Abstract
The analgesic effects of peripheral nerve blocks can be prolonged with the placement of perineural catheters allowing repeated injections of local anaesthetics in humans. The objectives of this study were to evaluate the clinical suitability of a perineural coiled catheter (PCC) at the sciatic nerve and to evaluate pain during the early post-operative period in dogs after tibial plateau levelling osteotomy. Pre-operatively, a combined block of the sciatic and the femoral nerves was performed under sonographic guidance (ropivacaine 0.5%; 0.3 mL kg−1 per nerve). Thereafter, a PCC was placed near the sciatic nerve. Carprofen (4 mg kg−1 intravenously) was administered at the end of anaesthesia. After surgery, all dogs were randomly assigned to receive four injections of ropivacaine (group R; 0.25%, 0.3 mL kg−1) or NaCl 0.9% (group C; 0.3 mL kg−1) every 6 h through the PCC. Pain was assessed by use of a visual analogue scale (VAS) and a multi-dimensional pain score (4Avet) before surgery (T-1), for 390 min (T0, T30, T60, T120, T180, T240, T300, T360 and T390) as well as 1 day after surgery (Day 1). Methadone (0.1 mg kg−1) was administered each time the VAS was ≥40 mm or the 4Avet was ≥5. At T390 dogs received buprenorphine (0.02 mg kg−1). Data were compared using Mann–Whitney rank sum tests and repeated measures analysis of variance. Regardless of group allocation, 55% of dogs required methadone. VAS was significantly lower at T390 (P = 0.003), and at Day 1 (P = 0.002) and so was 4Avet at Day 1 (P = 0.012) in group R than in group C. Bleeding occurred in one dog at PCC placement and PCC dislodged six times of 47 PCCs placed. Minor complications occurred with PCC but allowed four repeated administrations of ropivacaine or saline over 24 h in 91.5% of the cases.
Introduction
Tibial plateau levelling osteotomy (TPLO) is a common surgical procedure to correct instability of the stifle joint due to a rupture of the cranial cruciate ligament in dogs. The procedure is invasive as it involves osteotomy to decrease the angulation of the tibial plateau (Slocum & Slocum 1993). Animals undergoing this procedure might suffer from moderate to severe post-operative pain, especially if perioperative pain management is inadequate.
In the last decade, various loco-regional anaesthesia techniques have been described in small animals to improve peri-operative analgesia. Intra-articular and epidural injections as well as peripheral nerve blocks have all been investigated to improve post-operative well-being (Hoelzler et al. 2005; Gurney & Leece 2014). Injections of local anaesthetics at the femoral and the sciatic nerves have been shown to be as effective as an epidural injection of the same drugs to treat post-operative pain (Caniglia et al. 2012). The technique of ultrasound-guided injection of local anaesthetics at the sciatic and the femoral nerves for hind limb surgery has been described (Campoy et al. 2010) and applied in clinical canine patients (Rohrbach et al. 2015).
Ropivacaine is a long-acting amide with an improved safety profile compared to bupivacaine (cardiotoxicity) if large doses of local anaesthetics are administered (Kuthiala & Chaudhary 2011). In rats, 0.2 mL of ropivacaine administered at the sciatic nerve has been reported to act for 107–129 min depending on the concentration injected (Dyhre et al. 1997). The exact block duration of ropivacaine in dogs after a perineural injection at the sciatic and the femoral nerves remains to be determined.
Based on clinical experience (Rohrbach et al. 2015), symptoms indicating pain are often increased in dogs undergoing TPLO one day after surgery even though epidural anaesthesia or sciatic and femoral nerve blocks had been applied. One explanation might be the loss of the analgesic effects of the pre-operatively performed block at this time point.
In humans, the beneficial effects of peripheral nerve blocks have been prolonged with the placement of a perineural catheter allowing repeated or even continuous administration of local anaesthetics (Watson et al. 2005). Prolonged peripheral nerve blocks promote a faster short-term functional recovery and a better quality of analgesia compared to the systemic administration of analgesic drugs (Liu & Salinas 2003). The post-operative administration of levo-bupivacaine at the femoral nerve through a perineural catheter led to lower pain scores during 4 days when compared to saline (Williams et al. 2006).
A frequent problem with the handling of straight perineural catheters is dislodgement. With the aim to reduce this problem, a perineural coiled catheter (PCC) has been recently designed and applied in a human cadaveric study where those catheters were placed in the paravertebral space (Luyet et al. 2012). In a previous study conducted by our group, a superior intraoperative analgesic effect was demonstrated when only the sciatic nerve was blocked in dogs undergoing TPLO if compared to the femoral nerve (Rohrbach et al. 2015). Due to its lateral position, the sciatic nerve might be preferred over the femoral nerve to ensure a correct long-term anatomical position of PCC because movements of animals in the post-operative phase cannot be easily restricted as in humans.
