Highlights
- HPLC method for determination thiol-containing amino acids in rat plasma.
- Decreased plasma cysteine/cystine in acute and chronic stages of chemoconvulsant-induced epilepsy.
- Depletion of plasma cysteine/cystine can be ameliorated by a catalytic antioxidant.
- Measurement of plasma cysteine/cystine could serve as redox biomarkers in epilepsy.
Abstract
Currently the field of epilepsy lacks peripheral blood-based biomarkers that could predict the onset or progression of chronic seizures following an epileptogenic injury. Thiol/disulfide ratios have been shown to provide a sensitive means of assessing the systemic redox potential in tissue and plasma. In this study, we utilized a rapid, simple and reliable method for simultaneous determination of several thiol-containing amino acids in plasma using HPLC with electrochemical detection in kainic acid (KA) and pilocarpine rat models of epilepsy. In contrast to GSH and GSSG levels, the levels of cysteine (Cys) were decreased by 42% and 62% and cystine (Cyss) were increased by 46% and 23% in the plasma of KA- and pilocarpine-injected rats, respectively after 48 h. In chronically epileptic rats, plasma cysteine was decreased by 40.4% and 37.7%, and plasma GSSG increased by 33.8% and 35.0% following KA and pilocarpine, respectively. Treatment of rats with a catalytic antioxidant, 60 min after KA or pilocarpine significant attenuated the decrease of plasma Cys/Cyss ratios at the 48 h time point in both models. These observations suggest that the decreased cysteine and ratio of Cys/Cyss in plasma could potentially serve as redox biomarkers in temporal lobe epilepsy.
Graphical abstract
Keywords
Epilepsy ; Biomarker ; Cysteine ; Glutathione ; Catalytic antioxidant ; HPLC
1. Introduction
Peripheral biomarkers for epilepsy are notably absent from the tools available to inform its diagnosis and/or treatment. Although a few studies have suggested peripheral biomarkers in human epilepsies, the field lacks biomarkers that diagnose, predict, identify drug response and guide clinical trials [2] . Importantly, biomarkers of epileptogenesis are lacking. While it is recognized that only a fraction of individuals go on to develop epilepsy after severe brain injury, no tests are currently available to identify an at-risk group after injury. Ideally, blood-derived biomarker(s) collected in the phases immediately post-insult, epileptogenic period and chronic epilepsy period could inform diagnosis and/or treatment. Candidate biomarkers may be identified from knowledge of the pathogenesis of epilepsy development. In particular, processes such as inflammation, oxidative stress, gene regulation and metabolic alterations which are known mediators of epileptogenic insults may reveal key biomarker candidates [4] and [15] .
There has been significant progress in developing peripheral biomarkers indicative of redox changes in various disease such as atherosclerosis, cardiovascular diseases, chronic neurodegenerative diseases [1] and [3] . Redox alterations have been shown to occur in animal models of epilepsies and may contribute to seizure genesis, epileptogenesis and comorbidities [17] . Thiol/disulfide ratios provide a sensitive means of assessing the systemic redox potential of tissue and plasma in health and disease [11] . Cysteine is an important amino acid in proteins that undergoes reversible oxidation/reduction under biologic conditions and has been shown to not only control protein function, but also serve as a biomarker of oxidative damage. The ratio of cysteine/cystine (Cys/Cyss) has been proposed to serve as a biomarker of redox status of cells, tissues and plasma in animals and humans (see review [3] ). We have previously shown that epileptogenic injury causes oxidative damage and alterations in the brain tissue glutathione redox couples. Specifically, we have shown that the ratios of glutathione redox couple i.e. reduced glutathione: glutathione disulfide (GSH: GSSG) and/or reduced coenzyme A (CoASH) and its glutathione disulfide (CoASSG) i.e. CoASH: CoASSG are decreased in the kainic acid (KA) and pilocarpine models of TLE [10] , [18] and [14] . The goal of this study was to determine plasma thiol redox status in two chemoconvulsant models of epilepsy.
2. Materials and methods
2.1. Reagents
All chemicals were purchased from Sigma Aldrich. Kainic acid was purchased from AG Scientific, Inc. (New York, NY USA). Manganese (III) meso -tetrakis (di-N-ethylimidazole) porphyrin designated as AEOL 10150 was provided by Aeolus Pharmaceuticals through a research agreement with the University of Colorado.
