
Optimal conditions for conversion to biodiesel were identified as 0.26 v/v methanol:oil, 1% w/v KOH, for 1 h at 60°C which provided a product with 95.49% fatty acid methyl ester content. A key conclusion from this study was that the resulting biodiesel meet diesel standards for Europe (i.e., EN 14214), China (i.e., GB252-2000), and America (i.e., ASTM D6751).
As an alternative approach to a two-stage treatment, Wang et al. [42] reported a more aggressive methanolysis-utilizing trap grease from Guangzhou Environmental Protection Agency with an acid value of 100 mg/g and 0.8% water. Using methanol and sulfuric acid, they were able to achieve an ester content in the product of 89.67% employing 35.0 methanol:oil ratio and 11.3% acid catalyst stirred for 4.59 h at 95°C. Calcium oxide was then used to neutralize the acid, the calcium sulfate was removed by filtration and the glycerol was decanted. The excess methanol was recovered by a low-temperature distillation and the remaining biodiesel was purified to Korean regulatory biodiesel standards via two distillation towers.
A review of the open literature and web indicates several companies are now actively pursuing the conversion of trap grease FOG to biodiesel although much of the technologies involved are proprietary. The initial processing of trap FOG prior to conversion has not been extensively reported in the research literature but a recent report indicates that prior to esterification of FFA in FOG the trap grease needs to be screened to remove solids, degummed, sulfur depleted, and dried [50]. It also needs to be stated that the conversion of trap grease FOG to biodiesel will be accompanied with a substantial water fraction that needs to be environmentally disposed in an acceptable manner. Hence, cositing this process with a waste water treatment system is one of the most preferred industrial options to pursue. Finally, purification of the FAME/biodiesel is often accomplished via distillation.
A pilot plant demonstration of converting trap FOG to biodiesel has been reported in detail by Chakrabarti et al. [51] on a 200–400 L. This batch process employed interceptor grease trucked to the East Bay Municipal Utility District (Oakland, CA) after being pretreated by a third party to extract the brown grease. The conversion of the FFA to FAME was accomplished using methanol and H2SO4 and if the level of FFA was >1.5–2.0% a second esterification was performed. The esterified FOG was then transesterified with sodium methoxide. The crude biodiesel was water washed, treated with a magnesium silicate absorbent, and filtered. All batches meet the total acid number and glycerin limits for ASTM D6751 method. The most significant challenge was the sulfur limits as the trap FOG feedstock had sulfur levels of 300–400 ppm and ~200 ppm was found in the product. Clearly, this is of concern as recent ultra low sulfur diesel regulations have a 15 ppm sulfur specification for highway diesel fuel (see Federal register, 2001). The researchers were able to address these levels by preforming vacuum distillation and treating with activated charcoal yielding a reported sulfur value of 12 ppm. This methodology was viewed as costly and clearly further research is needed to facilitate S-removal in a more cost effective manner.
Testing the generated biodiesel as B20 or B100 using diesel dump trucks was viewed overall as successful and the drivers were satisfied with the performance although it was mentioned that there was an increased frequency of fuel filter changes with the factors contributing to this not determined. The experimental parameters measured in these trials allowed an evaluation of net energy generation which was reported as 1120 kJ/L-FOG and GHG emission reduction of 0.48 kg CO2/L-FOG.
Research Directed at Improving FOG to FAME
Given the environmental and financial interests in converting trap FOG to biodiesel, there are continuing research efforts at improving the overall efficiency and ease of this process. One approach to simplifying the process is to use a solid-acid catalyst which would facilitate acid recovery. Huang et al. [52] studied the use of Amberlyst-15 as a replacement for sulfuric acid. Employing trap grease (KOH value of 100 mg/g and 0.8% water) with a 27:1 molar ratio of methanol to FFA, and 10% Amberlyst-15 at 95°C for 3 h the conversion of FFA to methyl esters was reported at 98.73%. Equally impressive was the fact that the Amberlyst-15 could be reused for another 10 trials with no loss in activity. These results indicate a substantial improvement over an earlier reported study by Gan et al. [53] that reported conversion efficiencies of 60% with less rigorous conditions.