For those reasons, the current study was planned with the aim to evaluate the clinical feasibility of PCC placement near the sciatic nerve and to gather preliminary evidence on the analgesic efficacy of ropivacaine delivered via this route.
According to our hypothesis, a PCC will allow repeated injections of ropivacaine at the sciatic nerve and potentially lead to better analgesia than injections of NaCl 0.9% in the early post-operative phase as well as on the morning following surgery.
Material and methods
Animals
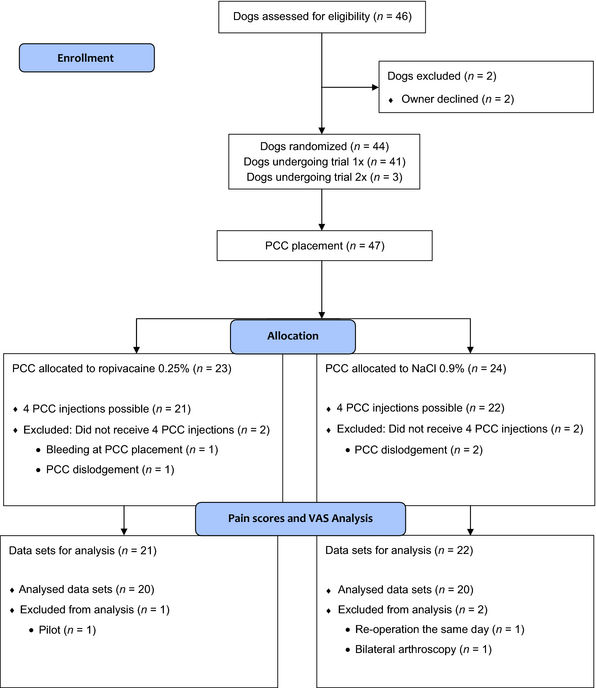
Forty four client-owned dogs were included in the study, three of whom underwent the trial twice (bilateral surgery; minimal interval of 10 weeks between surgeries). The trial was stopped when a complete data set of 40 procedures had been achieved. The patient flow diagram is presented in Fig. 1. Dogs were included in the study if a TPLO was planned, if they were classified as ASA I or II according to the scale of the American Society of Anaesthesiologists, if bodyweight was >15 kg and if the signed owner consent was obtained. All data were collected at the Small Animal Clinic of the Vetsuisse Faculty of the University of Bern. The trial was approved by the Committee for Animal Experiments of the Canton of Bern, Switzerland (BE 83/12).
|
|
|
Figure 1. Patient flow diagram of dogs undergoing tibial plateau levelling osteotomy eligible for the study in the time frame of October 2012 to September 2013. The diagram was adapted from CONSORT 2010 (transparent reporting of trials). PCC, perineural coiled catheters. |
Anaesthetic protocol
An intravenous (IV) access was established by placement of a catheter into the left or right cephalic vein 20 min after an intramuscular injection of acepromazine (0.02 mg kg−1; Prequillan; Arovet AG, Dietikon, Switzerland). General anaesthesia was induced with propofol (Propofol 1% MCT; Fresenius Kabi, Bad Homburg, Germany) injected IV titrated to effect. The trachea was intubated using an appropriately sized cuffed silicone tube. An alveolar concentration of 1.3% isoflurane (Attan Isoflurane; Provet, Lyssach, Switzerland) was targeted to maintain an adequate level of anaesthetic depth and delivered in a rebreathing system (Primus anaesthetic machine; Dräger, Lübeck, Germany) in 100% oxygen for the maintenance of anaesthesia.
Monitoring included cardiovascular and respiratory parameters as electrocardiography, oxygen saturation (SpO2), respiratory rate (fR), end tidal carbon dioxide concentration (FE’CO2), end tidal isoflurane concentration (FE’Iso) and oesophageal temperature. Whenever the cannulation of the metatarsal artery was successful, blood pressure was measured invasively. Otherwise non-invasive oscillometric blood pressure monitoring was applied. All parameters were recorded every 5 min. Spontaneous ventilation was tolerated as long as FE’CO2 was not exceeding 6.6 kPa (50 mmHg); otherwise mechanical ventilation was initiated to maintain normocapnia. Fluids were delivered at the rate of 10 mL kg−1 h−1 throughout anaesthesia (Plasma Lyte A; Baxter, Volketswil, Switzerland) until recovery. Further measures to maintain all vital parameters in a physiological range were performed according to individual needs. A modified Robert Jones bandage was applied to the operated limb before recovery. Following endotracheal extubation, carprofen (4 mg kg−1; Carprofen; Zoetis Schweiz, Zürich, Switzerland) was administered IV to all dogs.