2.2. Kainic acid and pilocarpine treatment
Adult, male Sprague-Dawley rats (~300 g), purchased from Harlan Laboratories (Indianapolis, Indiana) were used in all experiments. Upon arrival, rats were group housed on a 12/12 light/dark cycle with ad libitum access to both food and filtered water. Animal studies were carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institute Animal Care and Committee (IACUC) at the University of Colorado Anschutz Medical Campus. After one week of acclimation, rats were treated with kainic acid (11 mg/kg, s.c.) or pilocarpine (350 mg/kg, s.c.) to induce SE. In pilocarpine treated rats were injected with scopolamine (1 mg/kg, i.p.) 30 min prior to pilocarpine to limit peripheral cholinergic effects and diazepam (10 mg/kg, i.p.) 90 min after pilocarpine to terminate SE. All rats were visually monitored during SE and behavioral seizures were scored using a modified Racine scale [9] . Briefly, seizures were scored on the following scale: P1- freezing behavior, P2-head nodding, P3- unilateral forelimb clonus, P4- bilateral forelimb clonus with rearing, and P5- bilateral forelimb clonus with rearing, falling, and/or hindlimb clonus. Only rats experiencing SE defined as: at least P3 or higher seizures followed by a period of continuous seizure activity for at least 90 min, were included in further analyses as SE is the best predictor for the development of chronic epilepsy. Animals were sacrificed at 48 h (acute seizures) or 12 weeks (spontaneous chronic seizures) after initial treatment. In spontaneous chronic seizures group, only the rats exhibiting P3 or higher chronic seizures by video monitoring were used. In acute seizure group, some KA or pilocarpine-treated rats were administered with AEOL 10,150 5 mg/kg, s.c. at 60 min after KA or pilocarpine and thereafter every 4 h until sacrifice. Rats injected with KA or pilocarpine that did not reach behavioral P3 score were called “non-responders”.
2.3. Plasma preparation
Blood (~0.5 ml) was drawn with a 23-gauge heparinized needle and syringe. Add blood from the syringe into a tube containing 10 μl heparin (100 units/ml in saline, heparin sodium salt, sigma H3393). Invert tube gently (to avoid hemolysis) 2 times to mix. The blood tubes were centrifuged at 13,000 rpm 4 °C for 5 min to remove RBCs as soon as possible. Transfer 100 or 200 μl of supernatant (plasma) to a new tube with cold same volume 2% PCA/0.2 M boric acid. Samples stored at −80 °C until assay.
2.4. Measurement thiols and disulfides in plasma
The levels of cystine, cysteine, homocysteine, methionine, glutathione, glutathione disulfide, ascorbic acid and tyrosine in plasma were measured with an ESA (Chelmsford, MA) 5600 CoulArray HPLC equipped with eight electrochemical cells following the principle of company instruction (Dionex Application Brief 131) and method prescribed obviously [5] with some modification. Potentials of the electrochemical cells were set at 400/500/600/650/700/750/800/850 mV vs. Pd. Analyte separation was conducted on YMC-Pack ODS-A C-18 analytical column (Waters Inc., 4.6 mm×250 mm; 5 µm particle size). A two-component gradient elution system was used with component A of the mobile phase composed of 50 mM NaH2 PO4 pH 2.7, 1.0 mM 1-octanesulfonic acid and component B composed of 50 mM NaH2 PO4 pH 2.7, 1.0 mM 1-octanesulfonic acid and 50% methanol. 100% A is run at 0.4 ml /min from 0 to 10 min at initial time and a linear gradient to 100% A 0.6 ml/min is run over the period from 10 to 18 min From 18 to 20 min, the conditions are maintained at 100% A 0.6 ml/min then is run a linear gradient to 92% A, 8% B 0.6 ml/min from 20 to 28 min and returned back 100% A 0.4 ml/min from 28 to 32 min Equilibration time for the next run is 8 min The sample tubes containing 1:1 ratio plasma and 2% PCA/0.2 M boric acid were centrifuged at 13,000g , 4 °C for 10 min to pellet protein. An aliquot (20 μl) of the supernatant was injected into the HPLC.
2.5. Statistical analyses
All data is expressed as mean±SEM. Statistical differences were analyzed by one-way ANOVA with post hoc multiple comparison test (Tukey test) or Students t -test (two-tailed). P values less than 0.05 were considered statistically significant. All analyses were performed using GraphPad Prism 5 software.