An interesting alternative solid-acid approach was recently published using magnetic iron-coated nano-sized acid catalyst (iron nanoparticles were coated with poly(glycidyl metharcylate) and sulfonic acid groups on the surface) [54]. This catalyst was shown to readily convert FFA in waste grease (i.e., 0.2 g/experiment from Singapore sewage system, FFA:14% wt, 0.14% moisture) in 96% yield to the corresponding FAME within 2 h at 70°C using a 4% loading of catalyst and a methanol:oil weight ratio 1.4. The authors provided data indicating that this nano-catalyst provided superior performance to Amberlyst-15 which was proposed to be due to the high surface area and ease of accessible to the acid groups of the nano-catalyst. The magnetic property of this catalyst was also shown to help facilitate catalyst recovery using a strong magnetic field.
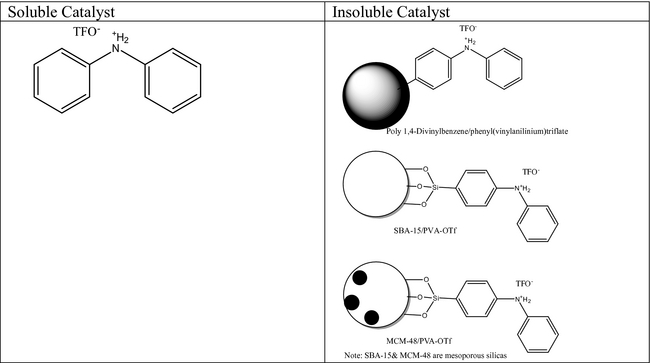
Along the same approach, Ngo et al. [55] reported a series of homogenous and heterogenous catalysts that were capable of converting trap grease FFA and acyl-glycerides to the corresponding FAME in >95% yield (see Fig. 4). The efficiency of conversion was observed with trap grease samples from Atlanta, GA (0.18% H2O) and San Francisco, CA (0.27% H2O) at 125°C for 2 h with 20 molar equivalent of methanol and 0.5–5.0% catalyst. Experimental results did indicate that the homogenous catalyst worked slightly better than the heterogeneous catalyst.
|
|
|
Figure 4. Catalyst used for conversion of trap grease fats, oils, and greases (FOG) to FAME. Employed 200 mg trap FOG/experiment; catalyst 0.5–5.0% |
The heterogeneous catalysts were effective in the generation of FAME and also facilitated recovery of the catalyst by simply filtration. As an alternative, several studies have examined using methanol and trap FOG under supercritical conditions. Operating at temperatures of 275–325°C, the supercritical methanol technology is catalyst free and benefits from a homogeneous reaction under these conditions. The drawback of this procedure includes high capital and energy costs and a need for a high molar ratio of methanol to grease [56, 57].
Given the diversity of materials in trap FOG and the presence of water it is only natural to examine alternative biological routes. For example, the application of a whole-cell biocatalyst based on Rhizopus oryzae (ATCC10260) was shown to yield 55% biodiesel from trap FOG (employing 12 mL FOG/experiment) with a 72-h transesterification reaction using no excess methanol in a water-containing system at room temperature [58]. Building on this success, Yan et al. [59] has reported a novel tandem lipase system that is especially tailored to esterifying FFA to FEMA and transesterify acylgylcerides to FAME in one-pot. Using trap FOG grease (2 g/experiment, 21.7% FFA) from Singapore, methanol (1:1 mole ratio to grease) and recombinant E. coli wet or dry cells were stirred at 30°C for 6–72 h. Two additional aliquots of methanol (same volume as used at time 0) were added at 6 and 12 h. The recombinant E. coli capable of coexpressing Candida antarctica and Thermomyces lanuginosus lipases was shown to convert the trap FOG grease to biodiesel in 95% yield after 72 h. This approach was shown to be more effective than using individual expressed lipases and furthermore the recombinant E. coli cells could be recycled five times and retain 75% productivity. This biological approach in the future could be very attractive given the mild conditions, ease by which the lipases could be generated and well established methodologies for disposal of biological catalysts.