Nerve blocks
Prior to surgery, a block of the sciatic and the femoral nerves was performed under sonographic guidance (M-Turbo; SonoSiteTM, Bothell, WA, USA) in all dogs as described by Campoy et al. (2010). After aseptical preparation of the injection sites while the dog was positioned in lateral recumbency, wherein the limb to be operated was placed uppermost, a Tuohy needle (18 G × 100 mm; Pajunk, Geisingen, Germany) was inserted using the in-plane ultrasound-guided technique for both blocks. Ropivacaine (Ropivacain 0.5%; Fresenius Kabi, Germany) 0.3 mL kg−1 (maximum 10 mL) was injected slowly under real-time visualization in close proximity to each nerve if neither blood could be aspirated nor a high resistance could be felt.
PCC placement
After the injection of ropivacaine at the sciatic nerve, the PCC (SonoLong Curl Sono, 20G × 90 cm; Pajunk, Germany) was inserted blindly through the Tuohy needle previously placed for the ropivacaine injection until it reached the distal Tuohy needle orifice. This could be identified by the first marking on the PCC reaching the needle hub entrance. Subsequently, the PCC was further advanced by approximately 2.5 cm (second marking reaching the needle hub entrance) to enable the catheter to form its coil within the ropivacaine bubble. The needle was then withdrawn carefully with the non-dominant hand while the dominant hand was used to keep the PCC in place to avoid any dislodgement. The PCC was fixed to the skin with instant adhesive medical glue (Histoacryl; B. Braun, Rubi, Spain) before being covered with auto-adhesive foil, equipped with an antibacterial filtre and taped dorsally over the lumbar area.
Intraoperative assessment of nociception and surgery
The dogs were positioned carefully on the surgery table to avoid dislodgement of the previously placed PCC. Prior to the start of the surgical stimulation, heart rate (HR), mean arterial pressure (MAP) and fR were recorded in intervals of 5 min over 15 min and the mean values were considered as baseline values. The surgical procedure consisted of an arthroscopy of the stifle joint followed by osteotomy of the tibia according to a standard procedure (Slocum & Slocum 1993). During surgery, an increase of ≥30% of two of the three parameters (HR; MAP; fR) was considered indicative of nociception and led to an administration of fentanyl (3 μg kg−1 IV). Post-operative radiographs were taken whenever indicated before recovery.
Post-operative treatment
After endotracheal extubation, each subject was randomized to receive either four injections of 0.3 mL kg−1 of ropivacaine 0.25% (group R, n = 23) with a maximum of 10 mL dog−1 per injection site or the equivalent volume of NaCl 0.9% (group C, n = 24) through the PCC every 6 h starting at this time point (T0). For randomization, a person other than the main investigator (VM) drew the group assignment from an envelope containing equal numbers of both groups. The syringes containing either ropivacaine 0.25% or saline were not labeled to allow blinding. Whenever the data generated by a certain dog had to be excluded from the analysis (Fig. 1), the randomization paper was returned to the envelope by the same person (HR). The trial was stopped when the data collection for 20 TPLO procedures per group was completed.
Post-operative evaluations
A modified multi-dimensional pain scale (4Avet; 0 = no pain, 15 = maximal pain) and a visual analogue scale (VAS; 0 = no pain, 100 = maximal pain) were applied at the pre-defined time points (T). The scores were recorded before premedication (T-1) and over 390 min (T0, T30, T60, T120, T180, T240, T360 and T390) starting at the time point of endotracheal extubation (T0) and a last time on the morning after surgery (Day 1). The evaluation at T0 was performed prior to the first injection through the PCC. The last evaluation at Day 1 was performed 1 h after the last injection into the PCC. The 4Avet was evaluated prior to the VAS.
At the same time points, the course of the blocks was evaluated by use of a modified Bromage score (Bromage 1965) and a sensory score in each dog. With the modified Bromage score, the motor function (0 = complete weight bearing, 1 = partial weight bearing, 2 = no weight bearing) was assessed and with the sensory score the sensation at the distal limb was tested by clamping the third toe with a needle holder (0 = no reaction to toe clamping, 1 = mild reaction to toe clamping, 2 = strong reaction to toe clamping). The modified Bromage score and the sensory score were always applied after the VAS. All scores were completed by the same observer (VM) being unaware of the treatment during the entire study period. An evaluation of the local surgical area was not possible due to the bandage. The reaction to palpation of the surgical site applied over the bandage was a component of the multi-dimensional painscore (4Avet). Methadone (0.1 mg kg−1 IV) was administered as rescue analgesia if 4Avet ≥5 or VAS ≥40 mm at each evaluation time point (T0–T360), as such values were considered indicative of moderate pain. At T390, all dogs received buprenorphine 0.02 mg kg−1 subcutaneously, at midnight as well as on the day following surgery (Day 1) after the last pain evaluation. This protocol represents the routine analgesia protocol after TPLO at our institution. Even though the pain scores were not recorded during the night, a thorough clinical evaluation based on subjective clinical assessment was performed by a responsible veterinarian as for regular stationary patient in the hospital.
All dogs were discharged from the hospital after a bandage change 1 day after surgery in good condition without any sign of reduced motor function. Oral carprofen (4 mg kg−1) was continued for 7 days.
