Summary
Heterotopic pancreas is a congenital anomaly characterized by the presence of ectopic pancreatic tissue far from the pancreas. The treatment of heterotopic pancreas may include expectant observation, endoscopic resection, or surgery. The aim of this study was to describe the technique of cap-assisted endoscopic mucosal resection for the management of heterotopic pancreas of the stomach. Two patients, a 41-year-old woman and a 31-year-old man, were referred to us for the management of gastric subepithelial lesions. Endoscopic ultrasound was used in the female patient to disclose two small hypoechoic lesions arising from the submucosal layer. Cap-assisted endoscopic mucosal resection was performed in both patients without complications. Histopathological examination of the resected specimens showed heterotopic pancreatic tissue in the submucosal layer. Our technique is a suction, snaring, and cut method. This method does not need a special cap with a shallow circumferential lip on the inside and the snare does not need to be pre-looped. This technique allowed a histopathological confirmation of the suspected diagnosis in both patients.
Keywords
Cap-assisted technique ; Endoscopic mucosal resection ; Heterotopic pancreas ; Subepithelial lesions
Introduction
Heterotopic pancreas (HP) is an uncommon congenital anomaly characterized by the presence of ectopic pancreatic tissue far from the pancreas and without any anatomical or vascular communication with the pancreas. It occurs in around 2% of the general population [1] and is noted to be more common in men [2] . HP may occur throughout the gastrointestinal tract, but frequently involves the stomach and proximal small intestine. Most affected patients are asymptomatic, although a minority of patients may present with a variety of symptoms, such as epigastric pain [1] . Options for the treatment of HP in the stomach include surgery [1] ; [2] , endoscopic resection [3] ; [4] ; [5] ; [6] , or a “wait and see” strategy.
Endoscopic mucosal resection (EMR) is an important method that allows sessile lesions confined to the mucosa or with only minute submucosal invasion to be removed. However, it is often difficult to entrap a flat lesion with standard EMR. We describe here two cases of HP treated with cap-assisted EMR, a so-called “suction, snaring, and cut” method. This is a relatively simple approach for the management of HP.
Case reports
Case 1
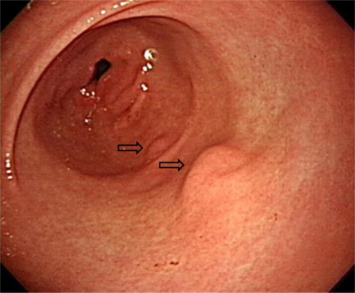
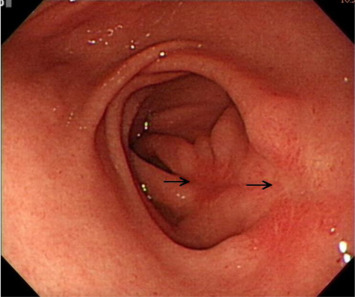
A 41-year-old woman presented to our gastrointestinal outpatient department with a 1-month history of postprandial abdominal fullness and early satiety. No significant abnormality was noted on physical examination and laboratory tests. Esophagogastroduodenoscopy (EGD) showed the presence of two small polypoid lesions (1.2 cm and 0.8 cm in size) with intact mucosa in the gastric antrum (Fig. 1 ). Endoscopic ultrasonography (EUS) indicated two oval, hypoechoic, and homogeneous lesions appearing to involve the muscularis mucosa and submucosa (Fig. 2 ). We suggested a “wait-and-see” strategy because a benign etiology was more favored. However, the patient was worried about the possibility of a malignant potential and decided, after discussion, to receive endoscopic resection. Informed consent was obtained and the patient was sedated with an intravenous administration of midazolam. We then performed cap-assisted EMR at our endoscopy unit.
|
|
|
Figure 1. Esophagogastroduodenoscopy shows two polypoid lesions in the antrum (1.2 cm and 0.8 cm; arrows). |
|
|
|
Figure 2. Endoscopic ultrasonography (Miniprobe 12 MHz, Olympus, Tokyo, Japan) shows an oval, well-defined, inhomogeneous hypoechoic tumor with an irregular outer margin involving the muscularis mucosa and submucosa layer (arrow). |
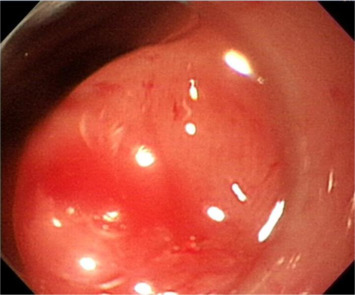
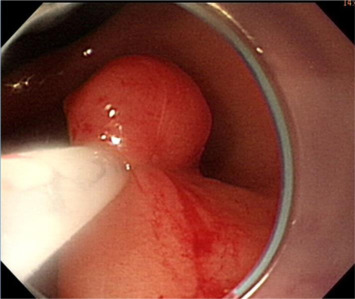
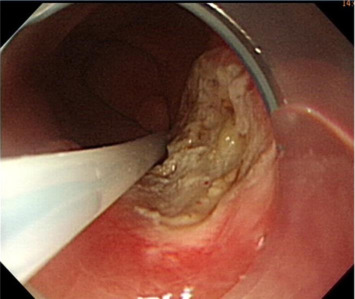
Initially, we administered a submucosal injection of diluted epinephrine along the margin of the larger lesion; however, we were unable to loop the snare around the lesion. A translucent plastic cap (straight distal attachment, MH-462, outer diameter 12.6 mm; Olympus, Tokyo, Japan) was first fixed on the tip of the endoscope and the cap was positioned on the target lesion. Suction was then applied to draw the flat lesion into the cap and, when the suction was released, the flat lesion became a pseudopolyp (Fig. 3 ). Next, we used an electrosurgical snare (oval shape, SD-9L-1, Olympus) to strangle the pseudopolyp immediately before when it became flat (Fig. 4 ). We then resected it with an electrosurgical generator (ERBE VIO 200D, settings with ENDO CUT Q model effect 3, duration 1, interval 5; Elektromedizin, Tübingen, Germany), resulting in an artificial ulcer without active bleeding or perforation (Fig. 5 ). We removed the smaller lesion smoothly by the same method and the total procedure time was 19 minutes. The patient fasted for 1 day and an intravenous proton pump inhibitor was given. Her course after EMR was uneventful and she was discharged 2 days later.