An interesting application of these technological approaches was just demonstrated by Ngo et al. [60] in which he grafted Candida antarctica and Thermomyces lanuginosus lipases to core-shell structured iron oxide magnetic nanoparticles. Using the same trap grease as Yan et al. used above, the grafted Candida antarctica and Thermomyces lanuginosus lipases in methanol converted the grease to FAME in 97% and 95% yields, respectively [53]. The bio-grafted catalysts could be readily recovered using an external magnetic field and then recycled.
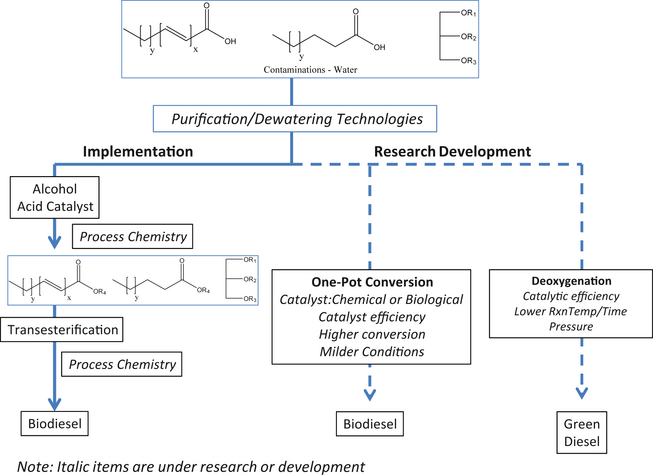
An alternative approach to the utilization of trap grease is to catalytically deoxygenate FOG molecules yielding a hydrocarbon fuel resource. Toba et al. [61] has reported the use of NiMo, NiW, and CoMo sulfide catalyst for hydrodeoxygenation for silylated trap grease. The former two catalysts showed high and stable hydrogenation activity, whereas CoMo suffered from deactivation. Hydrodeoxygenation was accomplished in +99% at 250–350°C for 3 h pressurized with 7 MPa of hydrogen (0.5 wt catalyst, 10 g FOG/experiment). The product yield of n-paraffin was 94.8% using NiMo/Al2O3 and 91.4% using NiMo/B2O3-Al2O3 and the remaining components were trace amounts of iso-paraffin and olefins. No alcohols, FFA, or esters were detected in the product mixture. In the long term, this approach may see increasing attention as the need for methanol esterification/transesterification reactions and its post processing is eliminated. Nonetheless, researchers need to identify milder conditions/improved catalysts so as to accelerate this process into routine process production. Figure 5 provides a general technology map of converting trap grease FOG to a fungible biofuel for today and the future.
|
|
|
Figure 5. Translational research needs in trap fats, oils, and greases to biofuels. |
As stated in the early part of this review, many governmental organizations have reported the benefits of collecting trap grease FOG before it enters sewer systems and indeed, in many parts of the world, high volume generators of FOG are mandated to collect this material which then needs to be processed in an environmentally acceptable manner. The conversion of this resource into biodiesel today, and possibly green diesel in the future, addresses the green city vision that includes a zero-waste policy that several major metropolitan areas are pursuing [62]. Hence, a confluence of events have propelled the commercialization of trap grease FOG to biofuels and as these technologies are implemented they will certainly benefit from pinch and LCA analysis [63, 64].
In summary, the advent of the green city movement and renewed emphasis on environmental sustainable technologies is providing strong interest in converting societal wastes to value added fuels, chemicals, and materials. Existing mandates for the collection and processing of trap grease FOG makes this a valuable resource for biodiesel production. Although the chemical constituents of trap FOG are variable, general trends are apparent and chemical conversion technologies for the synthesis of biodiesel can be readily accomplished. Current commercial production technologies make this an attractive option and ongoing research will further simplify this process and only accelerate the demand for this valuable resource.
Conflict of Interest
None declared.
References
- Clark, J. H., R. Luque, and A. S. Matharu. 2012. Green chemistry, biofuels, and biorefinery. Annu. Rev. Chem. Biomol. Eng.3:183–207.
- Ragauskas, A. J., C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, et al. 2006. The path forward for biofuels and biomaterials. Science311:484–489.
- Smith, A. M.2008. Prospects for increasing starch and sucrose yields for bioethanol production. Plant J.54:546–558.
- Graham-Rowe, D. 2011. Agriculture: beyond food versus fuel. Nature474:S6–S8.