Statistical analysis
Data analysis was performed using statistical software (Sigma Stat, Version 3.5; Systat Software, San Jose, CA, USA). All parameters were tested for normality using the Kolmogorov–Smirnov test. Students t-test was applied for the evaluation of demographic data. The pain scores were compared between groups at each time point using Mann–Whitney Rank Sum Test and a Bonferroni correcture was applied for T30–T390. A Bonferonni correcture was not applied for Day 1 as this time point was considered to be a separate time point and not influenced by the previous results. The comparison within groups was performed with repeated measures analysis of variance for the modified Bromage score and the sensory score. Pain evaluation scores and nerve block duration are expressed as the median [IQR]. A Fisher exact test was applied to compare consumption of intraoperative fentanyl with consumption of post-operative methadone. The significance was set at P ≤ 0.05.
Results
The dogs included in this study were recruited from October 2012 until September 2013. All animals were discharged from hospital the day after surgery without any visible residual effects from the loco-regional anaesthesia.
Demographic distribution
Dogs included in this study were classified into ASA I or II at the pre-anaesthetic clinical examination. No differences between groups regarding weight (R: 34 ± 9 kg; C: 34 ± 13 kg; P = 0.98) or gender (R, 2/20 male intact, 6/20 male castrated, 3/20 female, 9/20 female spayed; C, 2/20 male intact, 8/20 male castrated, 2/20 female, 8/20 female spayed) were identified. Dogs were significantly older in group R (7.2 ± 2.1 years) than in group C (5.8 ± 2.0 years, P = 0.034). Group R was composed of mixed-breed dogs (n = 6), Labrador retrievers (n = 2), Golden retrievers (n = 2) and Boxer (n = 2), with other breeds represented only once. Group C was composed of Bernese mountain dogs (n = 5) and Entlebucher mountain dogs (n = 2), with other breeds represented once.
Surgical procedure
The surgical procedure was preceded by an arthroscopy of the stifle joint in all but one dog. No differences were found between the two groups regarding the duration of the procedures (Table 1).
| Groups | Group R | Group C |
|---|---|---|
| Parameters | Median [IQR] | Median [IQR] |
| General anaesthesia | 220 [200–237] | 240 [225–260] |
| Block to endotracheal extubation | 172 [152–192] | 190 [172–210] |
| Block to arthroscopy start | 55 [50–70] | 62 [52–75] |
| Arthroscopy | 25 [24–31] | 30 [25–355] |
| TPLO to endotracheal extubation | 75 [65–95] | 100 [71–110] |
| IQR, interquartile ranges; TPLO, Tibial plateau levelling osteotomy. Group R received repeated injections of ropivacaine 0.25%; Group C received repeated injections of NaCl 0.9%. | ||
PCC placement, complications and removal
The PCC could easily be placed in all but one dog where mild bleeding was detected at T0. In this animal, the PCC was removed immediately and it was excluded from data analysis. In three dogs, the PCC was dislodged during the evaluation period leading to exclusion from data analysis as well. The PCC was easily removed 1 h after the last injection into the PCC by gently withdrawing the device after removing the self-adhesive foil in all animals. The bent shape of the catheter tip had remained in all PCCs and no knots were observed. In three dogs, dislodgement occurred just during the 1 h period before the PCC was removed at Day 1. In those dogs, all four injections through the PCC could be performed and they were not excluded from analysis. The patient flow diagram is presented in Fig. 1. E-collars were not applied after recovery to optimize the comfort of the animals. Four injections of ropivacaine or NaCl were administered every 6 h in 91.5% of the cases (43 of 47 placed PCCs). The overall total dislodgement rate over the entire study period was 14.9% (one PCC was removed due to bleeding and six PCC dislodgement of 47 placed PCCs).
Pain evaluation
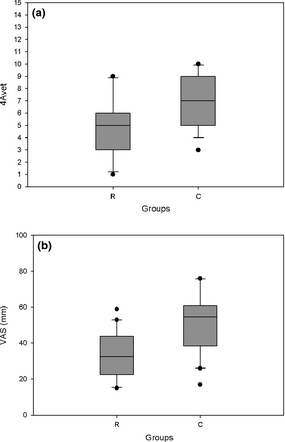
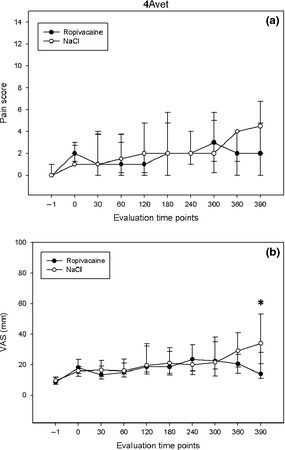
For 4Avet and VAS, no differences between groups could be detected from T-1 to T360 (Figs. 2a,b). The 4Avet was not significantly lower in group R than in group C at T390 after the Bonferroni correction (P < 0.007) (R: 2.0 [0–4.5], C: 4.5 [2.0–6.5], P = 0.017) (Fig. 2a). At Day 1, the 4Avet was significantly lower in group R than in group C (R: 5 [3–6], C: 7 [5–9], P = 0.012) (Fig. 3a) and so was the VAS at T390 (R: 14 [11–28], C: 34 [21–53], P = 0.003) (Fig. 2b) and Day 1 (R: 32 [23–43], C: 54 [39–60], P = 0.002) (Fig. 3b).