|
|
|
Figure 3. Suction is applied to draw the flat lesion into the cap and, on the release of suction, the flat lesion becomes a pseudopolyp. |
|
|
|
Figure 4. The elevation is immediately snared before it becomes flat and is removed using electrocauterization. |
|
|
|
Figure 5. Endoscopic mucosal resection is smooth and leaves an artificial ulcer without bleeding or perforation. |
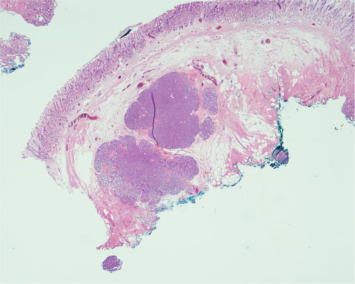
The histopathological examination from both resected specimens showed nests of HP tissue in the submucosal layer (Fig. 6 ). Although the margins of the resected specimens were not free of HP tissue, her EGD at follow-up 2 months later showed only two linear ulcer scars on the previous EMR sites without endoscopic evidence of a residual protruded lesion (Fig. 7 ).
|
|
|
Figure 6. Photomicrograph of the resection specimen showing nests of pancreatic tissue within the gastric submucosa; the resection margin is not free. Hematoxylin and eosin stain (×10 magnification). |
|
|
|
Figure 7. Esophagogastroduodenoscopy at follow-up shows two linear ulcer scars (arrows) without endoscopic evidence of a residual protruded lesion. |
Case 2
A 31-year-old man presented to our gastrointestinal outpatient department with a 2-week history of postprandial abdominal fullness and early satiety. EGD showed the presence of a flat, oval lesion with intact mucosa, approximately 1.2 cm in size, located on the greater curvature side of the gastric antrum. The patient opted for endoscopic resection of the mass after he was informed that this lesion most likely represented a benign submucosal tumor. Informed consent was obtained and we successfully performed cap-assisted EMR at our endoscopy unit (total procedure time 8 minutes). The patient fasted for 1 day and a proton pump inhibitor was administered intravenously. His course after EMR was uneventful and he was discharged 2 days after surgery.
The results of the histopathological examination of the resected specimen showed nests of heterotopic pancreatic parenchyma and ducts in the submucosa with a resection margin free of tumor. His EGD follow-up 6 months later showed only a linear ulcer scar without endoscopic evidence of a residual protruded lesion.
Discussion
HP, also known as pancreatic rest, is most often detected as an incidental finding during routine EGD. The typical endoscopic appearance in the stomach is like a firm round or oval umbilicated subepithelial nodule along the greater curvature situated several centimeters proximal to the pylorus. Most patients are asymptomatic, but symptoms may rarely occur due to the irritative effect of the secreted hormones or enzymes by the HP [2] . Asymptomatic HP can generally be followed expectantly, with treatment reserved for patients who are symptomatic, for enlarging lesions seen during follow-up, or to give a certain diagnosis. The management of this category of gastric subepithelial lesions is still controversial and often based on limited data or clinical experience, or both [7] . The optimal indication for resection of subepithelial lesions may include an increase in size during a follow-up period and symptomatic, potential malignant features on EUS, and for definitive histopathological diagnosis. HP in these two patients was discovered incidentally during EGD for investigation of early satiety and postprandial abdominal fullness. Nevertheless, endoscopic removal was requested by both patients to ensure a certain diagnosis.
The diagnosis of HP is not straightforward. Although central umbilication is commonly associated with this condition, it is unknown whether this endoscopic finding is characteristic and indicative of HP. The typical EUS image of HP in the stomach is a hypoechoic, heterogeneous submucosal mass, with the occasional involvement of the muscularis propria and mucosa. A ductal structure may also be discernible within the lesion. Although these EUS findings are suggestive of HP, the accuracy of EUS for the differential diagnosis of other subepithelial tumors (e.g., gastrointestinal stromal tumor) is limited [6] ; [8] ; [9] . In fact, neither of our two patients showed endoscopic findings typical of HP, such as central umbilication. We were unable to obtain a definitive diagnosis, although we believed that the lesions were benign.