- Foston, M., and A. J. Ragauskas. 2012. Biomass characterization: recent progress in understanding biomass recalcitrance. Ind. Biotechnol.8:191–208.
- Ferrell, J., and V. Sarisky-Reed. 2010. National algal biofuels technology roadmap. A technology roadmap resulting from the National Algal Biofuels Workshop Dec. 2008. Available at http://www1.eere.energy.gov/biomass/pdfs/algal_biofuels_roadmap.pdf (accessed March 30, 2012).
- Durbin, T. D., R. Cocker David, A. A. Sawant, K. Johnson, J. W. Miller, B. B. Holden, et al. 2007. Regulated emissions from biodiesel fuels from on/off-road applications. Atmos. Environ.41:5647–5658.
- Stelmachowski, M.2011. Utilization of glycerol, a by-product of the transesterification process of vegetable oils: a review. Adv. Chem. Res.9:59–92.
- Feasibility Report Small Scale Biodiesel Production. Available at http://www.wmrc.uiuc.edu/tech/small-scale-biodiesel.pdf (accessed March 30, 2013).
- Mittelbach, M., B. Pokits, and A. Silberholz. 1992. Production and fuel properties of fatty acid methyl esters from used frying oil. In Liquid Fuels from Renewable Resources: Proc. Alternative Energy Conference. St. Joseph, MI. 74–78.
- Horton, C.2007. Removal of free fatty acids from used cooking oil prior to biodiesel production. Brit. U.K. Pat. Appl. GB20060006533 20060331. GB 2436836 A 20071010.
- Bressler, D.2011. Methods for producing fuels and solvents substantially free of fatty acids. PCT Int. Appl. WO2011IB00464 20110224. WO 2011104626 A2 20110901
- Used and Waste Oil and Grease for Biodiesel. Available at http://www.extension.org/pages/28000/used-and-waste-oil-and-grease-for-biodiesel (accessed April 5, 2013).
- BLACKGOLD Converting our Crudest Wastes into our Cleanest Fuels. Available at http://www.blackgoldbiofuels.com/ (accessed April 5, 2013).
- FOGFUELS™ Fueling the Future™. Available at http://www.fogfuels.com/ (accessed April 5, 2013).
- Pacific Biodiesel. Available at http://www.biodiesel.com/ (accessed April 5, 2013).
- Bacigalupi, R. Turning trap grease, brown grease to biodiesel. Opportunity, process and issues. Available at http://www.gobiomass.com/article.cfm?id=28407&PageNum=2 (accessed April 5, 2013).
- Bevill, K.2008. A greasy alternative. Biodiesel Magazine. October 14, 2008.
- Restaurant Grease: Knowing Your Ohio EPA Regulations. 2010. Available at http://www.epa.ohio.gov/portals/41/sb/publications/restaurant.pdf (accessed April 5, 2013).
- Weiss, M.2007. Grease interceptor facts and myths. Plumbing Systems & Designs. Nov., 34- 39.
- Grease Interceptors A112.14.3 - 2000. Available at http://www.asme.org/products/codes—standards/grease-interceptors (accessed March 30, 2013).
- Gallimore, E., T. N. Aziz, Z. Movahed, and J. Ducoste. 2011. Assessment of internal and external grease interceptor performance for removal of food-based fats, oil, and grease from food service establishments. Water Environ. Res.83:882–892.
- Grease Interceptor Maintenance. Available at http://www.sanjoseca.gov/archives/163/GreaseInterceptorMaintenance_E.pdf; http://www.centralsan.org/documents/Grease_Interceptor_Maint_Fact_Sheet.pdf (accessed April 5, 2013).
- Understanding Grease Removal And The 25% Rule - City of Dallas. Available at http://www.dallascityhall.com/code_compliance/pdf/GreaseTrap_brochure.pdf (accessed April 5, 2013).
- Rintoul, S.2006. Stop FOG now. Water Environ. Lab. Solut.13:6–8.
- He, X., M. Iasmin, L. O. Dean, S. E. Lappi, J. J. Ducoste, and F. L. de los Reyes. 2011. Evidence for fat, oil, and grease deposit formation mechanisms in sewer lines. Environ. Sci. Technol.45:4385–4391.