|
|
|
Figure 2. Pain scores (4Avet) (a) and visual analogue scales (VAS) (b), from evaluation time point Day 1 (24 h post-operative) of dogs receiving repeated perineural injections of ropivacaine (n = 20) or NaCl 0.9% (n = 20) through a perineural coiled catheter. Data are presented as median [IQR] and outliers. Statistically significant difference between groups: P = 0.003 (a), P = 0.002 (b). |
|
|
|
Figure 3. Pain scores (4Avet) (a) and visual analogue scales (VAS) (b), from evaluation time points T-1 to T390 of dogs receiving repeated perineural injections of ropivacaine (n = 20) or NaCl 0.9% (n = 20) through a perineural coiled catheter. Data are presented as median [IQR]. *Statistically significant difference between groups (P < 0.007). |
In group C, the VAS was lower at T-1 than at T120, T180, T240, T300, T360 and Day 1 (P < 0.05). At Day 1, the VAS was higher than at T0, T30, T60 and T390 and so was 4Avet compared to all the other time points (P < 0.05) except T390. The 4Avet at T390 was higher than at T-1, T0, T30 and T60 (P < 0.05).
In group R, the VAS was lower at T-1 than at T120, T180, T240, T360 and Day 1 (P < 0.05). At Day 1, the VAS was higher than at T0, T30, T60, and T390 and so was 4Avet compared to all the other time points (P < 0.05). The 4Avet at T300 was higher than at T-1 (P < 0.05).
Evaluation of motor and sensory function
No difference between groups regarding the modified Bromage score could be detected at any evaluation time point. At the evaluation of Day 1, a score of 2 indicating no weight bearing was attributed to four dogs (R: n = 3; C: n = 1). In one of the three dogs allocated to group R, the sensory score was 0 indicating a complete lack of sensation. The dog was re-evaluated 5 h after the last ropivacaine injection when full weight bearing and sensation to toe clamping had returned. A significant difference regarding the sensory score was found at T360 (P = 0.031) and at T390 (P = 0.001). At these time points, animals receiving repeated injections of ropivacaine were less sensitive to toe clamping.
Rescue analgesia
In eight dogs (R: n = 5; C: n = 3), fentanyl had to be administered intraoperatively. A second dose was needed in three dogs (R: n = 2; C: n = 1). No correlation between the administration of intraoperative fentanyl and the requirement for post-operative methadone was found (P = 0.689). During the post-operative phase, 55% of the dogs received methadone independently from group allocation (R: n = 11; C: n = 11; Table 2). No difference between groups regarding methadone requirements could be detected. The block duration, defined as intervals between the pre-operative nerve block and administration of the first methadone bolus was not different between groups (R: 285 [238–383] min; C: 370 [279–429] min; P = 0.278).
| Methadone boli | Group R (number of dogs) | Group C (number of dogs) |
|---|---|---|
| 1 | 4 | 4 |
| 2 | 1 | 2 |
| 3 | 3 | 1 |
| 4 | 2 | 2 |
| 5 | 1 | 1 |
| 6 | 0 | 0 |
| 7 | 0 | 1 |
| Total number of dogs | 11/20 | 11/20 |
Discussion
According to our hypothesis, PCC allowed repeated injection of ropivacaine or NaCl 0.9%. Post-operative VAS was significantly lower at T390 and Day 1 and so was 4Avet at Day 1, but post-operative methadone consumption could not be reduced in group R compared to group C.
In the present study, the PCC could be easily placed in a clinical setting with a certain potential for dislodgement (14.9%). The repeated administrations of ropivacaine 0.25% at the sciatic nerve through a PCC led to lower pain scores at 6.5 h (T390) and one day after surgery (Day 1) compared to the control group receiving repeated administrations of NaCl 0.9% at the same nerve after TPLO.
Assessing pain in non-verbal patients is challenging despite the ongoing progress in pain evaluation (Sharkey 2013). In this study, the condition of the dogs during the post-operative period was assessed by use of two pain scores (4Avet and VAS). The VAS is a widely applied method to evaluate pain in animals, but it is only reliable over time if always performed by the same observer as conducted in our animals (Holton et al. 2001). A German translation of the 4Avet has been regularly used in our clinic with satisfactory results over the last years and this version has also been applied in our study. The fact that the VAS was evaluated after the 4Avet might have influenced the observer in the evaluation of the VAS. This might explain the very similar values of both scores and must be considered as a limitation of the chosen scoring technique. Ideally, VAS and 4Avet should have been evaluated by several observers.