3. Results
HPLC coupled with electrochemical detection enables the simultaneous determination of most thiol-containing amino acids, including cysteine, cystine, homocysteine, methionine as well as GSH and GSSG, and ascorbate in plasma. Separation of all peaks from biological samples and standards was achieved with distinct retention times and potentials (channels). All peaks observed in samples were additionally identified using known standards with retention times and ratios of peak distribution in different channels. The variation of retention time and ratio of distribution in different channels between standards and biological samples were <4%. Reproducibility of the method was evaluated by making six injections of different concentration standards (0.01–2 nmol) or different volume plasma solution (1–100 μl) per day in a 5-day period. Repeated regression analysis of the standard or samples of cystine, methionine and GSH reached R=0.99 and cysteine and GSSG reached R=0.97–0.98. Typical chromatographs were shown in Fig. 1 and Fig. 2 .
|
|
|
Fig. 1. The chromatograph of the standards from left to right: Cystine 1 nmol, Cysteine 0.4 nmol, homocysteine 0.4 nmol, Ascorbic acid (AA) 2 nmol, Methionine (Met) 1 nmol, GSH 0.4 nmol, Tyrosine 2 nmol and GSSG 0.2 nmol were loaded into the column. Channel 7 (blue) potential: 800 mv; Channel 4 (red) potential: 650 mv and Channel 2 (green) potential: 500 mv. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
Fig. 2.
The chromatograph of the plasma samples from left to right: Cystine, Cysteine, homocysteine, Ascorbic acid (AA), Methionine (Met), GSH, Tyrosine and GSSG. All of the peaks in plasma samples were double identified by standards with retention time and ratio of peak distribution in different potentials (channels).
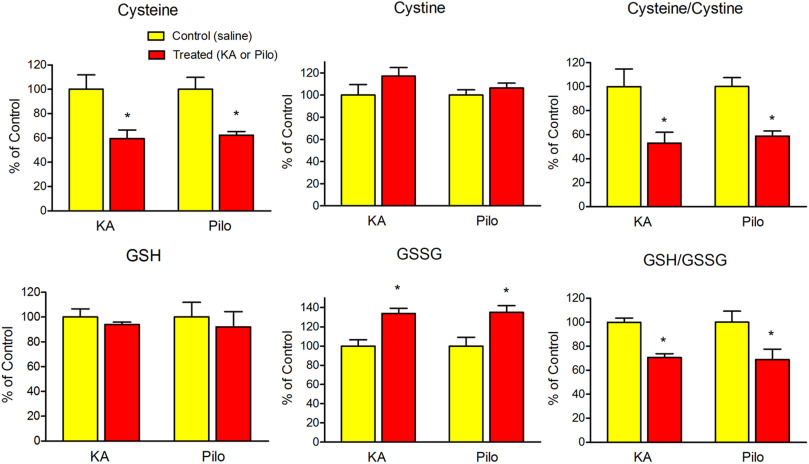
Evaluation of plasma cysteine and cystine 48 h after treatment with chemoconvulsants revealed decreases of 42% and 62% in cysteine levels and an increase of 46% and 23% in cystine levels in KA- and pilocarpine-treated rats, respectively. Ratios of cysteine/cystine (Cys/Cyss) were significantly decreased by 60.6% after KA and 69.5% after pilocarpine. However, the plasma levels of GSH and GSSG were not significantly altered in either model 48 h after injection of KA or pilocarpine. To verify the role of oxidative stress on altered cysteine and cystine levels, we determined the effect of a catalytic antioxidant, AEOL10150. Treatment of rats with AEOL10150 administered 60 min after KA or pilocarpine and every 4 h thereafter for 48 h abolished the decreased Cys/Cyss ratios. (Fig. 3 ).
|
|
|
Fig. 3. The levels of cysteine, cystine, cysteine/cystine, GSH, GSSG and GSH/GSSG in the plasma of rats 48 h after KA or pilocarpine. Saline or AEOL10150 5 mg/kg (dissolved in saline) was injected by s.c. route beginning 60 min after KA or pilocarpine and every 4 h thereafter until sacrifice. Bars represent mean+S.E.M. The control levels of cysteine, cystine, GSH and GSSG in plasma at 48 h after saline treatment are as follows: 5.66±0.31; 32.52±1.16; 3.91±0.11; 0.38±0.03, µM. *p<0.01 vs. control, #p<0.05 vs. KA+saline or pilocarpine+saline group, one-way, ANOVA, n=6 per group. |
In chronically epileptic rats displaying spontaneous behavioral seizures (12 week time point), the levels of plasma cysteine were significantly decreased by 40.4% and 37.7% after KA and pilocarpine administration, respectively. The levels of plasma cystine showed mild and statistically insignificant increases of 17.1% and 6.3% after KA and pilocarpine administration, respectively. Interestingly, GSSG levels were also increased by 33.8% and 35.0% in KA and pilocarpine models, respectively. However, no changes in GSH levels were observed (Fig. 4 ). Ratios of Cys/Cyss and GSH/GSSG were significantly decreased by 47.0% and 29.4% after KA and 41.2% and 31.1% after pilocarpine, respectively.