- Environmental Protection Agency. 2004. EPA report to congress. Available at http://www.epa.gov/npdes/pubs/csossoRTC2004_chapter04.pdf; Environmental Protection Agency. 2007b. EPA SSO Draft Fact Sheet. Available at http://www.epa.gov/npdes/pubs/sso_fact_sheet_model_permit_cond.pdf (accessed April 5, 2013).
- Fats, Oils & Grease To Green Fuel, Partanen, W.E. Available at http://www.ncsafewater.org/Pics/Training/AnnualConference/AC08TechnicalPapers/SpecialTopics/AC08ST_Tues1100_Partenan.pdf (accessed April 5, 2013).
- Fat, Oil and Grease Programme Dublin City Councils FOG Programme. Available at http://www.dublincity.ie/WATERWASTEENVIRONMENT/WASTEWATER/Pages/FatOil andGreaseProgramme.aspx (accessed April 5, 2013).
- Grease Interceptor Regulation. Available at http://www.metrovancouver.org/SERVICES/PERMITS/GREASETRAPREGULATION/Pages/default.aspx (accessed April 12, 2013).
- Grease Under Control at South East Water, Scoble, C., Day, N. 2002. Proceedings of the 65th Annual Water Industry Engineers and Operators' Conference. Available at http://www.wioa.org.au/conference_papers/02/paper7.htm (accessed April 5, 2012).
- Suto, P., D. M. D. Gray, E. Larsen, and J. Hake. 2006. Innovative anaerobic digestion investigation of fats, oils, and grease. Proc. Water Environ. Fed. Residuals Biosolids Manage.22:858–879.
- Long, J. H., T. N. Aziz, F. L. III de los Reyes, and J. J. Ducoste. 2012. Anaerobic co-digestion of fat, oil, and grease (FOG): a review of gas production and process limitations. Process Saf. Environ. Prot.90:231–245.
- Sheng, L.2012. ‘Gutter oil’ sewer problem drained. Global Times. Available at http://www.globaltimes.cn/content/714805.shtml (accessed April 5, 2012).
- Wiltsee, C. 1998. Urban Waste Grease Resource Assessment. Available at http://www.epa.gov/region9/waste/biodiesel/docs/NRELwaste-grease-assessment.pdf (accessed April 5, 2012).
- Wiltsee, G.1998. Urban waste grease resource assessment. NREL/SR-570-26141, Nov.
- Canakci, M.2007. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol.98:183–190.
- Nisola, G. M., E. S. Cho, H. K. Shon, D. Tian, D. J. Chun, E. M. Gwon, et al. 2009. Cell immobilized FOG-trap system for fat, oil, and grease removal from restaurant wastewater. J. Environ. Eng.135:876–884.
- Karnasuta, S., V. Punsuvon, C. Chiemchaisri, and K. Chunkao. 2007. Optimization of biodiesel production from trap grease via two-step catalyzed process. Asian J. Energy Environ.8:145–168.
- Neczaj, E., J. Bien, A. Grosser, M. Worwag, and M. Kacprzak. 2012. Anaerobic treatment of sewage sludge and grease trap sludge in continuous co-digestion. Global Nest J.14:141–148.
- Montefrio, M. J., T. Xinwen, and J. P. Obbard. 2010. Recovery and pre-treatment of fats, oil and grease from grease interceptors for biodiesel production. Appl. Energy87:3155–3161.
- Wang, Z. M., J. S. Lee, J. Y. Park, C. Z. Wu, and Z. H. Yuan. 2008. Optimization of biodiesel production from trap grease via acid catalysis. Korean J. Chem. Eng.25:670–674.
- Coker, C.2006. Composting grease trap wastes. Biocycle47:27–30.
- Robbins, D. M., O. George, and R. Burton. 2011. Developing programs to manage fats, oil, and grease (FOG) for local governments in India. V. World Aqua Congress Proceedings, New Delhi, India, 1–13.
- Ward, P. M. L.2012. Brown and black grease suitability for incorporation into feeds and suitability for biofuels. J. Food Prot.75:731–737.
- Vasudevan, P. T., and B. Fu. 2010. Environmentally sustainable biofuels: advances in biodiesel research. Waste Biomass Valorization1:47–63.