The population of dogs undergoing TPLO is very uniform in terms of animal size, weight, and pre-operative pain status; furthermore, the surgical procedure is highly standardized. Therefore, canine TPLO can be considered as an adequate model for the study of analgesia techniques under clinical conditions. According to Bufalari et al. (2012), non-steroidal anti-inflammatory drugs and buprenorphine are thought to provide sufficient analgesia for the first 8 h following TPLO. In the present study, the higher pain scores detected at Day 1 in group C let assume that the pain intensity after this surgery might be underestimated and that usually applied analgesic protocols might be insufficient to provide adequate pain relief at Day 1. Perineural catheters allowing prolonged regional analgesia seemed to reduce this problem as both scores were lower at T390 and at Day 1 in the ropivacaine group compared to the control group.
A study evaluating continuous perineural nerve blocks in 1416 human patients revealed effective post-operative analgesia in 96.3% of the cases recovering from orthopaedic surgery even if an elevation of the pain score was noted after 24 h (Capdevila et al. 2005). An increase in VAS and 4Avet scores 1 day after surgery was observed in our study in both groups as well.
In most human studies, analgesic drugs administered through perineural catheters have been provided as continuous rate infusions (Merritt et al. 2014). The continuous administration in a conscious dog can be challenging because the animals move and the risk of dislodgement of the catheter is increased. In humans, the post-operative opioid consumption was lower in patients receiving local anaesthetics over a femoral nerve catheter with an automated intermittent bolus technique compared to a continuous administration of the same drug (Hillegass et al. 2013). The influence of repeated bolus administrations compared to a continuous delivery of local anaesthetics on the quality and duration of the blocks in dogs has not been investigated to date.
The catheter seemed to remain in close proximity to the nerve during the complete evaluation time (24 h) as indicated by the lower pain score at Day 1 even though no diagnostic imaging was performed to confirm the exact anatomical location. The slight elevation of the pain score after 24 h compared to the early post-operative phase might be explained by the fact that ropivacaine was only provided to the sciatic nerve. At the medial side, the sensory innervation of the canine knee joint is provided by branches of the saphenous nerve (originating from the femoral nerve); at the lateral side, by branches of the common peroneal nerve (originating from the sciatic nerve) (O'Connor & Woodbury 1982). After a combined block of both the femoral and the sciatic nerves prior to surgery, the sciatic nerve was chosen for PCC placement and repeated drug administration during the post-operative period and should be considered as a limitation to the study. Presently, the result regarding the advantage of combining a sciatic nerve block with a femoral nerve block to treat acute pain in human patients recovering from total knee arthroplasty is considered to be inconclusive (Abdallah & Brull 2011) and justifies the decision to place a single PCC in our study.
Reduced post-operative morphine requirements were observed in human patients recovering from total knee arthroplasty receiving a constant rate infusion of levo-bupivacaine administered to the lumbar plexus (Watson et al. 2005). However, the decrease in morphine consumption in the mentioned study was only observed 24 h after surgery. When the analgesic effects of repeated injections of ropivacaine 0.75% twice daily at the saphenous nerve were compared to saline, no difference in the post-operative morphine consumption was noted, but the pain scores of the placebo group were higher (Andersen et al. 2013). The requirements for rescue analgesia during the first 360 min after endotracheal extubation did not vary between groups in our study. This can possibly be explained with regard to the long-lasting analgesic effects of the combined pre-operative block as suggested by the 4Avet and VAS scores, low in both groups between T0 and T360.
Dogs undergoing TPLO are usually treated with analgesic drugs prior to surgery. This might have influenced post-operative pain evaluation even though pre-operative analgesic drugs were discontinued after inclusion in the study. Additionally, the surgery might differ between the study subjects. The degree of osteoarthritis, the presence of meniscal tears, the degree of cruciate tears are all examples that might influence the degree of post-operative pain and should be considered as a substantial limitation of pain evaluation.
During the post-operative phase, each time a 4Avet score of ≥5 (33% of maximal value) or a VAS of ≥40 mm (40% of maximal value) was reached, methadone was administered as rescue analgesia. When the 4Avet was validated by Rialland et al. (2012), rescue analgesics were administered only if a score corresponding to 60% of the maximal value was obtained. The rather low threshold used in the present study to titrate rescue analgesia might also explain the lack of difference observed between groups in terms of methadone consumption.
For the intraoperative period, a complete sensory block was desired. Rescue fentanyl was administered to eight dogs and an incomplete block was suspected in these animals but no dog received more than two doses of fentanyl. Interestingly, no correlation between the administration of intraoperative and post-operative rescue drugs could be found; however, an incomplete intraoperative block could provide sufficient post-operative analgesia has been previously hypothesized by Vettorato et al. (2012).