|
|
|
Fig. 4. The levels of cysteine, cystine, cysteine/cystine, GSH, GSSG and GSH/GSSG in the plasma of rats 12 weeks after KA or pilocarpine treatment. Bars represent mean+S.E.M. The control levels of cysteine, cystine, GSH and GSSG in plasma at 12 weeks after saline treatment are as follows: 5.70±0.38; 32.61±1.69; 4.45±0.30; 0.54±0.03, µM. *p<0.01 vs. control group, t -test (two-tailed), n=5–7 per group. |
In non-responders, no changes were observed the levels of cysteine, cystine, GSH, or GSSG at 48 h or 12 weeks after injection of KA or pilocarpine. Furthermore, no changes in the levels of homocysteine, ascorbic acid, methionine or tyrosine were observed in either model at the 48 h or 12 week time point (data not shown).
4. Discussion
In this study, we report three major observations. 1) A HPLC method with electrochemical detection protocol provides a rapid, simple and reliable method that allows the simultaneous determination several thiols and thiol-containing amino acids in plasma. 2) The levels of cysteine and ratios of Cys/Cyss were significantly decreased at the 48 h and 12 week time points in the plasma of rats administered KA or pilocarpine. 3) Antioxidant treatment significantly inhibited the depletion of Cys/Cyss at the 48 h time point in both the KA and pilocarpine models confirming the role of oxidative stress.
Here we provide a simpler electrochemical detection method involving sample pretreatment that overrides pH adjustment and derivatization required for fluorescence detection. Repeated regression analysis of the standards and samples using this method showed high correlations coupled with low variability. Furthermore, HPLC with fluorescence detection method only measures cysteine, cystine, GSH and GSSG, whereas electrochemical detection allows measurement of additional thiol containing amino acids, such as homocysteine and methionine. Cysteine and methionine are the only amino acids in proteins capable of undergoing reversible oxidation/reduction under biologic conditions [6] . The method was validated in this study for a simultaneous determination of thiols containing amino acids in plasma.
GSH/GSSG and Cys/Cyss, the two major low-molecular-weight thiol/disulfide couples in mammalian plasma, are not in redox equilibrium with Cys/Cyss redox potential (Eh ) ~60 mV more oxidized than Eh of GSH/GSSG in plasma [6] and [8] . Human studies have revealed that the plasma Eh of Cys/Cyss is more readily oxidized in comparison to the plasma Eh of GSH/GSSG smoking, atherosclerosis, cardiovascular diseases and aging [7] , [12] , [1] and [3] . This is evident in our rats at 48 h time point after KA or pilocarpine-induced SE which revealed significant depletion cysteine, increased cystine and decreased Cys/Cyss ratio, but not GSH, GSSG or GSH/GSSG ratio in the plasma. In chronically epileptic rats (12 week time point), although both Cys/Cyss and GSH/GSSG ratio were decreased, the Cys/Cyss ratio was decreased to a greater magnitude than GSH/GSSG in both of models. Furthermore, regardless of the time point or model, decreased cysteine levels dictated the increased oxidative stress in plasma. The lack of changes in plasma cysteine in the plasma in “non-responder animals” suggests that oxidative stress occurs due to ongoing disease progression and is consistent with our previous observations in hippocampal tissue of “non-responder animals” [14] .
The ability of the catalytic antioxidant AEOL10150 to prevent changes in plasma cysteine, cystine, Cys/Cyss and GSSG suggests role of oxidative stress in their depletion. AEOL10150 is known to possess high superoxide dismutase (SOD), catalase activity and lipid peroxidation inhibitory activities and achieves sufficient plasma levels to exert antioxidant effects in rats [13] . Previous studies from our laboratory have demonstrated AEOL10150s efficacy against brain oxidative stress indices including decreased GSH/GSSG and increased 3-nitrotyrosine in KA and pilocarpine TLE rats models [14] and [16] . Taken together, these observations suggest that the decreased cysteine and ratio of Cys/Cyss in plasma can serve as sensitive biomarkers for oxidative damage in the KA and pilocarpine models of TLE. Furthermore, they may serve as surrogate biomarkers of disease progression, pharmacoresistance and/or treatment response in epilepsy patients.
Conflicts of interest
Dr. Patel is a consultant for Aeolus Pharmaceuticals which develops catalytic antioxidants for human diseases.