- Perego, C., and D. Bianchi. 2010. Biomass upgrading through acid-base catalysis. Chem. Eng. J.161:314–322.
- Diaz, L., M. E. Borges, 2012. Low-Quality Vegetable Oils as Feedstock for Biodiesel Production Using K-Pumice as Solid Catalyst. Tolerance of Water and Free Fatty Acids Contents. J. Agric. Food. Chem. 60:7928–7933.
- Kim, H. J., H. Hilger, and S. J. Bae. 2013. NiSO4/SiO2 catalyst for biodiesel production from free fatty acids in brown grease. Energy Eng.139:35–40.
- Bacigalupi, R.2010. Turning trap grease, brown grease to biodiesel. Opportunity, process and issues. Biomass Products & Technology, Aug.
- Chakrabarti, A. J., J. M. Hake, I. Zarchi, and D. M. D. Gray. 2008. 4Waste grease biodiesel production at a wastewater treatment plant. Water Environ. Fed.???:2770–2789.
- Huang, D., Z. Wang, and Z. Yuan. 2012. Pretreatment of trap grease with Amberlyst-15 for biodiesel production. Adv. Mater. Res.347–353:2528–2531.
- Gan, S., H. K. Ng, P. H. Chan, and F. L. Leong. 2012. Heterogeneous free fatty acids esterification in waste cooking oil using ion-exchange resins. Fuel Process. Technol.102:67–72.
- Zillillah, T. G., and Z. Li. 2012. Highly active, stable, and recyclable magnetic nano-size solid acid catalysts: efficient esterification of free fatty acid in grease to produce biodiesel. Green Chem.14:3077–3086.
- Ngo, H. L., Z. Xie, S. Kasprzyk, M. Haas, and W. Lin. 2011. Catalytic synthesis of fatty acid methyl esters from extremely low quality greases. J. Am. Oil Chem. Soc.88:1417–1424.
- Alsoudy, A., M. Hedeggard, T. Janajreh, and I. Janajreh. 2012. Influence on process parameters in transesterification of vegetable and waste oil – a review. Int. J. Res. Rev. Appl. Sci.10:64–77.
- Preprocessinc Grease Trap Waste to Feedstock to Fuel. Available at [www.preprocessinc.com/…/b0351bd5e310031f%204639473c97ed0abe.pdf www.preprocessinc.com/…/b0351bd5e310031f 4639473c97ed0abe.pdf]; http://www.extension.org/pages/28000/used-and-waste-oil-and-grease-for-biodiesel (accessed April 5, 2012).
- Jin, G., T. J. Bierma, C. G. Hamaker, R. Mucha, V. Schola, J. Stewart, et al. 2009. Use of a whole-cell biocatalyst to produce biodiesel in a water-containing system. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng.44:21–28.
- Yan, J., A. Li, Y. Xu, T. P. N. Ngo, S. Phua, and Z. Li. 2012. Efficient production of biodiesel from waste grease: one-pot esterification and transesterification with tandem lipases. Bioresour. Technol.123:332–337.
- Ngo, T. P. N., A. Li, K. W. Tiew, and Z. Li. In press. Efficient transformation of grease to biodiesel using highly active and easily recyclable magnetic nanobiocatalyst aggregates. Bioresour. Technol.
- Toba, M., Y. Abe, H. Kuramochi, M. Osako, T. Mochizuki, and Y. Yoshimura. 2011. Hydrodeoxygenation of waste vegetable oil over sulfide catalysts. Catal. Today164:533–537.
- Ferry, D.2011. Urban Quest ‘Zero’ Waste Wall Street J. September 11. Available at http://online.wsj.com/article/SB10001424053111904583204576542233226922972.html (accessed April 5, 2013).
- Ebrahim, M., and A. Kawari. 2000. Pinch technology: an efficient tool for chemical-plant energy and capital-cost saving. Appl. Energy65:45–49.
- Majer, S., F. Mueller-Langer, V. Zeller, and M. Kaltschmitt. 2009. Implications of biodiesel production and utilisation on global climate – a literature review. Eur. J. Lipid Sci. Technol.111:747–762.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?