Good mobility with minimal pain sensation in the post-operative phase was targeted in our study. To reach this goal, a ropivacaine concentration of 0.25% was chosen based on experience in humans as species-specific evidence is lacking (Brodner et al. 2007). The lower sensory scores at T360 and T390 in group R were achieved, assuming that the target was achieved. At Day 1, mobility based on the modified Bromage score was present in all but three dogs of the ropivacaine group. In humans, early mobility was present with a continuous block of the sciatic nerve using ropivacaine 0.2% in adults while a sensory block without motor block was provided with ropivacaine 0.25% in children (Casati et al. 2004; Petroheilou et al. 2012). In our study, the assessment of weight bearing as a criterion to evaluate the extent of motor block might not always be conclusive as the bandage applied to the operated leg of the dogs might have influenced the motor function.
An important limitation of this study lays in the fact that 4Avet, VAS, motor and sensory score have not been evaluated between T390 and Day 1. Conclusions about the prolonged effect of repeated injections of ropivacaine 0.25% during this period are not possible. Consequently, the lower 4Avet and VAS observed at Day 1 should be interpreted with care as the condition of the dogs overnight might have influenced these results. To objectify the efficacy of repeated injections of ropivacaine, all scores should have been evaluated more frequently and over a longer time period which was logistically not possible for a single observer. Including another observer to evaluate pain overnight would have been possibly helpful but would have increased the scores variability. Despite this limitation, the PCC seems to be an effective tool to reduce post-operative pain in dogs undergoing invasive orthopaedic surgery in which a standard analgesia protocol might fail to control pain at Day 1.
A lack of diagnostic imaging to confirm the position of the PCC over the study period is another limiting factor. According to Marhofer et al. (2013), the dislodgement rate of straight perineural catheters at the femoral nerve was 25% in healthy volunteers undergoing physical exercise. The catheter used in this study has previously been placed in the paravertebral space in human cadavers (Luyet et al. 2012), but until now clinical studies evaluating the efficacy of the coiled catheters or comparison with straight perineural catheters are lacking.
Complications associated with the use of perineural catheters are classified as major (abscess formation, nerve damage) and minor (bacterial colonization, dislodgement or accidental withdrawn of the catheter, bleeding) (Capdevila et al. 2005). In our study, some minor complications (bleeding, dislodgement) were observed and the overall dislodgement rate was 14.9% (PCC removed from its placement site). Higher patient numbers would be needed to be able to judge the safety of PCC placement and a longer follow up needed to evaluate abscess formation and nerve damage.
In conclusion, the PCC can be placed in the sciatic nerve of dogs allowing repeated administrations of ropivacaine or NaCl with a low rate of dislodgement. Similar studies under similar conditions examining PCC placement on the femoral nerve and comparing straight perineural catheters with PCCs are warranted.
Acknowledgement
The PCCs were generously donated by the company Pajunk, Geisingen, Germany.
Source of funding
None.
Conflicts of interest
None.
Contributions
None.
References
- Abdallah F.W. & Brull R. (2011) Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A systematic review. Regional Anesthesia and Pain Medicine36, 493–498.
- Andersen H.L., Gyrn J., Moller L., Christensen B. & Zaric D. (2013) Continuous saphenous nerve block as supplement to single-dose local infiltration analgesia for postoperative pain management after total knee arthroplasty. Regional Anesthesia and Pain Medicine38, 106–111.
- Brodner G., Buerkle H., Van Aken H., Lambert R., Schweppe-Hartenauer M.L., Wempe C.et al. (2007) Postoperative analgesia after knee surgery: a comparison of three different concentrations of ropivacaine for continuous femoral nerve blockade. Anesthesia and Analgesia105, 256–262.
- Bromage P.R. (1965) A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiologica Scandinavica Supplementum16, 55–69.
- Bufalari A., Maggio C., Cerasoli I., Morath U. & Adami C. (2012) Preemptive carprofen for peri-operative analgesia in dogs undergoing Tibial Plateau Leveling Osteotomy (TPLO): a prospective, randomized, blinded, placebo controlled clinical trial. Schweizer Archiv fur Tierheilkunde154, 105–111.
- Campoy L., Bezuidenhout A.J., Gleed R.D., Martin-Flores M., Raw R.M., Santare C.L.et al. (2010) Ultrasound-guided approach for axillary brachial plexus, femoral nerve, and sciatic nerve blocks in dogs. Veterinary Anaesthesia and Analgesia37, 144–153.
- Caniglia A.M., Driessen B., Puerto D.A., Bretz B., Boston R.C. & Larenza M.P. (2012) Intraoperative antinociception and postoperative analgesia following epidural anesthesia versus femoral and sciatic nerve blockade in dogs undergoing stifle joint surgery. Journal of the American Veterinary Medical Association241, 1605–1612.
- Capdevila X., Pirat P., Bringuier S., Gaertner E., Singelyn F., Bernard N.et al. (2005) Continuous peripheral nerve blocks in hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1,416 patients. Anesthesiology103, 1035–1045.
