Abstract
Clonorchis sinensis is endemic to Southeast Asia, Japan, China, and Taiwan. Those infected often have a history of consumption of raw fresh fish. Its manifestations can be asymptomatic of cholangitis, biliary stones, or cholangiocarcinoma. A 69-year-old male living in Chishan, Taiwan visited our hospital with obstructive jaundice. Noninvasive analyses, such as stool examination, abdominal ultrasound, and an abdominal computed tomography scan did not reveal clues of clonorchiasis. As the obstructive jaundice was unexplained, endoscopic retrograde cholangiopancreatography (ERCP) was then performed and a hepatic fluke was seen via aspiration of bile. Subsequently, the ova of C. sinensis were detected via microscopic examination of bile samples. Bile aspiration is not routine during an ERCP procedure; however, we suggest the bile aspiration can be diagnostically accurate for clonorchiasis, especially for patients with a suspicious infection.
Keywords
Aspiration ; Cholangiopancreatography ; Clonorchis sinensis ; Endoscopic retrograde
Introduction
Clonorchis sinensis , also known as the oriental or Chinese liver fluke, typically causes clonorchiasis. This parasite is endemic to Asian countries such as China, Hong Kong, Taiwan, Japan, Korea, and Vietnam [1] . Humans are the definitive hosts of C. sinensis , which is often transmitted by eating cysts in raw freshwater fish [2] . The adult worm lives predominantly in the biliary tract, gallbladder, and less frequently in pancreatic ducts [3] .
Patients with a heavy worm burden commonly suffer from right upper abdominal pain, fever, jaundice, anorexia, or diarrhea. Major complications include suppurative cholangitis, bile duct stone formation, and even cholangiocarcinoma [4] . Endoscopic retrograde cholangiopancreatography (ERCP) has an important role in differential diagnosis and intervention [5] . This report describes a case of a directly visualized adult worm of C. sinensis in aspirated bile during ERCP.
Case Report
A 69-year-old male, living in Chishan, a town in southern Taiwan, arrived at our hospital with upper abdominal fullness, general malaise, and a poor appetite for 2 weeks. Accompanying symptoms were yellow skin color, tea-colored urine, clay-colored stool, and weight loss of 6 kg over the past 2 months. He had diabetes mellitus, hypertension controlled by medication, and performed physical activity regularly for 10 years. He denied consuming alcohol or drugs. Physical examination on admission showed icteric sclera and clay-colored stool. However, there was no abdominal tenderness. The patient’s vital signs, including body temperature, pulse rate, and respiratory rate, were all within normal ranges. Initial laboratory data were as follows: aspartate aminotransferase level, 720 IU/L (normal range, 5–35 IU/L); alanine aminotransferase level, 702 IU/L (normal range, 0–40 IU/L); total bilirubin, 9.7 mg/dL (normal range, 0.2–1.6 mg/dL); alkaline phosphatase, 290 U/L (normal range, 3.7–5.3 U/L); albumin, 3.5 g/dL (normal range, 3.7–5.3 g/dL); normal prothrombin time; white blood cell count, 7660 cells/mm3 (normal range, 4000–9900 cells/mm3 ); hemoglobin, 11.2 g/dL (normal range, 13.5–17.8 g/dL); and platelet count, 243,000 cells/mm3 (normal range, 150,000–450,000 cells/mm3 ). The blood eosinophil count was 1%. Viral serology was negative for the hepatitis B surface antigen, but positive for the antibody for the hepatitis C virus. Serum α-fetoprotein level was 6.3 ng/mL (< 10 ng/mL), the carcinoembryonic antigen was 3.8 ng/mL (< 5.0 ng/mL), and the carbohydrate antigen 19-9 was 91.1 U/mL (< 37 U/mL). Routine stool analysis yielded no evidence of parasite ova. Renal function and electrolyte data were normal.