Histological diagnosis of HP is usually difficult when tissue specimens are obtained using standard endoscopic biopsy forceps [10] . Tissue from the deeper layers of the gastric wall can be obtained by taking multiple biopsy specimens of the same site using jumbo forceps (named as stacked, bite-on-bite, or tunneled biopsies), although the diagnostic yield appears to be limited with an increased risk of bleeding. In one previous study, the diagnostic yield of stacked biopsies was 42% (15/36 lesions) and the complication rate was 2.8% (1/36 cases complicated by bleeding) [7] . Some workers believe that it is difficult to obtain a definitive preoperative diagnosis of HP and therefore advocate surgical resection [2] . Although there are some reports of the surgical resection of HP [1] ; [2] ; [3] , EMR may be a less invasive alternative for the resection of accessible lesions.
However, there have only been a few reports [3] ; [4] ; [5] ; [6] that describe the use of EMR for HP resection. Two groups have described the use of cap-fitted EMR [3] ; [4] . The “inject, lift, and cut,” or “strip biopsy” EMR technique [5] was reported for the resection of six cases of HP. Sun and Wang [6] studied the use of EUS-guided injection and the “inject and cut” EMR technique for the resection of 16 upper gastrointestinal submucosal tumors, two of which were gastric HP tumors. Khashab et al [11] suggested that ligation-assisted EMR may be more operator-friendly than other EMR techniques. The concept of tissue capture is similar to the familiar variceal ligation technique.
This modified cap-assisted EMR technique is relatively simple to practice, but is not always applicable to the resection of various subepithelial lesions (SELs). In our experience, the limitations and drawbacks of this technique include layer, size, consistency, and unconfirmed complete resection of SELs. For example, we may only apply this technique to SELs on the superficial submucosal layer, which needs previous EUS layer delineation. We cannot suction the pseudopolyp into the transparent cap if the lesion is larger than the applied diameter. Only soft, not firm, lesions can be suctioned and snared (e.g., lipomas, cysts, or carcinoid tumors) rather than stromal tumors or tumors adherent to the muscularis propria layer. In addition, we cannot confirm complete resection of a SEL by this technique because the suction-induced pseudopolyp may regress prior to snaring and cutting.
We have presented here a simpler and more operator-friendly approach using modified cap-assisted EMR. This technique is called the “suction, snaring, and cut” method. The advantages of this method are the use of a reusable cap, not a special cap with a shallow, circumferential lip on the inside, and easy snare resection without technique-demanding pre-looping. This technique allowed a histopathological diagnosis of the suspected gastric HP in both our patients without complications.
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- [1] E.C. Lai, R.K. Tompkins; Heterotopic pancreas. Review of a 26 year experience; Am J Surg, 151 (1986), pp. 697–700
- [2] O.T. Ormarsson, I. Gudmundsdottir, R. Mårvik; Diagnosis and treatment of gastric heterotopic pancreas; World J Surg, 30 (2006), pp. 1682–1689
- [3] T.H. Lee, H.P. Wang, S.F. Huang, T.H. Wang, J.T. Lin; Endoscopic mucosal resection for treatment of heterotopic pancreas in the stomach; J Formos Med Assoc, 98 (1999), pp. 643–645
- [4] D.O. Faigel, D. Gopal, D.A. Weeks, C. Corless; Cap-assisted endoscopic submucosal resection of a pancreatic rest; Gastrointest Endosc, 54 (2001), pp. 782–784
- [5] T. Kojima, H. Takahashi, A. Parra-Blanco, K. Kohsen, R. Fujita; Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection; Gastrointest Endosc, 50 (1999), pp. 516–522
- [6] S. Sun, M. Wang; Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors; Endoscopy, 34 (2002), pp. 82–85
- [7] J.H. Hwang, S.D. Rulyak, M.B. Kimmey; American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses; Gastroenterology, 130 (2006), pp. 2217–2228
- [8] B. Brand, L. Oesterhelweg, K.F. Binmoeller, P.V. Sriram, S. Bohnacker, S. Seewald, et al.; Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract; Dig Liver Dis, 34 (2002), pp. 290–297
- [9] G.C. Hunt, P.P. Smith, D.O. Faigel; Yield of tissue sampling for submucosal lesions evaluated by EUS; Gastrointest Endosc, 57 (2003), pp. 68–72
- [10] C.Y. Hsia, C.W. Wu, W.Y. Lui; Heterotopic pancreas: a difficult diagnosis; J Clin Gastroenterol, 28 (1999), pp. 144–147
- [11] M.A. Khashab, O.W. Cummings, J.M. DeWitt; Ligation-assisted endoscopic mucosal resection of gastric heterotopic pancreas; World J Gastroenterol, 15 (2009), pp. 2805–2808
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?