- Casati A., Vinciguerra F., Cappelleri G., Aldegheri G., Grispigni C., Putzu M.et al. (2004) Levobupivacaine 0.2% or 0.125% for continuous sciatic nerve block: a prospective, randomized, double-blind comparison with 0.2% ropivacaine. Anesthesia and Analgesia99, 919–923, table of contents.
- Dyhre H., Lang M., Wallin R. & Renck H. (1997) The duration of action of bupivacaine, levobupivacaine, ropivacaine and pethidine in peripheral nerve block in the rat. Acta Anaesthesiologica Scandinavica41, 1346–1352.
- Gurney M.A. & Leece E.A. (2014) Analgesia for pelvic limb surgery. A review of peripheral nerve blocks and the extradural technique. Veterinary Anaesthesia and Analgesia41, 445–458.
- Hillegass M.G., Field L.C., Stewart S.R., Borckardt J.J., Dong L., Kotlowski P.E.et al. (2013) The efficacy of automated intermittent boluses for continuous femoral nerve block: a prospective, randomized comparison to continuous infusions. Journal of Clinical Anesthesia25, 281–288.
- Hoelzler M.G., Harvey R.C., Lidbetter D.A. & Millis D.L. (2005) Comparison of perioperative analgesic protocols for dogs undergoing tibial plateau leveling osteotomy. Veterinary Surgery34, 337–344.
- Holton L., Reid J., Scott E.M., Pawson P. & Nolan A. (2001) Development of a behaviour-based scale to measure acute pain in dogs. The Veterinary Record148, 525–531.
- Kuthiala G. & Chaudhary G. (2011) Ropivacaine: a review of its pharmacology and clinical use. Indian Journal of Anaesthesia55, 104–110.
- Liu S.S. & Salinas F.V. (2003) Continuous plexus and peripheral nerve blocks for postoperative analgesia. Anesthesia and Analgesia96, 263–272.
- Luyet C., Meyer C., Herrmann G., Hatch G.M., Ross S. & Eichenberger U. (2012) Placement of coiled catheters into the paravertebral space. Anaesthesia67, 250–255.
- Marhofer D., Marhofer P., Triffterer L., Leonhardt M., Weber M. & Zeitlinger M. (2013) Dislocation rates of perineural catheters: a volunteer study. British Journal of Anaesthesia111, 800–806.
- Merritt C.K., Mariano E.R., Kaye A.D., Lissauer J., Mancuso K., Prabhakar A.et al. (2014) Peripheral nerve catheters and local anesthetic infiltration in perioperative analgesia. Best Practice & Research Clinical Anaesthesiology28, 41–57.
- O'Connor B.L. & Woodbury P. (1982) The primary articular nerves to the dog knee. Journal of Anatomy134 (Pt 3), 563–572.
- Petroheilou K., Livanios S., Zavras N., Hager J. & Fassoulaki A. (2012) Sciatic lateral popliteal block with clonidine alone or clonidine plus 0.2% ropivacaine: effect on the intra-and postoperative analgesia for lower extremity surgery in children: a randomized prospective controlled study. BMC Anesthesiology12, 2.
- Rialland P., Authier S., Guillot M., Del Castillo J.R., Veilleux-Lemieux D., Frank D.et al. (2012) Validation of orthopedic postoperative pain assessment methods for dogs: a prospective, blinded, randomized, placebo-controlled study. PLoS One7, e49480.
- Rohrbach H., Casoni D., Müller N., Rytz U., Spadavecchia C. & Eichenberger U. (2015) Loco-regional analgesia in dogs undergoing tibia plateau levelling osteotomy (TPLO): ultrasound guided blocks of the sciatic and/or the femoral nerves versus epidural anaesthesia. Veterinary Anaesthesia and Analgesia42, A1–A40. Proceedings of the Veterinary Anaesthetists Meeting, 24-26th September 2014 Vienna.
- Sharkey M. (2013) The challenges of assessing osteoarthritis and postoperative pain in dogs. The AAPS Journal15, 598–607.
- Slocum B. & Slocum T.D. (1993) Tibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canine. The Veterinary Clinics of North America Small Animal Practice23, 777–795.
- Vettorato E., Bradbrook C., Gurney M., Aprea F., Clark L. & Corletto F. (2012) Peripheral nerve blocks of the pelvic limb in dogs: a retrospective clinical study. Veterinary and Comparative Orthopaedics and Traumatology25, 314–320.
- Watson M.W., Mitra D., McLintock T.C. & Grant S.A. (2005) Continuous versus single-injection lumbar plexus blocks: comparison of the effects on morphine use and early recovery after total knee arthroplasty. Regional Anesthesia and Pain Medicine30, 541–547.
- Williams B.A., Kentor M.L., Vogt M.T., Irrgang J.J., Bottegal M.T., West R.V.et al. (2006) Reduction of verbal pain scores after anterior cruciate ligament reconstruction with 2-day continuous femoral nerve block: a randomized clinical trial. Anesthesiology104, 315–327.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?