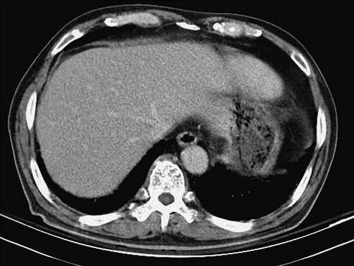
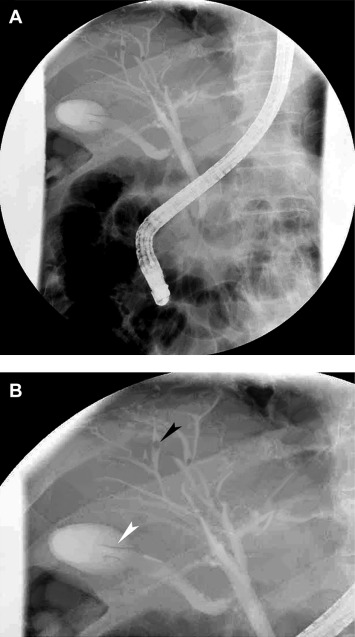
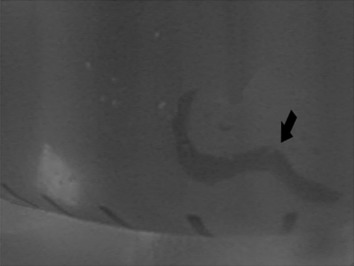
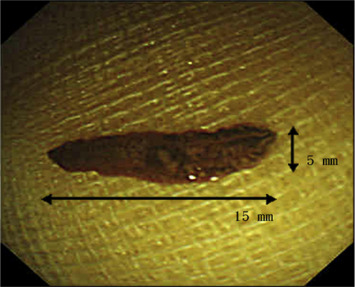
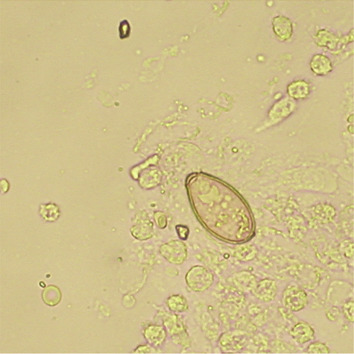
Abdominal sonography revealed gallbladder wall thickening without a gallstone and no significant liver lesions. Abdominal computed tomography did not show filling defects or abnormal dilatation of intrahepatic ducts and common bile duct (Figure 1 ). Due to the uncertain etiology of the obstructive jaundice, ERCP was applied. The ERCP revealed two falciform filling defects in the gallbladder, mild dilatation of peripheral intrahepatic ducts, and some suspicious small round filling defects in intrahepatic ducts (Figures 2 A and 2B). The bile was aspirated via a 10 mL syringe after successful cannulation using guide wire technique without prior contrast injection into biliary tracts. Then, a hepatic fluke was identified in the syringe (Figure 3 ). The worm was 15 mm in length × 3 mm in width, brown color, pointed anterior and rounded posterior shape, slender leaf-like appearance and with branched testes in the posterior one third (Figure 4 ). The fluke’s morphology was compatible with that of C. sinensis. The aspirated bile was stored in the test tube and was centrifuged at a speed of 3500 revolutions/min. We got the 0.5 mL bile debris at the bottom of the test tube for microscopic examination. Microscopic examination of the aspirated bile debris revealed several ovoid ova ( Figure 5 ), compatible with C. sinensis ova (light yellow color with a prominent shoulder rim of the operculum and comma-shaped terminal knob on the opposite side). A nasobiliary tube was inserted to release the obstructive jaundice. Praziquantel 25 mg/kg/dose three times daily at 4- to 6-hour intervals for 1 day was prescribed. One week after ERCP, no ovum was identified from aspirated bile via the nasobiliary tube. Subsequently, cholangiography was repeated and found no filling defect of the biliary tract (such as gallbladder and bile ducts). In addition, bilirubin levels kept within the normal limit after removal of the nasobiliary tube. The patient was discharged after a 10-day hospitalization. During a 6-months follow-up, he did not have symptoms of abdominal pain or jaundice.
|
|
|
Figure 1. No abnormal intrahepatic ducts dilatation and no biliary tree filling defects. |
|
|
|
Figure 2. (A) Mild dilatation of peripheral intrahepatic ducts was noted. (B) Two falciform defects in the gallbladder (black arrow head) with some round filling defects (white arrow head) were noted. (A magnified image from Figure 2 A). |
|
|
|
Figure 3. The black arrow shows a hepatic fluke in the syringe after bile aspiration via a catheter. |
|
|
|
Figure 4. The hepatic fluke was 15 mm × 3 mm in size, with brown pigmented, leaf-shaped slender appearance. |
|
|
|
Figure 5. Clonorchis sinensis ova had characteristics of light yellow color, prominent shoulder rim of operculum, and comma-shaped terminal knob on the opposite side (under 400× microscope field view). |
Discussion
Chan et al [5] noted that 39% of cases of C. sinensis infection were from an endemic area including Meinung and Chishan. In addition, 83% of those had a history of consuming raw fish. The Chishan area was where our patient resided, and he also had a habit of consuming raw freshwater fish. This history was a clue for clonorchiasis.