Acknowledgments
This work was funded by NINDS Grants UO1NS083422 (M.P) and R01NS086423 (MP) . This work was supported (in part) by a grant from the Associate Dean of Research Seed Grant Program, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado. The authors would like to thank Drs. Lindsey Gano, Jennifer Pearson and Shane Rowley for providing some of the plasma samples and help with the KA, pilocarpine and AEOL 10150 injections.
References
- [1] S. Ashfaq, J.L. Abramson, D.P. Jones, S.D. Rhodes, W.S. Weintraub, W.C. Hooper, V. Vaccarino, D.G. Harrison, A.A. Quyyumi; The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults; J. Am. Coll. Cardiol., 47 (2006), pp. 1005–1011
- [2] J. Engel Jr., A. Pitkanen, J.A. Loeb, F.E. Dudek, E.H. Bertram 3rd, A.J. Cole, S.L. Moshe, S. Wiebe, F.E. Jensen, I. Mody, A. Nehlig, A. Vezzani; Epilepsy biomarkers; Epilepsia, 54 (Suppl. 4) (2013), pp. S61–S69
- [3] Y.M. Go, D.P. Jones; Cysteine/cystine redox signaling in cardiovascular disease; Free Radic. Biol. Med., 50 (2011), pp. 495–509
- [4] M. Hegde, D.H. Lowenstein; The search for circulating epilepsy biomarkers; Biomark. Med., 8 (2014), pp. 413–427
- [5] P. Jandik, J. Cheng, J. Evrovski, N. Avdalovic; Simultaneous analysis of homocysteine and methionine in plasma; J. Chromatogr. B Biomed. Sci. Appl., 759 (2001), pp. 145–151
- [6] D.P. Jones, Y.M. Go, C.L. Anderson, T.R. Ziegler, J.M. Kinkade Jr., W.G. Kirlin; Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control; FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol., 18 (2004), pp. 1246–1248
- [7] D.P. Jones, V.C. Mody Jr., J.L. Carlson, M.J. Lynn, P. Sternberg Jr.; Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses; Free Radic. Biol. Med., 33 (2002), pp. 1290–1300
- [8] M. Kemp, Y.M. Go, D.P. Jones; Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology; Free Radic. Biol. Med., 44 (2008), pp. 921–937
- [9] L.P. Liang, Y.S. Ho, M. Patel; Mitochondrial superoxide production in kainate-induced hippocampal damage; Neuroscience, 101 (2000), pp. 563–570
- [10] L.P. Liang, M. Patel; Seizure-induced changes in mitochondrial redox status; Free Radic. Biol. Med., 40 (2006), pp. 316–322
- [11] S.E. Moriarty-Craige, D.P. Jones; Extracellular thiols and thiol/disulfide redox in metabolism; Annu. Rev. Nutr., 24 (2004), pp. 481–509
- [12] S.E. Moriarty, J.H. Shah, M. Lynn, S. Jiang, K. Openo, D.P. Jones, P. Sternberg; Oxidation of glutathione and cysteine in human plasma associated with smoking; Free Radic. Biol. Med., 35 (2003), pp. 1582–1588
- [13] H.C. O’Neill, C.W. White, L.A. Veress, T.B. Hendry-Hofer, J.E. Loader, E. Min, J. Huang, R.C. Rancourt, B.J. Day; Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide; Free Radic. Biol. Med., 48 (2010), pp. 1188–1196
- [14] J.N. Pearson, S. Rowley, L.P. Liang, A.M. White, B.J. Day, M. Patel; Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy; Neurobiol. Dis., 82 (2015), pp. 289–297
- [15] A. Pitkanen, J. Engel Jr.; Past and present definitions of epileptogenesis and its biomarkers; Neurotherapeutics, 11 (2014), pp. 231–241
- [16] S. Rowley, L.P. Liang, R. Fulton, T. Shimizu, B. Day, M. Patel; Mitochondrial respiration deficits driven by reactive oxygen species in experimental temporal lobe epilepsy; Neurobiol. Dis., 75 (2015), pp. 151–158
- [17] S. Rowley, M. Patel; Mitochondrial involvement and oxidative stress in temporal lobe epilepsy; Free Radic. Biol. Med., 62 (2013), pp. 121–131
- [18] S. Waldbaum, L.P. Liang, M. Patel; Persistent impairment of mitochondrial and tissue redox status during lithium-pilocarpine-induced epileptogenesis; J. Neurochem., 115 (2010), pp. 1172–1182
Document information
Published on 20/10/16
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?