In a laboratory study, peripheral eosinophilia accounted for 39% of patients with biliary symptoms and only 10.8% in patients without biliary symptoms [5] . Until now, fecal examination has been the standard method of diagnosis for human clonorchiasis [6] . However, fewer than half of the patients had ova in their stools, even when bile was positive for the ova [5] . In this patient, ova were not found in the stool. This might be due to chronic infestation or an insufficient number of ova in the stool. Detection of deoxyribonucleic acid from C. sinensis is another promising method for diagnosis. Its sensitivity was 71.0% and its specificity was 76.7% from a study in Thailand [7] . Serodiagnosis of C. sinensis by enzyme-linked immunosorbent assay is also practical and very common in Korea. It has a reported sensitivity of 87.8% with crude extract antigen [8] .
Adult worms generally reside in the medium and small intrahepatic bile ducts, resulting in intrahepatic bile duct obstruction, cholangitis, and multifocal periductal fibrosis. Computed tomography may reveal diffuse pipe-like dilatation of the peripheral intrahepatic ducts or leaf-like tiny filling defects in the biliary tree [9] .
An ERCP intervention is an effective modality for differential diagnosis and may be an appropriate release for obstructive jaundice. During ERCP, cholangiography may show characteristic findings of diffusely uniform dilatation of intrahepatic ducts, particularly in peripheral bile ducts and filamentous filling defects, as well as small, irregular filling defects in them [9] ; [10] .
In this patient, no ova were identified in the stool. Image studies, such as sonography and abdominal computed tomography, revealed no definite evidence of C. sinensis infection. The patient then underwent ERCP. The bile ducts were mildly dilated, especially the peripheral intrahepatic ducts. This finding was compatible with clonorchiasis [9] . The two falciform filling defects in the gallbladder hinted at the possibility of hepatic flukes or incomplete contrast filling of the gallbladder. As a diagnostic method, bile was aspirated, and a living worm was identified. Its appearance was compatible with that of C. sinensis . To the best of our knowledge, few reports have described direct diagnosis via bile aspiration when performing an ERCP procedure. In our hospital, endoscopists routinely perform bile aspiration for diagnostic surveillance when no definite etiology is identified by cholangiography. In summary, clonorchiasis should be included in the differential diagnosis list of unexpected obstructive jaundice, particularly in areas in which clonorchiasis is endemic. Routine analysis of aspirated bile may improve the diagnostic accuracy for live C. sinensis flukes during ERCP.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
The authors thank Ted Knoy for revision of English Grammar.
References
- [1] H.J. Rim; The current pathobiology and chemotherapy of clonorchiasis; Korean J Parasitol, 24 (Suppl) (1986), pp. 7–20
- [2] G.M. Park, T.S. Yong; Geographical variation of the live fluke, Clonorchis sinensis , from Korea and China based on the karyotypes, zymodeme and DNA sequences ; Southeast Asian J Trop Med Public Health, 32 (2001), pp. 12–16
- [3] P.H. Chan, T.B. Teoh; The pathology of Clonorchis sinensis infestation of the pancreas ; J Pathol Bacteriol, 93 (1967), pp. 185–189
- [4] S.T. Hong; Clonorchis sinensis; M.D. Miliotis, J.W. Bier (Eds.), International handbook of foodborne pathogens, Marcel Dekker, Inc., New York, Basel (2003), pp. 581–592
- [5] H.H. Chan, K.H. Lai, G.H. Lo, J.S. Cheng, J.S. Huang, P.I. Hsu, et al.; The clinical and cholangiographic picture of hepatic clonorchiasis; J Clin Gastroenterol, 34 (2002), pp. 183–186
- [6] S.T. Hong, Y. Fang; Clonorchis sinensis and clonorchiasis, an update ; Parasitol Int, 61 (2012), pp. 17–24
- [7] R.J. Traub, J. Macaranas, M. Mungthin, S. Leelayoova, T. Cribb, K.D. Murrell, et al.; A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand ; PLoS Negl Trop Dis, 3 (2009), p. e367
- [8] M.H. Choi, I.C. Park, S. Li, S.T. Hong; Excretory-secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA; Korean J Parasitol, 41 (2003), pp. 35–39
- [9] I.-H. Su, S.-Y. Chu, C.-M. Chen, K.-T. Pan, M.-Y. Hsu, R.-F. Shi, et al.; Imaging findings of liver clonorchiasis; J Radiol Sci, 36 (2011), pp. 145–151
- [10] J.W.C. Leung, J.Y. Sung, S.C.S. Chung, C. Metrewili; Hepatic clonorchiasis—a study by endoscopic retrograde cholangiopancreatography; Gastrointest Endosc, 35 (1989), pp. 226–231
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?